Abstract
Nontyphoidal Salmonella (NTS) species cause self-limiting diarrhea and sometimes severe disease. Antibiotic treatment is considered only in severe cases and immune-compromised patients. The beneficial effects of antibiotic therapy and the consequences for adaptive immune responses are not well understood. We used a mouse model for Salmonella diarrhea to assess the effects of per os treatment with ciprofloxacin (15 mg/kg of body weight intragastrically 2 times/day, 5 days) or parenteral ceftriaxone (50 mg/kg intraperitoneally, 5 days), two common drugs used in human patients. The therapeutic and adverse effects were assessed with respect to generation of a protective adaptive immune response, fecal pathogen excretion, and the emergence of nonsymptomatic excreters. In the mouse model, both therapies reduced disease severity and reduced the level of fecal shedding. In line with clinical data, in most animals, a rebound of pathogen gut colonization/fecal shedding was observed 2 to 12 days after the end of the treatment. Yet, levels of pathogen shedding and frequency of appearance of nonsymptomatic excreters did not differ from those for untreated controls. Moreover, mice treated intraperitoneally with ceftriaxone developed an adaptive immunity protecting the mice from enteropathy in wild-type Salmonella enterica serovar Typhimurium challenge infections. In contrast, the mice treated intragastrically with ciprofloxacin were not protected. Thus, antibiotic treatment regimens can disrupt the adaptive immune response, but treatment regimens may be optimized in order to preserve the generation of protective immunity. It might be of interest to determine whether this also pertains to human patients. In this case, the mouse model might be a tool for further mechanistic studies.
INTRODUCTION
Nontyphoidal Salmonella (NTS) species, such as Salmonella enterica serovar Typhimurium, are among the most common causative agents of food-borne diarrheal diseases worldwide. The typical disease symptoms, involving stomach cramps, nausea, and acute diarrhea, appear approximately 6 to 72 h after consumption of contaminated food or water (4, 43). S. Typhimurium-induced diarrhea is usually self-limiting and resolves within 5 to 7 days (9). Afterwards, the patients typically continue to shed the pathogen for a period of 2 to 8 weeks. In some individuals, the pathogen is able to establish a persistent infection (32), resulting in the excretion of Salmonella spp. for 6 months or even longer after the remission of the acute symptoms (2, 6). These asymptomatic carriers may pose a risk to their environment, as they can spread the pathogen, especially when workers in restaurants or in the food industry are affected (21).
Noncomplicated cases are generally treated by electrolyte and fluid replacement (25). Here, antimicrobial therapy is not recommended, as it does not shorten the length of diarrhea, reduces pathogen shedding only transiently, involves the risk of adverse drug reactions, and may even increase the rates of long-term shedding (25, 45). An additional problem arising from antibiotic treatment would be a disruption of an adaptive immune response, for which changes in antigen dosage or kinetics might be critical. For practical and ethical reasons, the protection from future disease is very difficult to study in human patients and the effect of antibiotic treatment on the adaptive immune response remains unknown.
In some cases, NTS can cause severe disease, i.e., severe diarrhea and extraintestinal infection (19, 25). Immune-compromised individuals, newborns, and the elderly may be at particular risk (e.g., see references 19, 25, 46, and 49). These patients are often treated with antibiotics (25, 45). However, it is not well understood to which extent this may foster the emergence of long-term asymptomatic Salmonella excreters or the emergence/spread of antibiotic resistance (16) or impair immune protection after reinfection with Salmonella.
Another common side effect is antibiotic-associated diarrhea (AAD), caused by an alteration of the gut microbiota composition, resulting in a possible overgrowth of opportunistic pathogenic bacteria such as Clostridium difficile (31). A repeated exposure to therapeutic doses of antimicrobials can even lead to long-term disruption of the gut flora (10, 11, 20). This side effect is not restricted to orally applied antibiotics. Parenteral application can also affect intestinal microbiota, presumably due to gut targeting through the biliary system (17).
Normally, the microbial ecosystem, consisting of about 1010 to 1012 bacteria (12), efficiently prevents invasion by foreign species. This has been extensively studied in the case of enteric pathogens and is known as colonization resistance (CR) (57). Clinical observations suggest that antibiotic treatment may increase the incidence of long-term asymptomatic NTS excreters (30, 41, 48). Furthermore, antibiotic therapy may increase the risk of infection with antibiotic-resistant bacteria (18) or disrupt beneficial effects of the microbiota on intestinal immune homeostasis (7, 39). This has resulted in an ongoing controversy on whether antibiotic treatment might interfere with the generation of a protective immune response (54). However, systematic studies of these potentially adverse phenomena are scarce, and we do not know whether they are causally linked or which of them are causally linked.
Here, we have employed a well-established mouse model for acute Salmonella diarrhea (29) to study the effects and side effects of antibiotic treatment on the disease and on pathogen shedding. The streptomycin mouse model has recently been adapted to recapitulate the key phases of a human NTS infection, including the acute enteropathy, the generation of a protective adaptive immune response, as well as elimination of the pathogen from the gut lumen. Moreover, a small fraction of mice develops into nonsymptomatic excreters (13).
We have employed this model to assess the effects of ciprofloxacin or ceftriaxone on the course of the Salmonella infection in S. Typhimurium-infected mice. The fluoroquinolone antibiotic ciprofloxacin is often recommended for treatment of severe Salmonella infections and for chronic carriers (25, 33, 35, 45), since it has excellent activity in vitro and in vivo against different Salmonella strains. Furthermore, extended-spectrum cephalosporins such as ceftriaxone are especially used for treating children, as they provide pharmacodynamic advantages and resistant strains are still not very frequent (8, 15). Specifically, we were interested in the effects of antibiotic treatment on the burden of the acute disease and on the incidence, the intensity, or the duration of pathogen excretion. Furthermore, we analyzed the effects of antibiotic treatment on the generation of a protective mucosal immune response.
MATERIALS AND METHODS
Animals.
Conventional, specified-pathogen-free (SPF) wild-type (wt) C57BL/6 mice (7 to 10 weeks old) were bred at the Rodent Center HCI (RCHCI, Zurich, Switzerland) under barrier conditions in individually ventilated cages (IVC; Ehret).
Ethics statement.
All animal experiments were approved (licenses 201/2007 and 223/2010 by the Kantonales Veterinäramt Zürich) and performed according to local guidelines (TschV, Zurich, Switzerland) and the Swiss animal protection law (TschG).
Infection experiments.
