Abstract
Malaria continues to have a significant impact on the health of the developing world. Efforts to combat this disease now focus on combination therapy in order to stem the emergence of resistant parasites. Continued efforts are needed to discover and develop new agents for use in combination antimalarial regimens. MK-4815 is a small molecule with antimalarial activity that was identified from a large pharmaceutical compound collection using a semiautomated version of a well-established in vitro assay for the erythrocytic stages of Plasmodium falciparum. In vitro studies indicate that the compound selectively accumulates in infected red blood cells and is most effective against the metabolically active late trophozoite/early schizont stages. A variety of drug-resistant field isolates of P. falciparum were found to be as sensitive to MK-4815 as the wild-type lines. MK-4815 is orally active in a P. berghei mouse model of acute malaria. In this model, where untreated animals succumb to infection 10 to 12 days postinfection, MK-4815 was completely curative when given as a single dose of 50 mg/kg, 2 doses of 25 mg/kg, or 4.5 doses of 12.5 mg/kg. In pharmacokinetic studies with mice and rhesus monkeys, MK-4815 demonstrated oral bioavailability and low clearance. In addition, MK-4815 is inexpensive to synthesize, an important characteristic for providing affordable antimalaria therapy to the developing world. The attractive biological and pharmaceutical profile of MK-4815 demonstrates its potential for use in combination with other agents in the fight against malaria.
INTRODUCTION
Over half of the world's population, in 109 countries, is at risk of contracting malaria. In 2009, there were an estimated 225 million cases of malaria, resulting in approximately 781,000 deaths (23). The Plasmodium falciparum parasite is responsible for most malaria infections and a majority of the deaths. Antimalarial drugs have saved millions of lives since quinine was first isolated from cinchona bark in 1820. However, the cost of therapy is a major issue, and the realistic price that can be expected for an effective antimalarial in poverty-stricken areas where the disease is endemic severely limits the global investment to discover, develop, and distribute new antimalarial drugs.
The most significant challenges to malaria control are drug resistance due to the extreme selective pressure on the malaria parasite in areas where the disease is endemic and suboptimal dosing due to patient compliance issues. Chloroquine is no longer effective in many regions, and alternative therapies are limited. The introduction of artemisinin, a sesquiterpene lactone peroxide extracted from the leaves of the shrub Artemisia annua (and related derivatives), has helped in addressing the global problem of resistant malaria parasites. Artemisinin combination therapies (ACTs) are the best antimalarial drugs currently available (12, 16, 18). Widespread resistance to almost all antimalarial agents has emerged, and resistance to ACTs has been reported (14, 18). The current World Health Organization strategy is to use antimalarials only in combination regimens in an effort to slow the rate of resistance development previously experienced with monotherapy (22). Attention to and funding of malaria drug discovery activities have increased over the past few years, but there are limited effective treatments for malaria apart from the ACTs, and new agents for use in combination therapy are still needed. Based on the criteria outlined by the Medicines for Malaria Venture (MMV), the suitable product profile of a new antimalarial agent includes “efficacy against drug-resistant strains, cure within 3 days (using single daily doses), low toxicity especially in children and pregnancy, low risk for emergence of resistance, adeptness in formulation and packaging, and a low cost of goods.” These criteria make the hurdle of developing a new antimalarial agent quite high, and MMV anticipates that only 1 to 2% of new antimalarial projects will enter late-stage clinical development (15).
There have been advancements over the years with respect to malaria biology, including the methodology for quantifying the efficacy of antimalarial drugs in vitro. Two very significant events in the history of malaria biology were the successful cultivation of P. falciparum parasites in vitro (20) and the discovery that 3H-hypoxanthine labeling could be used to specifically quantify total parasite loads (5). Partnering these developments with the advent of high-throughput screening technology has now provided the opportunity to take on this challenge. Through miniaturization of the P. falciparum 3H-hypoxanthine assay in a 96-well format and the application of semiautomated screening technology, the Merck proprietary compound collection was rapidly screened for compounds with antimalarial activity. These efforts resulted in the discovery of MK-4815, a compound with potent activity against multidrug-resistant P. falciparum in vitro and potent, curative oral efficacy against Plasmodium berghei malaria in mice.
