Abstract
Antiretroviral entry inhibitors are now being considered as vaginally administered microbicide candidates for the prevention of the sexual transmission of human immunodeficiency virus. Previous studies testing the entry inhibitors maraviroc and CMPD167 in aqueous gel formulations showed efficacy in the macaque challenge model, although protection was highly dependent on the time period between initial gel application and subsequent challenge. In this paper, we describe the sustained release of maraviroc and CMPD167 from matrix-type silicone elastomer vaginal rings both in vitro and in vivo. Both inhibitors were released continuously during 28 days from rings in vitro at rates of 100 to 2,500 μg/day. In 28-day pharmacokinetic studies in rhesus macaques, the compounds were measured in the vaginal fluid and vaginal tissue; steady-state fluid concentrations were ∼106-fold greater than the 50% inhibitory concentrations (IC50s) for simian human immunodeficiency virus 162P3 inhibition in macaque lymphocytes in vitro. Plasma concentrations for both compounds were very low. The pretreatment of macaques with Depo-Provera (DP), which is commonly used in macaque challenge studies, was shown to significantly modify the biodistribution of the inhibitors but not the overall amount released. Vaginal fluid and tissue concentrations were significantly decreased while plasma levels increased with DP pretreatment. These observations have implications for designing macaque challenge experiments and also for ring performance during the human female menstrual cycle.
INTRODUCTION
Orally administered antiretroviral drugs (ARVs) are important for reducing viral loads in human immunodeficiency virus (HIV)-infected individuals and preventing the worldwide spread of HIV-1 infection (6, 14). The vaginal application of ARVs as microbicides has also emerged as a key prevention strategy in protecting women against infection (7, 23, 36, 37, 41, 44). It is now widely acknowledged that microbicides should be based on ARVs similar to those used for treatment for reasons of potency and practicality (13, 20, 21). The positive outcome of the CAPRISA (Centre for the AIDS Programme of Research in South Africa) trial of the reverse transcriptase inhibitor (RTI) tenofovir formally validates the concept of ARV-based microbicides (17, 18).
In addition to RTIs, other classes of ARVs are worth evaluating as microbicides, in part to counter the increased spread of RTI-resistant viruses resulting from expanded treatment programs with the same drug class (42). Using more than one ARV class in a coformulated microbicide also could increase both the potency and breadth of protection (15, 34). Small-molecule CCR5 inhibitors represent one such class, exemplified by the licensed drug maraviroc (MVC), which binds the CCR5 coreceptor and prevents the cell entry of the most frequently transmitted HIV-1 strains (19, 24, 29, 35). Both MVC and another CCR5 inhibitor, CMPD167, have been shown to prevent the vaginal transmission of simian human immunodeficiency virus 162P3 (SHIV-162P3) to macaques when delivered vaginally in an aqueous gel formulation (46, 47).
A microbicide product must be delivered in a way that maximizes its use. In the CAPRISA trial, aqueous gel-formulated tenofovir provided ∼50% protection when applied at or around the time of intercourse (17). The incomplete protection observed resulted from limited adherence to the relatively complex BAT24 dosing regimen, in which a single vaginal gel dose was applied within 12 h before sex, followed by a second dose up to 12 h after sex, irrespective of the number of sex acts within that period (17, 18). A better strategy, particularly for women at high risk of infection, would be a microbicide administered independently of coitus (e.g., a once-daily gel) to maintain protective ARV concentrations between applications. We have shown that MVC can be formulated in a nonaqueous, silicone-based gel that sustains high concentrations in the macaque vagina for up to 24 h (11).
Vaginal rings represent an alternative delivery method for providing sustained drug release into the vaginal lumen and tissue (26, 48). A matrix-type vaginal ring releasing the RTI dapivirine continuously for 28 days is presently in clinical development and due to enter phase III testing in 2012 (26, 31). Here, we show that both MVC and CMPD167 can be formulated in similar vaginal ring devices and released both in vitro and in rhesus macaques. We also explored whether the pretreatment of the macaques with Depo-Provera (DP) influences release in vivo. DP is commonly used to synchronize the macaque menstrual cycle and thin the vaginal epithelium prior to viral challenge experiments, thereby increasing susceptibility to infection (27, 46, 47). Although DP is known to affect vaginal/cervical histology and physiology (12, 16, 28), it is unclear how relevant these changes are to virus transmission events and how they influence the pharmacokinetics of vaginal microbicides.
MATERIALS AND METHODS
Water solubility.