Salmonella infections were performed in individually ventilated cages at the RCHCI, Zurich, Switzerland, as previously described (50). In brief, wild-type C57BL/6 mice were pretreated with 20 mg of streptomycin by gavage, and 24 h later, the mice were inoculated with 5 × 107 CFU of an attenuated S. Typhimurium strain (S. Typhimuriumatt; strain SL1344 sseD::aphT Strepr Kmr) (22) (MIC of ampicillin, 32 μg/ml; MIC of ciprofloxacin, 0.05 μg/ml; MIC of ceftriaxone, 0.5 μg/ml; MIC of streptomycin, 5,000 μg/ml; MIC of kanamycin, 500 μg/ml). S. Typhimuriumatt was used, as it avoids death from systemic pathogen spread and thereby allows recapitulation of the different phases of the diarrheal NTS infection for ≥80 days (13). Starting at day 2 postinfection (p.i.), mice were treated 2 times per day with either 15 mg/kg of body weight ciprofloxacin (Bayer) (by gavage), 50 mg/kg ceftriaxone (Rocephin; Roche) (intraperitoneally [i.p.]), or phosphate-buffered saline (PBS; control) for a period of 5 days. Subsequently, mice were sacrificed at either day 5 postinfection or day 40 p.i. The antibiotic dosage was in the range of current standard therapy for laboratory animals and verified experimentally by monitoring bacterial shedding (i.e., its reduction) after antibiotic treatment.
For challenge infections (at day 40 p.i.), mice were treated with ampicillin (20 mg, by gavage) and infected 24 h later with a dose of 200 CFU of the respective ampicillin-resistant (pM973) wild-type strain (SL1344). Samples of cecal tissue were cryoembedded, and inflammation was quantified on cryosections (5 μm, cross-sectional) stained with hematoxylin and eosin (H&E). Pathogen colonization was assessed as described below.
Histology.
H&E-stained cecum cryosections were scored as described previously, evaluating submucosal edema, polymorphonuclear leukocyte infiltration, goblet cells, and epithelial damage and yielding a total score of 0 to 13 points (23).
Analysis of S. Typhimurium loads in cecal content, MLNs, and spleen.
Mesenteric lymph nodes (MLNs), spleen, and liver were removed aseptically and homogenized in cold PBS (0.5% Tergitol, 0.5% bovine serum albumin [BSA]). The cecum content was suspended in 500 μl cold PBS, and bacterial loads were determined by plating on MacConkey agar plates (50 μg ml−1 streptomycin) as described previously (53). Colonization levels of the challenge strain (carrying pM973, which encodes an ampicillin resistance marker) and the strain used for primary infection (S. Typhimuriumatt Kmr) were determined by selective plating (100 μg ml−1 ampicillin or 30 μg ml−1 kanamycin).
Gut wash preparation of secreted immunoglobulin A (sIgA).
The small intestine was flushed with 2 ml of a washing buffer containing PBS, 0.05 M EDTA, pH 8.0, and 66 μM phenylmethylsulfonyl fluoride. Intestinal washes were briefly vortexed and centrifuged at 14,000 rpm and 4°C for 30 min (Eppendorf centrifuge). Aliquots of supernatants were stored at −80°C.
Statistical analysis.
Statistical analysis was performed using the exact Mann-Whitney U test (Prism software, version 4.0c) or Fisher's exact test. A P value of <0.05 (two-tailed) was considered to be statistically significant. In mouse experiments, values were set to the minimal detectable value (10 CFU for cecum, 10 CFU for MLNs, 20 CFU for the spleen) for samples harboring no bacteria.
Immunoblot analysis.
The equivalent of 1 unit of the optical density at 600 nm/ml of an overnight broth culture of S. enterica serovar Enteritidis (wt strain 125109 [55]), Escherichia coli (Nissle 1917 strain, wild type; gift of Sören Schubert), S. Typhimuriumatt (SL1344 sseD::aphT [22]), or proteinase K-treated S. Typhimuriumatt (0.4 mg/ml, 1 h, 57°C; Gibco/Life Technologies) was pelleted by centrifugation (14,000 rpm, 2 min), and the supernatant was discarded. Cells were resuspended in Laemmli sample buffer (0.065 M Tris-HCl [pH 6.8], 2% [wt/vol] sodium dodecyl sulfate [SDS], 5% [vol/vol] β-mercaptoethanol, 10% [vol/vol] glycerol, 0.05% [wt/vol] bromophenol blue) and lysed for 5 min at 95°C. Equivalent amounts of the different lysates were loaded onto a 12% SDS-polyacrylamide gel, and proteins were separated by electrophoresis. Immunoblots were stained with mouse serum (diluted 1:200 in PBS) or intestinal lavage fluid specimens (diluted 1:20 in PBS) from S. Typhimuriumatt-infected and PBS-treated, ciprofloxacin-treated, or ceftriaxone-treated mice. A goat anti-mouse IgA horseradish peroxidase (HRP) conjugate (Southern Biotech) was used as secondary antibody, and an enhanced chemiluminescence kit (Amersham) was used to develop the blot.
Bacterial fluorescence-activated cell sorter (FACS) analysis.
Analysis was performed as described recently (47). Three-milliliter LB cultures of the tested strain were inoculated from single colonies of plated bacteria and cultured overnight at 37°C without shaking. One milliliter of culture was gently pelleted for 4 min at 7,000 rpm in an Eppendorf centrifuge and washed 3 times with sterile-filtered PBS (1% BSA, 0.05% sodium azide), before it was resuspended to yield a final density of 107 bacteria per ml. Mouse serum was diluted 1:20 in PBS (1% BSA, 0.05% sodium azide) and heat inactivated at 60°C for 30 min. The serum solution was then spun at 13,000 rpm in an Eppendorf centrifuge for 10 min to remove any bacterium-sized contaminants, and the supernatant was used to prepare serial dilutions (1:20, 1:60, 1:180). Twenty-five microliters of the serum dilution and 25 μl of the bacterial suspension were mixed and incubated at 4°C for 1 h. The bacteria were washed twice before staining with monoclonal fluorescein isothiocyanate–anti-mouse IgA (559354; BD Pharmingen), phycoerythrin–anti-mouse total IgG (715-116-151; Jackson ImmunoResearch Europe), and allophycocyanin–anti-mouse IgM (550676; BD Pharmingen). Following an hour of incubation, bacteria were washed once with PBS (1% BSA, 0.05% sodium azide) and then resuspended in PBS (2% paraformaldehyde) for analysis on a FACSCalibur flow cytometer using forward scatter and side scatter parameters in logarithmic mode. The data were analyzed using FlowJo software (Treestar). Analysis of specific IgA levels in intestinal lavage fluid specimens was performed using an identical protocol and 1:2, 1:6, and 1:18 dilutions of the respective gut washes.
RESULTS
Peroral ciprofloxacin treatment reduces S. Typhimuriumatt loads in the intestine and cures acute intestinal inflammation.
Before the adaptive immune response could be studied, we needed to establish the effects of antibiotic treatment in our mouse model. To analyze the effect of ciprofloxacin, we used the animal model depicted in Fig. 1. Conventional C57BL/6 mice were pretreated with streptomycin and infected with S. Typhimuriumatt (5 × 107 CFU by gavage). By day 1 p.i., the pathogen efficiently colonized the intestine (108 to 109 CFU/g feces; Fig. 2A) (24). In the experimental group, the mice were treated with ciprofloxacin (15 mg/kg, orally, 2 times/day) for 5 days starting at day 2 p.i. Fecal pathogen excretion was already significantly reduced after 1 day of treatment (<105 CFU/g feces; P < 0.05 for day 1 versus day 3 p.i.; Fig. 2A, closed symbols) and lasted throughout the 5-day period of the ciprofloxacin treatment. In 15/21 mice, we observed a complete elimination of fecal S. Typhimuriumatt shedding by day 5 p.i. (≤10 CFU/g, the detection limit in feces). In contrast, S. Typhimuriumatt shedding remained high in the feces of the controls (PBS, ≥108 CFU/g feces, day 5 p.i.; Fig. 2A, open symbols).