MATERIALS AND METHODS
Chemicals.
MK-4815 (2-aminomethyl-3,5-di-tert-butylphenol; Fig. 1) is a white crystalline powder with a molecular weight of 271.8. Tritium (phenol-6-3H; specific activity of 10.6 Ci/mol)-labeled MK-4815 was synthesized by Merck Research Laboratories. Chloroquine was purchased from Sigma-Aldrich (St. Louis, MO).
Fig 1.

Structure of MK-4815 (2-aminomethyl-3,5-di-tert-butylphenol).
Parasite maintenance and in vitro assay.
P. falciparum parasites were maintained in culture with human type O+ red blood cells (RBCs) and serum according to the methods described by Trager and Jensen (20). A modified version of the in vitro [3H]hypoxanthine incorporation assay described by Desjardins et al. (7) was used for determination of the MK-4815 IC50s and IC90s (effective compound doses required to inhibit incorporation by 50 and 90%, respectively) for the Dd2 and NF54 strains of P. falciparum. In order to screen a large compound collection using semiautomated methods, the assay conditions were modified so that the total volume of each well was reduced to 125 μl while still maintaining a 2 to 2.5% hematocrit level. Tritiated hypoxanthine (0.5 μCi/well) was added at the start of the 48-h incubation period. The in vitro activity of MK-4815 was also measured against an expanded panel of P. falciparum strains that included (i) the drug-sensitive 3D7 clone of the NF54 isolate; (ii) the chloroquine-resistant (CQ-R), pyrimethamine-resistant (PYR-R), and cycloguanil-resistant (CYC-R) K1 strain (Thailand); (iii) the CQ-R, PYR-R, and CYC-R V1/S strain (Vietnam); (iv) the CQ-R FCB strain (Colombia); and (v) the CQ-R FCR3 strain (The Gambia). Strains in the expanded panel were evaluated in a similar manner; however, [3H]hypoxanthine was included only during the final 24 h of the 48-h incubation period (4). Mefloquine-resistant organisms were not readily available at the time this testing was done.
Mouse efficacy studies.
P. berghei (strain KBG173) was maintained by routine passage in BALB/c mice. An animal model of infection was established with BALB/c mice (20 to 25 g; Taconic Farms) infected by intraperitoneal (i.p.) injection (0.5-ml volume) of 106 P. berghei-infected RBCs. In most studies, treatment (oral gavage, 0.2-ml volume) was initiated 2 h postinfection. MK-4815 was given in a 10% dimethyl sulfoxide (DMSO) solution (0.2-ml volume), once or twice daily for up to 5 days (see figure legends for specific dosing parameters for each experiment). In this model, 100% of the untreated mice succumb to infection 10 to 12 days postinfection (>50% parasitemia). Efficacy was determined by observing survival and by monitoring parasitemia in mice via microscopic examination of Giemsa-stained smears of tail blood (21). In all studies, mice were examined for at least 70 days postinfection. Parasitemia in surviving mice was monitored every few days for a month and then weekly thereafter. Mice were considered parasite free after three consecutive tail blood samples showed no detectable parasitemia. All animal studies adhered to the guidelines set forth by the Merck Institutional Animal Care and Use Committee for the humane treatment of animals in research.
Effects of MK-4815 on early- and late-stage P. falciparum parasites.
Synchronized cultures of P. falciparum were prepared (10) by suspending culture pellets in a 1:10 ratio of RBCs (pellet volume) to 5% d-sorbitol and incubating them for 5 min at 37°C. The pellet was suspended and washed twice with equal volumes of RPMI 1640. This procedure lyses all of the parasites but those in the ring stage (13). To determine the effect of MK-4815 on ring stage parasites, 1-ml aliquots of the culture were exposed to 500 ng/ml MK-4815 and incubated for 0.5, 1, 2, 4, 7, or 16 h. After incubation, the aliquots were washed three times with RPMI 1640 and used in the [3H]hypoxanthine assay described earlier. In order to evaluate the effects on later stages of parasite development, synchronized cultures were incubated for 22 h before being exposed to MK-4815 for 1, 2, 4, 7, or 16 h and then washed and assayed as described above. The data are expressed as the percent inhibition of [3H]hypoxanthine incorporation relative to that in untreated cultures.