MVC and CMPD167 were supplied by ViiV Healthcare and the International Partnership for Microbicides, respectively. Water solubilities were determined in quadruplicate using the shake-flask method. Excess compound (∼25 mg) was added to 10 ml high-performance liquid chromatography (HPLC)-grade water, vortexed (1 min), and placed in an orbital incubator (37°C, 60 rpm; Infors HT Unitron, Switzerland) for 72 h. Samples were stored at room temperature for a further 24 h before filtering (0.2-μm cellulose acetate membrane syringe filter; minisart; Sartorius Stedim Biotech, France) and HPLC analysis (Waters 1525 binary HPLC pump, 717 Plus autosampler, in-line degasser AF unit, and 2487 dual λ absorbance detector) with a Luna C18(2) 100 Å, 5-μm column (150 by 4.60 mm) (Phenomenex, United Kingdom) at 30°C. For MVC samples, gradient elution involved a mobile phase of 10 mM KH2PO4 buffer, pH 3.0 (solvent A), and acetonitrile (solvent B) with a 1.0-ml/min flow rate (0 to 4 min with 70 to 20% solvent A; 4.0 to 4.5 min with 20 to 70% solvent A; and 4.5 to 7.0 min with 70% solvent A). MVC was detected at 3.3 min (210 nm). For CMPD167, isocratic HPLC was performed with a mobile phase of 0.1% trifluoroacetic acid in water-acetonitrile (70:30), a flow rate of 1.0 ml/min, and detection at 210 nm. The retention time was 6.4 min.
pKa and log P values.
Ionization constants and log P values were measured with a Sirius T3 instrument (Sirius Analytical Instruments, United Kingdom). Titrations were performed in 0.15 M KCl under nitrogen at 25°C. Apparent ionization constants (pKa) were determined in methanol-water mixtures, extrapolating to 0% methanol to give aqueous pKa values (1–3, 38, 43).
Vaginal ring manufacture.
Matrix-type, silicone elastomer, macaque-sized vaginal rings loaded with 400 mg micronized CMPD167 or MVC were manufactured by reaction injection molding. Each CCR5 inhibitor was mixed (1 min, 3,000 rpm; SpeedMixer DAC 150 FVZ-K; Synergy Devices, United Kingdom) into both parts A and B of silicone elastomer LSR9-9508-30 (Nusil Technology). The active parts were combined (1:1, wt/wt), speed mixed (1 min, 3000 rpm), injected into stainless steel molds in a laboratory-scale ring-making machine, and cured (3 min, 80°C). The rings, weighing 1.85 ± 0.01 g, measured 25.0 and 6.0 mm in external and cross-sectional diameters, respectively. Similar control rings have previously been shown to fit rhesus macaques optimally and caused no irritation (33).
In vitro release testing from vaginal rings.
Individual rings were placed into screw-top glass bottles containing either 50 or 25 ml simulated vaginal fluid (SVF) (days 1 to 4 and days 7 to 28, respectively). SVF mimics the chemical composition of vaginal fluid, including pH and osmolarity matched to normal vaginal fluid (32). The bottles were placed in an orbital shaking incubator (37°C, 60 rpm, throw 25 mm), and the release medium was sampled (5 ml) regularly during the 28-day study period. The release medium was completely replaced with fresh warmed medium after each sampling time point. The samples then were quantified for MVC or CMPD167 concentration using HPLC as described above, and daily release-versus-time profiles were plotted.
Macaque pharmacokinetic studies.
Twenty-four female cycling rhesus macaques (4 to 14 years) were housed at Tulane National Primate Research Center in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH (30a). The Tulane University Institutional Animal Care and Use Committee approved research. Twelve macaques were dosed once intramuscularly with 30 mg DP 1 month before ring placement (45). The other 12 were not treated. Twelve macaques per group (six given DP, six not treated) were fitted with vaginal rings loaded with 400 mg CMPD167 or MVC. Vaginal fluid and blood were collected immediately before the rings were atraumatically placed in the vagina. Additional vaginal fluid and blood samples were collected after 1, 4, and 8 h and then at 1, 2, 3, 4, 7, 10, 14, 21, and 28 days. Vaginal fluid was sampled by placing a preweighed Weck-Cel sponge into the vagina and allowing it to absorb (5 min) before removal and immediate transport to a laboratory for processing. The sponges were reweighed to calculate the collected vaginal fluid weight. The sponge tips were removed and placed into Spin-X tubes containing a 40-μm filter separating top and bottom chambers. Extraction buffer (300 μl) containing 0.25 M NaCl, 0.2% sodium azide, and protease inhibitors (Calbiochem) were added to the top well, and the tubes were centrifuged (13,000 × g, 15 min). The top chambers were removed and the samples stored (−80°C) prior to MVC or CMPD167 quantification. Blood samples were treated with EDTA before plasma was separated by centrifugation (1,000 × g, 20 min) and stored (−80°C) prior to analysis. Pinch biopsy specimens were sampled as previously described (11).
Quantification of CMPD167 and MVC in biological samples.