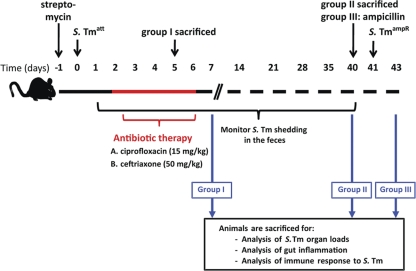
Fig 1.
Model system to study effects of antibiotic treatment of Salmonella infection. At day 0, streptomycin-treated mice were infected with S. Typhimuriumatt (S. Tmatt; 5 × 107 CFU intragastrically). At day 2 p.i., the mice were treated with either ciprofloxacin (15 mg/kg by gavage 2 times/day) or ceftriaxone (50 mg/kg i.p.) for 5 days. A first group of mice (group I) was sacrificed at day 5 p.i. to assess the impact of antibiotic treatment on gut inflammation and S. Typhimuriumatt colonization of the gut. S. Typhimuriumatt excretion was analyzed in groups II and III until day 40 p.i. At day 40 p.i., group II was sacrificed to determine intestinal inflammation and the presence of S. Typhimuriumatt-specific antibody levels in serum and gut washes of immunized mice. Group III was pretreated with ampicillin (20 mg orally) and challenged with ampicillin-resistant wt S. Typhimurium Ampr (200 CFU orally) at day 40 p.i. The degree of S. Typhimurium-induced gut inflammation was analyzed 2 days postchallenge.
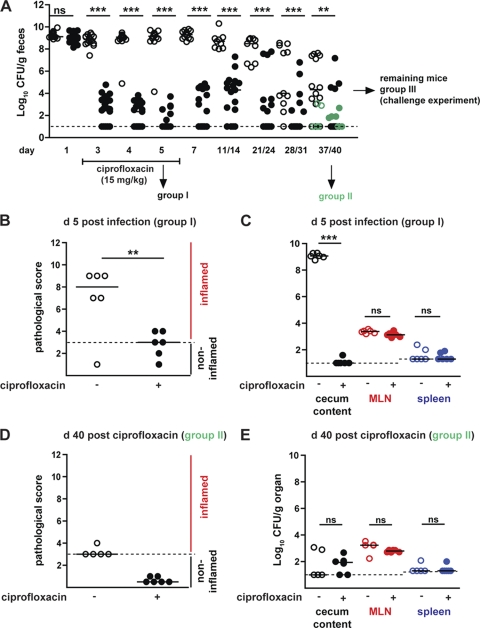
Fig 2.
Effects of ciprofloxacin treatment on the course of the primary diarrheal S. Typhimuriumatt infection. (A) Time course of fecal S. Typhimuriumatt shedding over a time period of 40 days. Streptomycin-treated mice were infected with S. Typhimuriumatt. At day 2 p.i., S. Typhimuriumatt-infected mice were treated with either PBS (control group; open circles) or ciprofloxacin (15 mg/kg by gavage 2 times/day; black circles) for 5 days, and S. Typhimurium shedding was monitored (5 < n < 23 individual mice per time point). (B) A group of mice (group I) was sacrificed at day 5 p.i., and cecum pathology was analyzed. (C) S. Typhimuriumatt loads in cecum content (black circles), MLN (red circles), and the spleen (blue circles) at day 5 p.i. (D) A group of mice was sacrificed at day 40 p.i. (group II, in green). Cecum pathology at day 40 p.i. of control and ciprofloxacin-treated mice. (E) S. Typhimuriumatt loads in cecum content (black), MLN (red), and the spleen (blue) at day 40 p.i. Dashed lines, detection limit for feces, cecum content, and MLN (10 CFU/g) and for spleen (20 CFU/g); black bars, median; **, P < 0.005; ***, P < 0.0005; ns, not significant.
Group I was sacrificed at day 5 p.i. in order to analyze intestinal pathology and colonization levels in cecal contents, MLNs, and spleens. Interestingly, 3 days of ciprofloxacin treatment was sufficient to cure the acute mucosal inflammation in all S. Typhimuriumatt-infected mice (6/6 mice with cecum pathological scores of ≤4), while the PBS-treated controls displayed pronounced intestinal inflammation (5/6 mice with cecal pathological scores of ≥7; Fig. 2B). In contrast, total MLN colonization (103 CFU/g) was not affected by the antibiotic treatment. The systemic spread (spleen) is low in all groups of mice. This is expected for the attenuated S. Typhimurium strain (S. Typhimuriumatt) used in this study (24) (≤20 CFU/g, the detection limit in spleen; Fig. 2C).
Groups II and III allowed the analysis of long-term effects of the 5-day ciprofloxacin treatment. After terminating the ciprofloxacin treatment, fecal shedding increased in some animals but generally remained significantly lower than that in the PBS-treated controls. In the latter group, fecal shedding declined significantly between days 11/14 and day 40 p.i. (Fig. 2A, open symbols). This is in line with earlier work and probably attributable to competitive replacement of S. Typhimuriumatt by the regrowing normal gut flora (13). Group II was sacrificed at day 40 p.i. to analyze intestinal pathology and colonization levels in cecal contents, MLNs, and spleens (Fig. 2D and E). No significant differences were detected with respect to cecal lumen colonization or pathogen loads in the MLNs or the spleens (P > 0.05; Fig. 2E). Furthermore, no sign of overt mucosal disease was evident in either group of mice (pathology score, ≤3; Fig. 2D). Finally, the ciprofloxacin-treated mice did show a slight but nonsignificant reduction of asymptomatic excreter frequency (1/17 mice; ≥105 CFU/g feces; pathology score, ≤3) compared to the nontreated controls (5/19 mice; Fig. 2) (P = 0.18, Fisher's exact test). Thus, ciprofloxacin treatment reduced fecal shedding and mucosal pathology (Fig. 2B and C) at day 5 p.i. but did not affect pathogen loads in the mesenteric lymph nodes or the spleen.
Mice treated with ciprofloxacin per os are not protected from disease upon challenge with wt S. Typhimurium.
Adaptive mucosal immune responses, i.e., sIgA specific for the O antigen of lipopolysaccharide (LPS), can protect from disease, if the same Salmonella serovar is encountered a second time (13, 40, 58). This is generally regarded as a beneficial consequence of the primary infection. However, it has remained unclear whether antibiotic treatment of the primary infection might compromise the generation of protective adaptive immunity.
To analyze the impact of peroral ciprofloxacin treatment on the generation of protective mucosal immunity, we performed a challenge experiment with the remaining animals whose results are shown in Fig. 2A (group III). At day 39 p.i., these S. Typhimuriumatt-infected mice, which had been treated (or not) with ciprofloxacin, were pretreated with ampicillin, as described in Materials and Methods. This transiently suppressed the microbiota which had regrown in these mice and eliminated any S. Typhimuriumatt bacteria which may have persisted in the gut. One day later, the animals were challenged with wt S. Typhimurium (ampicillin resistant, 200 CFU by gavage) (13). Using this type of immunization challenge protocol, S. Typhimuriumatt-immunized mice were generally protected against wt S. Typhimurium-inflicted disease, although wt S. Typhimurium could still colonize the gut lumen within 2 days after challenge. This observation was confirmed in the PBS control group presented here (Fig. 3, open symbols). In contrast, mice which had been treated with ciprofloxacin during the primary infection showed significantly higher levels of mucosal inflammation (7/10 mice with cecum pathology scores of ≥5; Fig. 3A). Those results indicated that peroral ciprofloxacin therapy may interfere slightly but significantly with the generation of a protective mucosal immune response.
Fig 3.
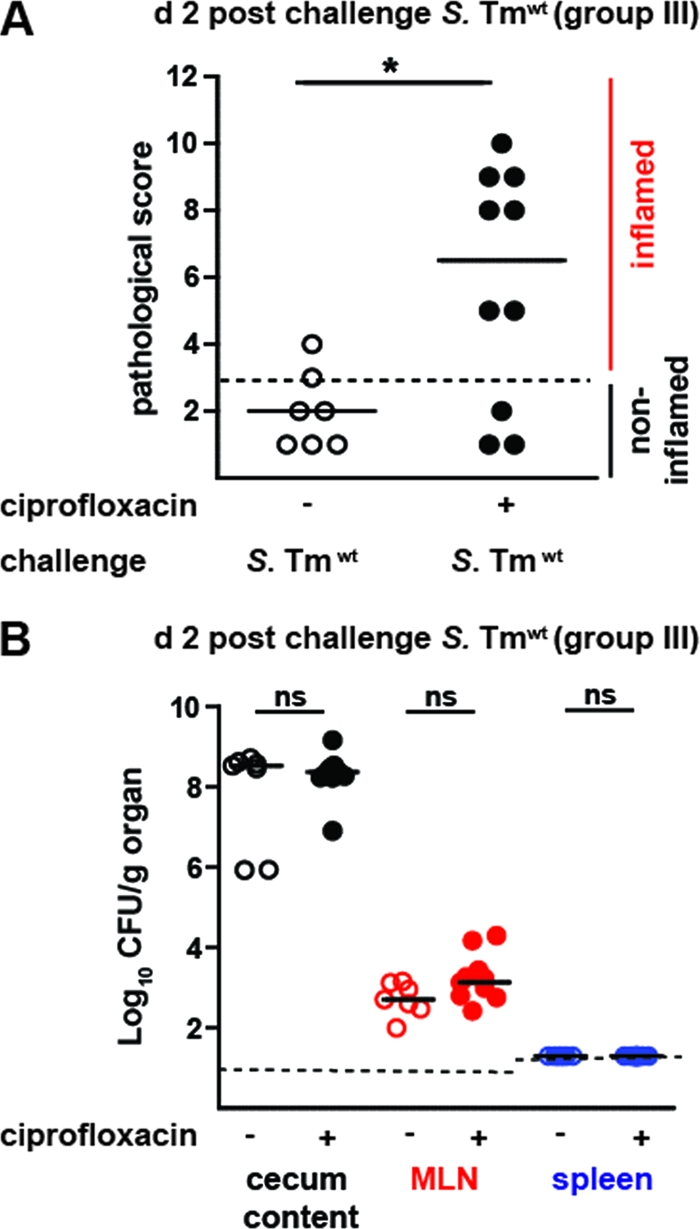
Ciprofloxacin-treated mice display reduced protection in challenge infections. (A) S. Typhimuriumatt-immunized mice had been treated with PBS (control mice; open circles) or ciprofloxacin (black circles). At day 40 p.i., the animals were challenged with wt S. Typhimurium (200 CFU intragastrically, group III). At day 2 postchallenge, inflammation of the cecal mucosa was assessed (a score of ≤3 indicates no inflammation; dashed line). (B) wt S. Typhimurium loads in the cecum content (black circles), MLN (red circles), and the spleen (blue circles). Dashed line, detection limits; black bars, median; *, P < 0.05; ns, not significant.
Effect of parenteral ceftriaxone therapy on the course of a primary S. Typhimuriumatt infection.
Our data presented above indicated that ciprofloxacin might compromise protection from reoccurring S. Typhimurium infections. Therefore, we turned to parenteral treatment with ceftriaxone, an extended-spectrum cephalosporin recommended for treatment of S. Typhimurium-induced bacteremia (15).
To analyze the effect of parenteral ceftriaxone treatment on the short-term benefits and long-term consequences for adaptive immune responses, we performed similar experiments as described above for ciprofloxacin. As expected, the parenteral treatment with ceftriaxone (50 mg/kg, i.p.) significantly reduced S. Typhimuriumatt excretion within 1 day after the onset of therapy (<106 CFU/g; P < 0.05 for day 1 versus day 3 p.i.; Fig. 4A). Within 3 days, ceftriaxone treatment eliminated S. Typhimuriumatt shedding (13/15 mice; ≤10 CFU/g feces) and reduced mucosal inflammation (median cecum pathology score, 4; Fig. 4B). Similar to the peroral ciprofloxacin therapy, the parenteral ceftriaxone therapy did not significantly affect S. Typhimuriumatt levels in the MLNs (103 CFU/g) at day 5 p.i. (Fig. 4C). These data indicated that both treatments provided equivalent therapeutic benefits during the acute phase of the infection.
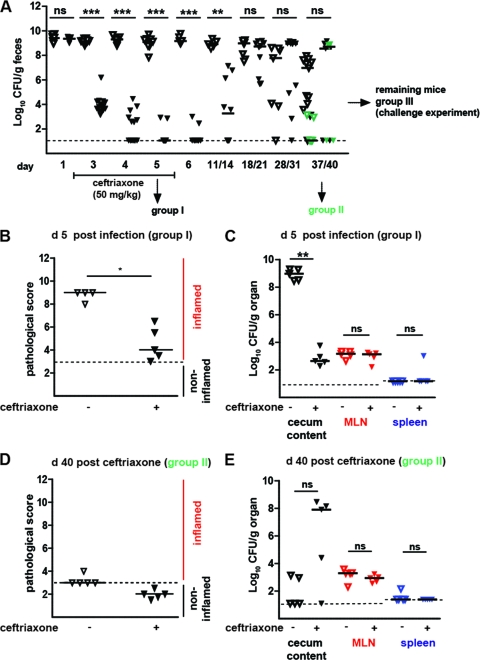
Fig 4.