Plasma, vaginal fluid, and vaginal tissue concentrations of CMPD167 and MVC were quantified by gradient reverse-phase HPLC (Prominence; Shimadzu) coupled to a triple-quadrupole mass spectrometer (API3200; Applied Biosystems). The methods are detailed in the supplemental material.
Quantification of vaginal ring CMPD167 and MVC content before and after use.
The amounts of CMPD167 and MVC remaining in the rings (extracted drug content, E) after in vitro release testing or use in macaques were quantified by solvent extraction. The initial amounts of inhibitor (measured loading, Lm) in rings from the same production batch, but not used in release studies, were measured similarly to allow the calculation of the total amount released from each ring (R) as R = Lm − E. Rings were weighed, cut into sections, and refluxed (2 h) in a mixture comprising 95 ml dichloromethane and 5 ml internal standard solution (5 mg/ml norethindrone in methanol). A 10-ml aliquot of extraction solution was evaporated to dryness (Buchi Syncore Polyvap, Oldham, United Kingdom), reconstituted in 10 ml methanol, diluted 1:20 in methanol, and assayed by HPLC as described previously for solubility quantification.
Antiviral activity of CMPD167 and MVC in vitro.
Antiviral activity was assessed using both rhesus macaque and human peripheral blood mononuclear cells (PBMC) as described previously (45).
Statistical analyses.
Statistical analyses used GraphPad Prism software. Data were analyzed using either one- or two-way analysis of variance depending on the number of variables to be compared. Significance was noted when P < 0.05.
RESULTS
Antiviral activity of CMPD167 and MVC.
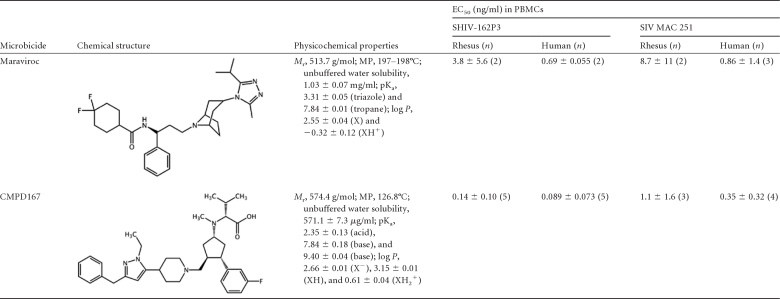
Maraviroc and CMPD167 inhibited the replication of SHIV-162P3 and SIVmac251 in human and macaque PBMCs, with 50% effective concentrations (EC50s) in the range of 0.1 to 10 ng/ml (Table 1). Both compounds were less potent against both viruses in macaque than in human PBMCs. For MVC, the EC50 differential was ∼5- to 10-fold; for CMPD167 it was ∼1.5- to 3-fold. Of the two challenge viruses, SHIV-162P3 was more sensitive to both inhibitors, by 1.2- to 7.8-fold depending on the virus and cell type.
Table 1.
Chemical structures, experimental physicochemical parameters, and in vitro antiviral properties for CMPD167 and maraviroca
Mr, relative molecular size; MP, melting point; pKa, experimentally determined ionization constants; log P, experimentally determined octanol-water partition coefficients; PBMCs, peripheral blood mononuclear cells.
Water solubility, pKa, and log P values for CMPD167 and MVC.
For both compounds, the experimentally determined values for water solubility, acid dissociation constants, and octanol/water partition coefficients (log P; presented for each ionization state of the molecules) are summarized in Table 1. Each CCR5 inhibitor is classified as slightly soluble; they exist in different ionization states depending on the environmental pH.
For MVC, the conjugate acid species of the basic triazole and tropane groups have pKa values of 3.31 and 7.84, respectively. The neutral form of MVC (i.e., with neither group protonated) exists predominantly above pH 8 and has a log P of 2.55 (i.e., hydrophobic). The monoprotonated form (XH+) (Table 1), which will predominate in the normal vaginal pH range for both humans (pH 4 to 5) (32, 39) and rhesus macaques (pH 6 to 7) (40), has a log P of −0.32 (i.e., hydrophilic). CMPD167 contains one acidic (pKa = 2.35) and three basic moieties, two of which provide measurable pKa values of 7.84 and 9.40. At normal vaginal pH, the carboxylic acid species and the basic groups are all ionized. Under these conditions, the CMPD167 molecule contains three ionized species (XH2+) overall, with a log P value of 0.61.
For comparison, various steroidal drugs in marketed vaginal ring devices (i.e., Estring, estradiol; Femring, estradiol-3-acetate; Nuvaring, etonogestrel and ethinyl estradiol; and Progering, progesterone) are nonionizable, with log P values of >2. All of these drugs are efficiently absorbed, as required for their particular clinical indications. At normal vaginal pH, the monoprotonated form of MVC, and more so the highly ionized CMPD167, might be less well absorbed than the nonionized steroidal drugs.