Effects of ceftriaxone therapy course of primary diarrheal S. Typhimuriumatt infection. (A) Time course of fecal S. Typhimuriumatt shedding. Streptomycin-treated mice were infected with S. Typhimuriumatt. At day 2 p.i., the infected mice were treated with either PBS (control group; open triangles) or ceftriaxone (50 mg/kg i.p.; black triangles) for 5 days, and S. Typhimurium excretion was monitored over a period of 40 days (5 < n < 29 individual mice per time point). Dashed line, detection limit (≤10 CFU/g) for S. Typhimuriumatt in feces. (B) At day 5 p.i., mice from the control and the ceftriaxone-treated groups were sacrificed (group I) and cecum pathology was analyzed. Dashed line, detection limit (a cecum pathology score of ≤3). (C) S. Typhimuriumatt loads in cecum content (black triangles), MLN (red triangles), and the spleen (blue triangles) at day 5 p.i. (D) Cecum pathology of control and ceftriaxone-treated mice sacrificed at day 40 p.i. (group II). (E) S. Typhimuriumatt loads in cecum content (black triangles), MLN (red triangles), and the spleen (blue triangles) at day 40 p.i. (group II). Black bars, median; *, P < 0.05; **, P < 0.005; ***, P < 0.0005; ns, not significant.
Using groups II and III, we also analyzed the effect of the parenteral ceftriaxone treatment on the later stages of the infection and protective immune responses. After cessation of ceftriaxone therapy, fecal shedding remained low for at least 5 days. However, at later time points, a massive relapse of fecal S. Typhimuriumatt shedding was observed in most animals and reached levels equivalent to those of the PBS-treated control group (Fig. 4A). By day 40 p.i., fecal shedding had declined in most of the animals from either group and did not differ significantly between the two groups (P ≥ 0.05; Fig. 4A).
In group II, no intestinal inflammation was detected at day 40 p.i. either in ceftriaxone-treated mice (5/5 mice with cecum pathology scores of ≤3) or in control animals (4/5 mice with cecum pathology scores of ≤3; Fig. 4D). Furthermore, we did not observe differences in colonization levels in MLNs or spleens. Finally, the frequency of asymptomatic excreters did not differ significantly between the two groups (>105 CFU/g feces; no pathology; ceftriaxone group, 3/5 mice; control group, 7/18 mice [P = 0.62, Fisher's exact test]). In conclusion, parenteral ceftriaxone treatment provided similar therapeutic benefits as the peroral ciprofloxacin treatment during the primary infection.
Effect of parenteral ceftriaxone treatment on elicitation of protective immunity.
Next, we analyzed whether parenteral ceftriaxone therapy affects the generation of a protective mucosal immune response. Groups of ceftriaxone-treated mice and control animals were challenged with wt S. Typhimurium as described above (group III; animals from the experiment whose results are shown in Fig. 4A). Two days postchallenge, S. Typhimuriumatt levels in the cecum content, MLNs, and the spleen were quantified and the pathology of the cecal mucosa was assessed. As expected, both groups had high pathogen levels in the cecal lumen and did not display significant differences in MLN or spleen colonization. Nevertheless, the control mice challenged with wt S. Typhimurium displayed no signs of overt cecum inflammation at day 2 postchallenge (Fig. 5A). The same held true for the ceftriaxone-treated mice. These findings indicated that parenteral ceftriaxone therapy did not interfere with the generation of a protective mucosal immune response.
Fig 5.
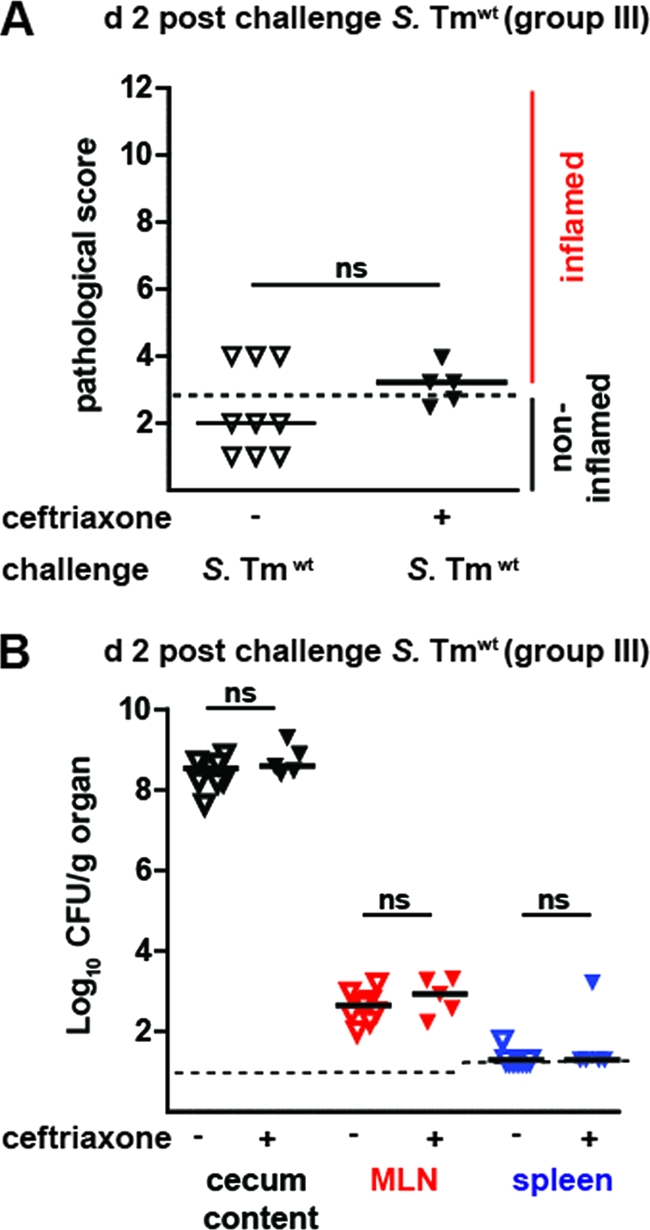
Ceftriaxone treatment does not interfere with the generation of a protective immune response. (A) Ceftriaxone-treated mice are protected from gut inflammation. Mice which had been exposed to S. Typhimuriumatt and treated with PBS (control mice; open triangles) or ceftriaxone (black triangles) were analyzed in challenge infections. At day 39, animals were pretreated with ampicillin (20 mg orally) and challenged with wt S. Typhimurium (200 CFU orally) at day 40 p.i. Cecum inflammation was determined at 2 days postchallenge (a score of ≤3 indicates no inflammation; dashed line). (B) wt S. Typhimurium loads in the cecum content (black triangles), MLN (red triangles), and spleen (blue triangles). Dashed line, detection limit; black bars, median; ns, not significant.
S. Typhimurium-specific antibody levels present in ceftriaxone- and ciprofloxacin-treated animals.
O-antigen-specific sIgA is produced upon mucosal infection with isolates of the family Enterobacteriaceae, and it is crucial for preventing enteropathy. Therefore, we reasoned that O-antigen-specific sIgA levels might be affected in different ways by the two antibiotic therapies tested (13, 36, 40, 56), thus explaining the different effects of peroral ciprofloxacin and parenteral ceftriaxone treatment on the generation of a protective immune response. To address this hypothesis, we determined the kinetics of the S. Typhimurium-specific humoral immune response using FACS assays and Western blot analysis.
First, we monitored the S. Typhimurium-specific humoral immune response by measuring specific Ig via surface staining of live, intact bacteria in a flow cytometric assay, as described recently (13). In the PBS-treated controls, S. Typhimurium-specific IgG and IgA were detectable in the serum and S. Typhimurium-specific sIgA was detectable in the gut washes at day 40 postinfection (Fig. 6A, top). Equivalent responses were observed in the gut washes from mice after ciprofloxacin or ceftriaxone therapy (Fig. 6A, bottom and middle).
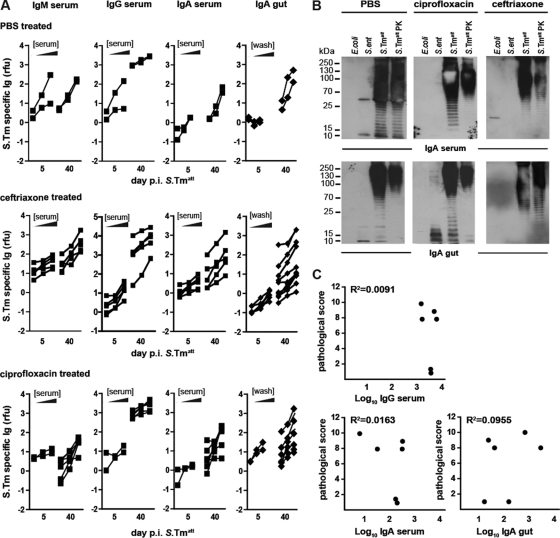
Fig 6.
S. Typhimurium-specific antibody levels present in ceftriaxone- and ciprofloxacin-treated animals. Antibody responses were analyzed in mice at days 5 and 40 p.i. with S. Typhimuriumatt (groups I and II from Fig. 2 and 4). (A) Antibodies from serum or gut washes which were directed against the surface of S. Typhimuriumatt were analyzed by bacterial FACS (2 < n < 10 samples per group; see Materials and Methods). The y axes indicate S. Typhimuriumatt-specific lg levels (relative fluorescence units [rfu]), and the x axes indicate the respective dilutions of serum (1:20, 1:60, 1:180) or gut wash (1:2, 1:6, 1:18). (B) Western blot analysis. Serum and gut wash from control or ciprofloxacin- or ceftriaxone-treated mice (day 40 p.i.) were analyzed by immunoblotting against different bacterial lysates (E. coli, S. Enteritidis, S. Typhimuriumatt, S. Typhimuriumatt digested with proteinase K [PK]). Specific IgA or slgA was detected with an HRP-labeled secondary antibody. The results are representative of experiments with 2 different animals. (C) Antibody levels do not correlate with protection in ciprofloxacin-treated mice (n = 6). The cecum pathology score was plotted against the highest value (1:20 dilution for serum and 1:2 dilution for gut wash) of S. Typhimuriumatt-specific IgG, IgA, and sIgA detected by bacterial FACS.
Then, we analyzed the bacterial antigens recognized by the humoral immune response. It is known that S. Typhimurium-specific Ig targets surface structures, like the LPS O antigen (13, 26, 40), flagella (27), or outer membrane proteins (37, 44). The antigens recognized by the serum IgA and the sIgA (at day 40 p.i.) were analyzed by Western blotting (Fig. 6B). Serum IgA and sIgA of the control group and the ciprofloxacin- or ceftriaxone-treated mice recognized specifically the O antigen of S. Typhimurium (protease-resistant ladder-like bands in the Western blot), a highly repetitive sugar structure of the LPS coating the surface of NTS. The antibody response was indeed pathogen specific for all groups, as LPSs of E. coli and S. enteritidis, both of which harbor different LPS O antigens, were not recognized. Thus, the production of S. Typhimurium O-antigen-specific IgA and sIgA was detectable in all groups, indicating that the humoral response was targeting the same antigenic surface structure of the pathogen.
Finally, we analyzed a possible correlation between the amounts of pathogen-specific IgG, serum IgA, or sIgA (from Fig. 6A) and the degree of mucosal inflammation observed upon pathogen challenge of the respective animals (from Fig. 5A). However, no significant correlation was observed for any of the antibody classes (Fig. 6C). Hence, using the techniques described above, we could not establish that the lack of protection in mice treated with ciprofloxacin per os (but not with ceftriaxone i.p.) is attributable to impaired humoral immune responses. However, we cannot exclude the possibility that differences in antibody avidity or low-abundance antigens which have escaped detection by our methods might be responsible.
DISCUSSION
While antibiotic therapy is not indicated in uncomplicated cases of NTS infection, treatment is recommended in complicated cases, i.e., in immune-compromised patients or cases of severe systemic spread. However, the effects of antibiotic treatment on the generation of an adaptive immune response are not understood. We have employed a well-established mouse model for acute NTS diarrhea and analyzed the effects of two commonly used antibiotic regimes.
In the case of peroral ciprofloxacin treatment, the mice showed an impaired protection from enteropathy in challenge infections with wt S. Typhimurium. The reasons for this effect remain to be established. In contrast, parenteral ceftriaxone treatment did not interfere with the generation of a protective immune response. These results suggest that antibiotic therapy can disrupt the adaptive immune response but treatment can be optimized to preserve a potentially beneficial immune response, at least in the mouse model.
In control experiments, we could demonstrate that both peroral treatment with ciprofloxacin and parenteral treatment with ceftriaxone were able to cure acute mucosal pathology and alleviate fecal pathogen shedding. Compared to nontreated controls, we did not observe negative effects with respect to the levels of fecal shedding, the frequency of asymptomatic carriers, or the amounts of pathogen-specific antibodies generated by 40 days p.i.
In the mouse model, we observed in many animals a relapse of gut colonization by the pathogen within a few days after ending antibiotic treatment. This is in line with clinical studies which demonstrate a transient reduction during the antibiotic treatment, followed by a relapse of fecal shedding a few days after the end of the therapy (46). It does not seem surprising that pathogens can grow up in the gut lumen after the end of the therapy, as antibiotic treatment is known to disrupt the normal population structure of the microbiota in humans and mice (1, 3, 5, 10, 11, 14, 28, 34, 42), and these effects are known to alleviate colonization resistance, i.e., pathogen growth inhibition conferred by the normal microbiota (13, 42, 52, 57). Pilot experiments performed in mice with a gut microbiota of low complexity (LCM mice) that is incapable of conferring colonization resistance (51) would argue in favor of this hypothesis. In S. Typhimuriumatt-infected LCM mice, peroral ciprofloxacin treatment cured the acute disease and efficiently suppressed fecal pathogen shedding, just as observed in the conventional mice (see Fig. S1 in the supplemental material). However, after the end of the antibiotic treatment, every single animal suffered from a rebound of pathogen shedding. This was at least partially suppressed if the mice were exposed to conventional microbiota (see Fig. S1B in the supplemental material). Our observations are in line with the notion that the normal microbiota can interfere with the rebound of the pathogen after the end of an antibiotic treatment. However, at present we do not know the microbiota species and the underlying mechanism(s) responsible.