Release of CMPD167 and MVC from vaginal rings in vitro.
The vaginal rings released both compounds into SVF continuously during a 28-day period (Fig. 1). Typical of matrix-type rings, where the solid drug particles are distributed throughout the ring, the daily amounts released decreased with time, in accordance with half-life (t1/2) kinetics (cumulative release versus t1/2 plots were linear; r2 > 0.999). MVC release rates were ∼10-fold greater than those for CMPD167; thus, after 28 days, 127 mg MVC was released, but only 13 mg CMPD167. This differential may be attributed to differences in the silicone elastomer permeability of the compounds and/or their solubility in SVF.
Fig 1.

In vitro mean daily release (with SD; n = 4) versus time profiles for silicone elastomer matrix-type vaginal rings containing 400 mg of maraviroc or CMPD167. Simulated vaginal fluid was used as the release medium with complete replacement at all sampling time points (50 ml for days 1 to 3, 25 ml thereafter).
Release of CMPD167 and MVC from vaginal rings in the macaque.
Vaginal fluid, vaginal tissue biopsy samples, and plasma concentrations were quantified during 28 days in both DP-treated and untreated macaques (Fig. 2). High concentrations of both CCR5 inhibitors were present in vaginal fluid within 24 h after ring insertion and changed little during the next 28 days. In both groups of animals, the mean vaginal fluid concentrations were significantly higher (∼2- to 3-fold) for MVC than for CMPD167. The vaginal fluid concentrations were ∼104- to 106-fold greater than in vitro 50% inhibitory concentrations (IC50s) for the inhibition of SHIV-162P3 infection of rhesus macaque PBMC (Table 1). Vaginal fluid concentrations at most time points were significantly greater (∼2- to 5-fold) in untreated macaques than in those pretreated with DP.
Fig 2.
Mean concentrations (with SD; n = 6) of maraviroc and CMPD167 measured in the vaginal fluid (A and D), vaginal tissue (B and E), and blood plasma (C and F) of rhesus macaques during a 28-day period of continuous ring placement. Vaginal tissue and plasma samples were not recorded on day 28 for CMPD167 rings. Note that the scales on the y axes are not always the same for the two inhibitors.
Vaginal tissue biopsy specimens were sampled infrequently during the dosing period to avoid excessive perturbation to the local environment. The tissue concentrations broadly tracked those in vaginal fluid but were much lower overall, at 1,000 to 20,000 ng/g (Fig. 2B and E). The tissue levels of both compounds were significantly greater in untreated animals, with MVC concentrations being higher than those of CMPD167. MVC tissue concentrations declined steadily with time from an early peak. Whether the low tissue level of CMPD167 on day 1 compared to that at day 7 is a true finding requires confirmation.
Mean plasma levels of both inhibitors were significantly greater in the DP-treated animals (Fig. 2C and F). Indeed, in the absence of DP, plasma MVC was below the limit of detection (<0.5 ng/ml), and only minimal concentrations (mean, 0 to 0.2 ng/ml) of CMPD167 were observed from day 7 onwards. When DP was used, early peaks were observed for both CCR5 inhibitors, but they were higher for CMPD167 (4.1 ng/ml, 24 h) than MVC (1.4 ng/ml, 8 h). Plasma concentrations of each compound then steadily declined and were 1.0 ng/ml for CMPD167 on day 14 and undetectable for MVC by day 21. The mean plasma concentrations of CMPD167 and MVC (range, 0.1 to 5 ng/ml) in the DP recipients are comparable to their IC50s (Table 1) but are at least four orders of magnitude lower than those measured in vaginal fluid.
Concentration ratios for CMPD167 and MVC at representative time points summarize the pharmacokinetic data (Table 2). The DP:noDP ratios describe the influence of DP pretreatment on inhibitor levels in the three biological compartments; the hormone increases plasma levels while reducing vaginal fluid and tissue levels for both compounds. Independently of DP pretreatment, the compartment ratios (VF:VT, VT:BP, and VF:BP, where VF is vaginal fluid, VT is vaginal tissue, and BP is blood plasma) show that inhibitor concentrations increase according to the trend BP<VT<VF. The VF levels ranged from 8- to 108-fold higher than VT levels, while VT levels were 302 to 52,600 times higher than BP levels.
Table 2.