In spite of their equivalent effects on the primary infection, both therapeutic approaches differed in their effect on the generation of a protective adaptive immune response. In challenge infections, the mice treated with ciprofloxacin per os were poorly protected from enteropathy, while animals parenterally treated with ceftriaxone were protected. However, both treatments allowed the generation of S. Typhimurium-specific sIgA (Fig. 6), an antibody class secreted into the gut lumen and protecting by pathogen aggregation, retardation of pathogen growth, and limiting of pathogen access to the intestinal epithelium (13, 40). We speculate that antibodies produced by mice perorally treated with ciprofloxacin might be impaired in their function. This qualitative defect might be explained in the light of previous data demonstrating that mucosal inflammation is important for the generation of a protective humoral immune response against S. Typhimurium (13, 38). Antibiotic therapy reduces pathogen/antigen densities and, at least in the mouse model, mucosal inflammation (Fig. 2 and 4). On the basis of this, one may speculate that peroral ciprofloxacin (but not parenteral ceftriaxone) treatment may have reduced local antigen levels and/or inflammation below the levels required for the generation of a robust protective immunity, i.e., somatic hypermutations required for generating high-affinity antibodies. To resolve this issue, analysis of kinetic differences in the antigen levels, the cessation of inflammation, and the efficiency of somatic hypermutation between ciprofloxacin- and ceftriaxone-treated mice would need to be undertaken.
However, we cannot rule out alternative explanations for the different protection in S. Typhimurium challenge infections after ciprofloxacin and ceftriaxone treatments. Distinct effects of the two classes of drugs on the community structure of the gut microbiota and differences in application forms (i.p. versus oral) might influence pharmacokinetics and thereby the timing and/or intensity of the inducing stimulus. It would be interesting to assess how later onset of treatment may affect cure, shedding, and protection after reexposure to the same pathogen.
In summary, our study verified that ciprofloxacin (per os) and ceftriaxone (i.p.) have equivalent effects during the primary infection. However, ciprofloxacin (but not ceftriaxone) treatment seems to impair the generation of protective immunity. In future studies, it might be of interest to analyze whether this is also true for the human infection.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Bärbel Stecher and the members of the W.-D. Hardt lab for critical discussions and to the staff of the RCHCI, in particular, S. Freedrich and T. Weber, for expert help in the mouse experiments.
This work was funded in part by a grant from the Novartis Stiftung für Medizinisch-Biologische Forschung to W.-D.H.
Footnotes
Published ahead of print 21 February 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Ambrose NS, Johnson M, Burdon DW, Keighley MR. 1985. The influence of single dose intravenous antibiotics on faecal flora and emergence of Clostridium difficile. J. Antimicrob. Chemother. 15:319–326 [DOI] [PubMed] [Google Scholar]
- 2. Balfour AE, Lewis R, Ahmed S. 1999. Convalescent excretion of Salmonella enteritidis in infants. J. Infect. 38:24–25 [DOI] [PubMed] [Google Scholar]
- 3. Bergan T, et al. 1986. Pharmacokinetics of ciprofloxacin and effect of repeated dosage on salivary and fecal microflora. Antimicrob. Agents Chemother. 29:298–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyd JF. 1985. Pathology of the alimentary tract in Salmonella typhimurium food poisoning. Gut 26:935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brumfitt W, Franklin I, Grady D, Hamilton-Miller JM, Iliffe A. 1984. Changes in the pharmacokinetics of ciprofloxacin and fecal flora during administration of a 7-day course to human volunteers. Antimicrob. Agents Chemother. 26:757–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buchwald DS, Blaser MJ. 1984. A review of human salmonellosis. II. Duration of excretion following infection with nontyphi Salmonella. Rev. Infect. Dis. 6:345–356 [DOI] [PubMed] [Google Scholar]
- 7. Cash HL, Whitham CV, Behrendt CL, Hooper LV. 2006. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313:1126–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang HR, Vladoianu IR, Pechere JC. 1990. Effects of ampicillin, ceftriaxone, chloramphenicol, pefloxacin and trimethoprim-sulphamethoxazole on Salmonella typhi within human monocyte-derived macrophages. J. Antimicrob. Chemother. 26:689–694 [DOI] [PubMed] [Google Scholar]
- 9. Coombes BK, et al. 2005. Analysis of the contribution of Salmonella pathogenicity islands 1 and 2 to enteric disease progression using a novel bovine ileal loop model and a murine model of infectious enterocolitis. Infect. Immun. 73:7161–7169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dethlefsen L, Huse S, Sogin ML, Relman DA. 2008. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 6:e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dethlefsen L, Relman DA. 2011. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. U. S. A. 108(Suppl. 1):4554–4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eckburg PB, et al. 2005. Diversity of the human intestinal microbial flora. Science 308:1635–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Endt K, et al. 2010. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog. 6:e1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Enzensberger R, Shah PM, Knothe H. 1985. Impact of oral ciprofloxacin on the faecal flora of healthy volunteers. Infection 13:273–275 [DOI] [PubMed] [Google Scholar]
- 15. Fey PD, et al. 2000. Ceftriaxone-resistant Salmonella infection acquired by a child from cattle. N. Engl. J. Med. 342:1242–1249 [DOI] [PubMed] [Google Scholar]
- 16. Giraud E, Baucheron S, Cloeckaert A. 2006. Resistance to fluoroquinolones in Salmonella: emerging mechanisms and resistance prevention strategies. Microbes Infect. 8:1937–1944 [DOI] [PubMed] [Google Scholar]
- 17. Giuliano M, Barza M, Jacobus NV, Gorbach SL. 1987. Effect of broad-spectrum parenteral antibiotics on composition of intestinal microflora of humans. Antimicrob. Agents Chemother. 31:202–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Glynn MK, et al. 2004. Prior antimicrobial agent use increases the risk of sporadic infections with multidrug-resistant Salmonella enterica serotype Typhimurium: a FoodNet case-control study, 1996-1997. Clin. Infect. Dis. 38(Suppl. 3):S227–S236 [DOI] [PubMed] [Google Scholar]
- 19. Gomez HF, Cleary TG. 1998. Salmonella, p 1321–1334 In Feigin RD, Cherry JD. (ed), Textbook of pediatric infectious diseases, 4th ed W. B. Saunders Company, Philadelphia, PA [Google Scholar]
- 20. Gorbach SL. 1993. Perturbation of intestinal microflora. Vet. Hum. Toxicol. 35(Suppl 1):15–23 [PubMed] [Google Scholar]
- 21. Greig JD, Todd EC, Bartleson CA, Michaels BS. 2007. Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 1. Description of the problem, methods, and agents involved. J. Food Prot. 70:1752–1761 [DOI] [PubMed] [Google Scholar]