CMPD167 and maraviroc concentration ratios within and between the various biological compartments as a function of Depo Provera or no-Depo Provera treatmenta
| Biological compartment and treatment type | Concn ratio on day: |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 |
7 |
14 |
28 |
|||||
| C167 | MRV | C167 | MRV | C167 | MRV | C167 | MRV | |
| DP:noDP | ||||||||
| BP | 68.8 | NA | 8.53 | NA | 5.47 | NA | NA | NA |
| VT | 0.777 | 0.0541 | 0.136 | 0.189 | 0.951 | 0.224 | NA | 0.863 |
| VF | 0.572 | 0.0677 | 0.189 | 0.281 | 0.338 | 0.422 | 0.629 | 0.314 |
| DP | ||||||||
| VF:VT | 45.0 | 52.9 | 28.1 | 31.9 | 8.38 | 58.2 | NA | 39.3 |
| VT:BP | 302 | 1.50 E3 | 841 | 1.41 E4 | 2.83 E3 | 1.09 E4 | NA | NA |
| VF:BP | 1.36 E4 | 7.94 E4 | 2.36 E4 | 4.49 E5 | 2.94 E4 | 6.34 E5 | NA | NA |
| noDP | ||||||||
| VF:VT | 61.1 | 42.3 | 20.2 | 21.5 | 23.6 | 30.9 | NA | 108 |
| VT:BP | 2.68 E4 | NA | 5.26 E4 | NA | 1.63 E4 | NA | NA | NA |
| VF:BP | 1.63 E6 | NA | 1.06 E6 | NA | 3.85 E5 | NA | NA | NA |
BP, blood plasma; VT, vaginal tissue; VF, vaginal fluid; DP, Depo-Provera treatment; noDP, no Depo-Provera treatment; NA, ratio not available owing to a zero value for one or more concentration parameter.
Residual drug content of rings after release in vitro and in macaques.
Residual drug content measurements showed that ∼3-fold greater amounts of MVC compared to those for CMPD167 were delivered to macaques during 28 days (Fig. 3). The differential is quantitatively consistent with the better release of MVC from the rings in vitro (Fig. 1 and 3) and with the pharmacokinetic study results (Fig. 2). However, the total mass of each compound lost from the rings was very similar for both DP-treated and untreated macaques (Fig. 3). Hence, DP influences the biodistribution of the compounds after release rather than release per se. Moreover, the amounts of each compound released during 28 days in vitro and in the macaques are comparable (Fig. 3). The in vitro release testing model using SVF is therefore reasonably predictive of what happens under in vivo conditions.
Fig 3.

Calculated total mass of maraviroc and CMPD167 delivered to macaques (MAC+DEPO and MAC−DEPO) and released in vitro (calc) based on the measurement of initial and residual drug contents performed by a solvent extraction method postuse. The in vitro data set refers to the amount of each compound released based on the measured cumulative drug release from in vitro release testing.
DISCUSSION
The physicochemical properties of drug molecules are fundamental to understanding their absorption characteristics, irrespective of the administration route. A small molecular volume (approximated by a molecular size of <500 Da), a noncharged structure (according to pKa values), and a relatively hydrophobic character (defined by log P) are all conducive to increased tissue absorption via passive transport mechanisms. Depending on its mechanism of action, a microbicide needs to be present in sufficient quantities in vaginal fluid and/or nearby tissues. Blood levels should be low to avoid driving the emergence of resistant variants in infected users and to minimize systemic toxicity. High vaginal fluid and/or tissue levels can only be achieved for a microbicide-loaded vaginal ring if the active compound (i) is effectively released from the ring, (ii) has adequate solubility in vaginal fluid, and (iii) can partition from the vaginal fluid into the nearby tissue. All three processes are ultimately governed by the compound's physicochemical properties under the conditions present in the vagina. Here, we measure the relevant physicochemical properties of microbicide candidates CMPD167 and MVC and use that information to help interpret pharmacokinetic data.
MVC was more water soluble than CMPD167 (∼1 and ∼0.5 mg/ml, respectively; Table 1), which was reflected by the in vitro release data (Fig. 1) and macaque vaginal fluid levels (Fig. 2A and D). However, aqueous solubility is not the only parameter governing in vitro release and vaginal fluid levels. Drugs are normally incorporated into silicone elastomer vaginal rings as solid micronized dispersions. A variable fraction remains in the solid state, acting as a drug microreservoir within the matrix to replenish what has been released. Moreover, the drug is almost always present in the nonionized (neutral) state, since no water is present to induce ionization. For the drug to be released, however, at least some must dissolve within the hydrophobic elastomer. Consequently, when the ring is inserted, a concentration gradient is established across the interface between its surface and the vaginal fluid. If the volume of the solvated molecule is sufficiently small relative to the pore size of the elastomer matrix, passive diffusion occurs. Accordingly, the silicone elastomer solubility of the drug, its initial loading in the ring, and its molecular volume are all key factors influencing release (25). The molecular size and log P values for the neutral forms of MVC (X) and CMPD167 (XH) are similar (Table 1). Hence, their differential water solubilities may explain why MVC is released better in vitro and in vivo.