- 22. Hapfelmeier S, et al. 2004. Role of the Salmonella pathogenicity island 1 effector proteins SipA, SopB, SopE, and SopE2 in Salmonella enterica subspecies 1 serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 72:795–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hapfelmeier S, et al. 2008. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in ΔinvG S. Typhimurium colitis. J. Exp. Med. 205:437–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hapfelmeier S, et al. 2005. The Salmonella pathogenicity island (SPI)-1 and SPI-2 type III secretion systems allow Salmonella serovar Typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J. Immunol. 174:1675–1685 [DOI] [PubMed] [Google Scholar]
- 25. Hohmann E. 2011. Approach to the patient in nontyphoidal Salmonella in a stool culture. In Calderwood SB, Baron EL. (ed), UpToDate, the clinical information service on the web and mobile devices. UpToDate, Inc., Waltham, MA [Google Scholar]
- 26. Iankov ID, et al. 2001. Monoclonal antibodies of IgA isotype specific for lipopolysaccharide of Salmonella enteritidis: production, purification, characterization and application as serotyping reagents. FEMS Microbiol. Lett. 196:215–221 [DOI] [PubMed] [Google Scholar]
- 27. Iankov ID, et al. 2002. Production and characterization of monoclonal immunoglobulin A antibodies directed against Salmonella H:g,m flagellar antigen. FEMS Immunol. Med. Microbiol. 33:107–113 [DOI] [PubMed] [Google Scholar]
- 28. Jimenez-Valera M, Gonzalez-Torres C, Moreno E, Ruiz-Bravo A. 1998. Comparison of ceftriaxone, amikacin, and ciprofloxacin in treatment of experimental Yersinia enterocolitica O9 infection in mice. Antimicrob. Agents Chemother. 42:3009–3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaiser P, Diard M, Stecher B, Hardt WD. 2012. The streptomycin mouse model for Salmonella diarrhea: functional analysis of the microbiota, the pathogen's virulence factors, and the host's mucosal immune response. Immunol. Rev. 245:56–83 [DOI] [PubMed] [Google Scholar]
- 30. Kazemi M, Gumpert TG, Marks MI. 1973. A controlled trial comparing sulfamethoxazole-trimethoprim, ampicillin, and no therapy in the treatment of Salmonella gastroenteritis in children. J. Pediatr. 83:646–650 [DOI] [PubMed] [Google Scholar]
- 31. Kelly CP, Pothoulakis C, LaMont JT. 1994. Clostridium difficile colitis. N. Engl. J. Med. 330:257–262 [DOI] [PubMed] [Google Scholar]
- 32. Kingsley RA, Baumler AJ. 2000. Host adaptation and the emergence of infectious disease: the Salmonella paradigm. Mol. Microbiol. 36:1006–1014 [DOI] [PubMed] [Google Scholar]
- 33. Lahdevirta J. 1989. Ciprofloxacin in the elimination of enteric Salmonella carriage stage. Scand. J. Infect. Dis. Suppl. 60:112–115 [PubMed] [Google Scholar]
- 34. Ljungberg B, Nilsson-Ehle I, Edlund C, Nord CE. 1990. Influence of ciprofloxacin on the colonic microflora in young and elderly volunteers: no impact of the altered drug absorption. Scand. J. Infect. Dis. 22:205–208 [DOI] [PubMed] [Google Scholar]
- 35. Lolekha S, Patanachareon S, Thanangkul B, Vibulbandhitkit S. 1988. Norfloxacin versus co-trimoxazole in the treatment of acute bacterial diarrhoea: a placebo controlled study. Scand. J. Infect. Dis. Suppl. 56:35–45 [PubMed] [Google Scholar]
- 36. Maaser C, et al. 2004. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect. Immun. 72:3315–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. MacLennan CA, et al. 2010. Dysregulated humoral immunity to nontyphoidal Salmonella in HIV-infected African adults. Science 328:508–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martinoli C, Chiavelli A, Rescigno M. 2007. Entry route of Salmonella typhimurium directs the type of induced immune response. Immunity 27:975–984 [DOI] [PubMed] [Google Scholar]
- 39. Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122:107–118 [DOI] [PubMed] [Google Scholar]
- 40. Michetti P, Mahan MJ, Slauch JM, Mekalanos JJ, Neutra MR. 1992. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect. Immun. 60:1786–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nelson JD, Kusmiesz H, Jackson LH, Woodman E. 1980. Treatment of Salmonella gastroenteritis with ampicillin, amoxicillin, or placebo. Pediatrics 65:1125–1130 [PubMed] [Google Scholar]
- 42. Perrin-Guyomard A, et al. 2005. Impact of residual and therapeutic doses of ciprofloxacin in the human-flora-associated mice model. Regul. Toxicol. Pharmacol. 42:151–160 [DOI] [PubMed] [Google Scholar]
- 43. Santos RL, et al. 2001. Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect. 3:1335–1344 [DOI] [PubMed] [Google Scholar]
- 44. Singh SP, Williams YU, Klebba PE, Macchia P, Miller S. 2000. Immune recognition of porin and lipopolysaccharide epitopes of Salmonella typhimurium in mice. Microb. Pathog. 28:157–167 [DOI] [PubMed] [Google Scholar]
- 45. Sirinavin S, Garner P. 2000. Antibiotics for treating salmonella gut infections. Cochrane Database Syst. Rev. 2:CD001167. [DOI] [PubMed] [Google Scholar]
- 46. Sirinavin S, Jayanetra P, Lolekha S, Layangkul T. 1988. Predictors for extraintestinal infection in Salmonella enteritis in Thailand. Pediatr. Infect. Dis. J. 7:44–48 [DOI] [PubMed] [Google Scholar]
- 47. Slack E, et al. 2009. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science 325:617–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith ER, Badley BW. 1971. Treatment of Salmonella enteritis and its effect on the carrier state. Can. Med. Assoc. J. 104:1004–1006 [PMC free article] [PubMed] [Google Scholar]
- 49. Sperber SJ, Schleupner CJ. 1987. Salmonellosis during infection with human immunodeficiency virus. Rev. Infect. Dis. 9:925–934 [DOI] [PubMed] [Google Scholar]
- 50. Stecher B, et al. 2008. Motility allows S. Typhimurium to benefit from the mucosal defence. Cell. Microbiol. 10:1166–1180 [DOI] [PubMed] [Google Scholar]
- 51. Stecher B, et al. 2010. Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. PLoS Pathog. 6:e1000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stecher B, Hardt WD. 2008. The role of microbiota in infectious disease. Trends Microbiol. 16:107–114 [DOI] [PubMed] [Google Scholar]
- 53. Stecher B, et al. 2007. Salmonella enterica serovar Typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 5:e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Su H, et al. 1999. The effect of doxycycline treatment on the development of protective immunity in a murine model of chlamydial genital infection. J. Infect. Dis. 180:1252–1258 [DOI] [PubMed] [Google Scholar]
- 55. Suar M, et al. 2006. Virulence of broad- and narrow-host-range Salmonella enterica serovars in the streptomycin-pretreated mouse model. Infect. Immun. 74:632–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Uren TK, et al. 2005. Vaccine-induced protection against gastrointestinal bacterial infections in the absence of secretory antibodies. Eur. J. Immunol. 35:180–188 [DOI] [PubMed] [Google Scholar]
- 57. van der Waaij D, Berghuis-de Vries JM, Lekkerkerk Lekkerkerk V. 1971. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J. Hyg. (Lond.) 69:405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wijburg OL, et al. 2006. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J. Exp. Med. 203:21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.