MVC and CMPD167 release in vivo yielded vaginal fluid concentrations as high as 830,000 and 180,000 ng/ml, respectively (Fig. 2 and 3). Such concentrations are 105- to 106-fold greater than the in vitro IC50s against SHIV-162P3 infection of macaque PBMC. The significantly (2- to 3-fold) higher MVC concentrations are consistent with in vitro release data (Fig. 1) and residual drug content data (Fig. 3). The amounts of the inhibitors released from the rings were independent of DP use; hormone treatment appears not to influence release per se but rather the subsequent biodistribution. Thus, DP pretreatment reduced the concentrations of both compounds in the vaginal fluid and tissue, but conversely more reached the plasma, particularly at early time points. Presumably, the thinner vaginal epithelium permits increased penetration of compounds from the vaginal lumen into the bloodstream. Nonetheless, blood levels were generally very low, particularly in macaques not given DP. The highest plasma concentrations of CMPD167 (low-ng/ml range) were similar to in vitro IC50s and broadly comparable to plasma dapivirine levels in human females using matrix rings (31). Whether the transient appearance in the plasma of a low level of a topically delivered compound could drive resistance development systemically in women who are unknowingly HIV-1 infected is uncertain.
MVC and CMPD167 were slightly less active against virus replication in macaque than human PBMC, with CMPD167 being the marginally more potent inhibitor (Table 1). 3H-MVC binding assays yielded mean equilibrium dissociation constant (KD) values of 1.36 and 0.86 nM for macaque and human CCR5, respectively, although the inhibitor dissociated ∼10-fold more quickly from macaque CCR5 (30). The IC50s for MVC inhibition of chemokine binding to macaque and human CCR5 were 17.5 and 7.2 nM (30); for CMPD167 they are 7.5 and 4.5 nM (M. Springer, personal communication). Thus, the binding and infection inhibition assays are consistent; both CCR5 inhibitors interact a little less efficiently with macaque than human CCR5, with CMPD167 being moderately more active. CCR5 inhibitors could fail to protect macaques on quantitative grounds while still being effective in humans under comparable conditions.
Other than by conducting an efficacy trial involving thousands of women, only macaque challenge experiments can address whether vaginal rings can protect against infection. We are presently addressing this issue by quantifying local drug concentrations at the time of challenge after delivery using both gels and vaginal rings. However, the sustained presence of a CCR5 inhibitor such as MVC, as provided by a vaginal ring, may be beneficial in a way that is not mimicked by a gel. When oral CCR5 inhibitors are given transiently to HIV-1-infected people, the unusually long delay before plasma viremia rebounds posttherapy is suggestive of prolonged effects on the availability of CCR5 for virus entry (9). Measurements of CCR5 occupancy on PBMC after the oral dosing of macaques with MVC support this view (1). In relation to the design of vaginal challenge studies, the lower vaginal fluid inhibitor concentrations in the DP-treated animals argue against using this challenge model. However, the alternative method, weekly challenges without DP use, requires most control animals to become infected during the 28-day period of ring use. Infection rates in vaginal challenge studies without DP use are highly variable (4, 5, 22), with possible influences being the macaque subspecies (Indian versus Chinese), the challenge stock and dose, and the use of antibiotics to eliminate vaginal flora (4, 5, 22). We are conducting studies to understand how best to challenge macaques that have vaginal rings inserted and hence gain an understanding of the protective potential of this coitally independent method of inhibitor delivery.
Overall, our results have implications for the use of DP in the macaque challenge model, at least in relation to the release and biodistribution of ARVs from vaginal rings. Whether contraceptive hormones influence the thickness of the vaginal epithelium in women is more controversial. It is worth considering whether and how the performance of vaginal rings as ARV delivery devices vary during the natural menstrual cycle of women. For example, in response to fluctuating levels of steroid hormones, cervicovaginal fluid volumes increase significantly at the beginning of the fertile phase, peaking just before ovulation before decreasing again afterwards (8, 10). The thickness of the vaginal epithelium also changes significantly during the normal menstrual cycle. As these factors could affect the release and biodistribution of ARVs from vaginal rings, some specific research on this topic is warranted.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (award U19 AI76982). We appreciate the donations of maraviroc and CMPD167 by ViiV Healthcare and the International Partnership for Microbicides, respectively. We thank Marty Springer for advice on the chemistry of CMPD167 and the provision of IC50s for maraviroc inhibition of chemokine binding.
No conflicts of interest are declared for any of the authors.
R. K. Malcolm, R. S. Veazey, J. P. Moore, and R. J. Shattock are principal or primary coinvestigators on the U19 grant, with responsibility for designing, coordinating, and reporting all aspects of the work. R. K. Malcolm and J. P. Moore had lead responsibility for drafting the manuscript, with contributions from all other authors. S. M. Fetherston, I. Major, and P. Boyd performed vaginal ring manufacture, in vitro release testing, and residual drug content extractions. D. J. Murphy and S. M. Fetherston completed the physicochemical characterization of the entry inhibitors (log P, water solubility, and pKa). P. J. Klasse and T. J. Ketas performed in vitro antiviral efficacy experiments. L. Goldman and L. Geer performed all of the bioanalytics associated with the macaque pharmacokinetic study (i.e., quantification of vaginal fluid, vaginal tissue, and plasma levels). R. S. Veazey, L. A. Doyle, and K. K. Rasmussen were responsible for all aspects of the macaque handling and sampling.
Footnotes
Published ahead of print 13 February 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Aung W, et al. 2011. Pharmacokinetic and pharmacodynamic profile of maraviroc in rhesus macaques after a single oral dose, abstr, O_10. Abstr. 6th Int. Workshop HIV Transm [Google Scholar]
- 2. Avdeef A, Comer JEA, Thomson SJ. 1993. pH-metric log P. Glass electrode calibration in methanol-water, applied to pKa determination of water-insoluble substances. Anal. Chem. 65:42–49 [Google Scholar]
- 3. Benet LZ, Goyan JE. 1967. Potentiometric determination of dissociation constants. J. Pharm. Sci. 56:665–680 [DOI] [PubMed] [Google Scholar]
- 4. Bomsel M, et al. 2011. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity 34:269–280 [DOI] [PubMed] [Google Scholar]
- 5. Cheng-Mayer C, et al. Delay of simian human immunodeficiency virus infection and control of viral replication in vaccinated macaques challenged in the presence of a topical microbicide. AIDS 25:1833–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen MS, et al. 2011. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 365:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhawan D, Mayer KH. 2006. Microbicides to prevent HIV transmission: overcoming obstacles to chemical barrier protection. J. Infect. Dis. 193:36–44 [DOI] [PubMed] [Google Scholar]
- 8. Eschenbach DA, et al. 2000. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin. Infect. Dis. 30:901–907 [DOI] [PubMed] [Google Scholar]
- 9. Fätkenheuer G, et al. 2005. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat. Med. 11:1170–1172 [DOI] [PubMed] [Google Scholar]
- 10. Flynn AM, McCarthy AM, Docker M, Royston JP. 1988. The temporal relationship between vaginal fluid volumes obtained with the Rovumeter vaginal aspirator and the fertile phase of the cycle. Hum. Reprod. 3:201–205 [DOI] [PubMed] [Google Scholar]
- 11. Forbes CJ, et al. Non-aqueous silicone elastomer gels as a vaginal microbicide delivery system for the HIV-1 entry inhibitor maraviroc. J. Control. Rel. 156:161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fotherby K, Koetsawang S, Mathrubutham M. 1980. Pharmacokinetic study of different doses of Depo Provera. Contraception 22:527–536 [DOI] [PubMed] [Google Scholar]
- 13. Grant RM, et al. 2008. Whither or wither microbicides? Science 321:532–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grant RM, et al. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 363:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heneine W. 2010. When do minority drug-resistant HIV-1 variants have a major clinical impact? J. Infect. Dis. 201:647–649 [DOI] [PubMed] [Google Scholar]
- 16. Jain J, et al. 2004. Pharmacokinetics, ovulation suppression and return to ovulation following a lower dose subcutaneous formulation of depo-provera. Contraception 70:11–18 [DOI] [PubMed] [Google Scholar]
- 17. Karim QA, et al. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karim SSA, Kashub ADM, Werner L, Karim QA. 2011. Drug concentrations after topical and oral antiretroviral preexposure prophylaxis: implications for HIV prevention in women. Lancet 378:279–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ketas TJ, et al. 2007. Entry inhibitor-based microbicides are active in vitro against HIV-1 isolates from multiple genetic subtypes. Virology 364:431–440 [DOI] [PubMed] [Google Scholar]
- 20. Klasse PJ, Shattock RJ, Moore JP. 2006. Which topical microbicides for blocking HIV-1 transmission will work in the real world? PLoS Med. 3:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klasse PJ, Shattock RJ, Moore JP. 2008. Anti-retroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu. Rev. Med. 59:455–471 [DOI] [PubMed] [Google Scholar]
- 22. Lagenaur LA, et al. Prevention of vaginal SHIV transmission in macaques by a live recombinant Lactobacillus. Mucosal Immunol. 4:648–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lederman MM, Offord RE, Hartley O. 2006. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat. Rev. Immunol. 6:371–382 [DOI] [PubMed] [Google Scholar]
- 24. MacArthur RD, Novak RM. 2008. Reviews of anti-infective agents. Maraviroc: the first of a new class of antiretroviral agents. Clin. Infect. Dis. 47:236–241 [DOI] [PubMed] [Google Scholar]
- 25. Malcolm K, et al. 2003. Influence of silicone elastomer solubility and diffusivity on the in vitro release of drugs from intravaginal rings. J. Control. Rel. 90:217–225 [DOI] [PubMed] [Google Scholar]
- 26. Malcolm RK, Woolfson AD, Toner CF, Morrow RJ, McCullagh SD. 2005. Long-term, controlled release of the HIV microbicide TMC120 from silicone elastomer vaginal rings. J. Antimicrob. Chemother. 56:954–956 [DOI] [PubMed] [Google Scholar]
- 27. Marx P, et al. 1996. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 2:1084–1089 [DOI] [PubMed] [Google Scholar]
- 28. Miller L, et al. 2000. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet. Gynecol. 96:431–439 [DOI] [PubMed] [Google Scholar]
- 29. Moore JP, Kuritzkes DR. 2009. A pièce de resistance: how HIV-1 escapes small molecule CCR5 inhibitors. Curr. Opin. HIV AIDS 4:118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Napier C, et al. 2005. Molecular cloning and radioligand binding characterization of the chemokine receptor CCR5 from rhesus macaque and human. Biochem. Pharmacol. 71:163–172 [DOI] [PubMed] [Google Scholar]
- 30a. National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 31. Nel A, et al. 2009. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J. Acquir. Immune Defic. Syndr. 51:41–423 [DOI] [PubMed] [Google Scholar]
- 32. Owen DH, Katz DF. 1999. A vaginal fluid simulant. Contraception 59:91–95 [DOI] [PubMed] [Google Scholar]
- 33. Promadej-Lanier N, et al. 2009. Development and evaluation of a vaginal ring device for sustained delivery of HIV microbicides to non-human primates. J. Med. Primatol. 38:263–271 [DOI] [PubMed] [Google Scholar]
- 34. Schader SM, Colby-Germinario SP, Schachter JR, Xu H, Wainberg MA. 2011. Synergy against drug-resistant HIV-1 with the microbicide antiretrovirals, dapivirine and tenofovir, in combination. AIDS 25:1585–1594 [DOI] [PubMed] [Google Scholar]
- 35. Seibert C, Sakmar TP. 2004. Small-molecule antagonists of CCR5 and CXCR4: a promising new class of anti-HIV-1 drugs. Curr. Pharmacol. Design 10:2041–2062 [DOI] [PubMed] [Google Scholar]
- 36. Shattock RA, Moore JP. 2003. Inhibiting HIV-1 sexual transmission. Nat. Rev. Microbiol. 1:25–34 [DOI] [PubMed] [Google Scholar]
- 37. Shattock RJ, Warren M, McCormack S, Hankins CA. 2011. AIDS. Turning the tide against HIV. Science 333:42–43 [DOI] [PubMed] [Google Scholar]
- 38. Slater B, McCormack A, Avdeef A, Comer JEA. 1993. pH-metric log P. Comparison of partition coefficients determined by HPLC and potentiometric methods to literature values. J. Pharm. Sci. 83:1280–1283 [DOI] [PubMed] [Google Scholar]
- 39. Smith KPB. 1993. Estrogens and the urogenital tract. Studies on steroid hormone receptors and a clinical study on a new estradiol releasing vaginal ring. Acta Obstet. Gynecol. Scand. 72(Suppl. 157):S1–S26 [PubMed] [Google Scholar]
- 40. Spear GT, et al. 2010. Identification of rhesus macaque genital microbiota by 16S pyrosequencing shows similarities to human bacterial vaginosis: implications for use as an animal model for HIV vaginal infection. AIDS Res. Hum. Retrovir. 26:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stone A. 2002. Microbicides: a new approach to preventing HIV and other sexually transmitted infections. Nat. Rev. Drug Discov. 1:977–985 [DOI] [PubMed] [Google Scholar]
- 42. Supervie V, García-Lerma JG, Heneine W, Blower S. 2010. HIV, transmitted drug resistance, and the paradox of preexposure prophylaxis. Proc. Natl. Acad. Sci. U. S. A. 107:12381–12386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takács-Novák K, Box KJ, Avdeef A. 1997. Potentiometric pKa determination of water-insoluble compounds. Validation study in methanol/water mixtures. Int. J. Pharm. 151:235–248 [Google Scholar]
- 44. Turpin JA. 2002. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin. Investig. Drugs 11:1077–1097 [DOI] [PubMed] [Google Scholar]
- 45. Veazey RS, et al. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9:343–346 [DOI] [PubMed] [Google Scholar]
- 46. Veazey RS, et al. 2005. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature 438:99–102 [DOI] [PubMed] [Google Scholar]
- 47. Veazey RS, et al. 2010. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 coreceptor. J. Infect. Dis. 202:739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Woolfson AD, Malcolm RK, Morrow RJ, Toner CF, McCullagh SD. 2006. Potential use of vaginal rings for prevention of heterosexual transmission of HIV: a controlled-release strategy for HIV microbicides. Am. J. Drug Deliv. 4:7–20 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.