Uptake of [3H]MK-4815 by infected RBCs.
P. berghei parasites were harvested from infected female BALB/c mice (40 to 60% parasitemia) after CO2 euthanasia. Blood was collected in phosphate-buffered saline (PBS) containing heparin. Cells were washed in PBS and centrifuged (4°C) at 1,500 × g for 5 min. The pellet was suspended in twice the volume of PBS and passed over a column containing cellulose powder (Whatman CF-11) for removal of leukocytes and platelets. P. berghei-infected RBCs in the eluant were washed in PBS. Control uninfected mouse RBCs were prepared in the same way.
RBC fractions infected with developmental-stage-specific parasites (of P. falciparum from in vitro cultures and P. berghei from infected mice) were enriched using a variation of the Percoll gradient method described by Dluzewski et al. (8). A 90% Percoll bottom layer was overlaid with a 60% Percoll cushion, and infected RBCs were applied to the top. After centrifugation at 8,000 × g for 10 min, two of the three prominent fractions were harvested. The top layer was enriched for mature trophozoite/schizont-infected RBCs, while the middle layer consisted largely of infected RBCs containing ring and early trophozoite stage parasites. The two fractions of infected RBCs were collected, washed with PBS or RPMI 1640, and utilized in the [3H]MK-4815 uptake studies described below.
Equal numbers (∼4 × 107) of RBC fractions that were uninfected, or infected with developmental-stage-specific parasites were incubated with [3H]MK-4815 at room temperature for 20 min. Specific uptake was determined by competition with a 1,000-fold molar excess of unlabeled MK-4815. The cells were centrifuged and washed twice with ice-cold Hanks balanced salt solution. Pellets were suspended in 100 μl 0.1% Triton X-100 and 6 μl 30% hydrogen peroxide and incubated for 10 min at 37°C. Scintillation solution was added, and samples were counted for 2 min each using a Beckman scintillation counter.
Pharmacokinetics of MK-4815 in mice and rhesus monkeys.
The single-dose pharmacokinetic parameters of MK-4815 were determined for the mouse and rhesus monkey. BALB/c mice were dosed with MK-4815 (2 mg/kg orally [p.o.] or 1 mg/kg intravenously [i.v.]) formulated in ethanol-polyethylene glycol (PEG) 400-H2O (5:15:80). Samples were taken by cardiac puncture at 0, 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, and 24 h. Blood was collected in heparinized tubes, and the plasma was separated by centrifugation. Plasma drug concentrations were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) following protein precipitation with acetonitrile. Rhesus monkeys were dosed with MK-4815 (2 mg/kg p.o. or 1 mg/kg i.v.) formulated in ethanol-PEG 400-water (20:25:55 at 2 mg/ml). Serial blood samples were taken at 0, 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, and 24 h. Blood was collected in heparinized tubes and separated by centrifugation. Plasma drug concentrations were determined by LC-MS/MS following solid-phase extraction on Waters Oasis hydrophilic-lipophilic-balanced reversed-phase sorbent. Standard pharmacokinetic parameters were calculated for MK-4815 in both preclinical species.
RESULTS
MK-4815 inhibition of P. falciparum viability in vitro.
From an empirical whole-cell screening of a synthetic chemical sample collection, MK-4815 (2-aminomethyl-3,5-di-tert-butylphenol, Fig. 1) was identified as an inhibitor of P. falciparum growth and development in human RBCs. Using the incorporation of [3H]hypoxanthine as a measure of parasite viability shows that MK-4815 is as potent as chloroquine against the chloroquine-sensitive NF54 strain of P. falciparum (IC50s, 38 and 35 ng/ml, respectively) and more potent than chloroquine against the chloroquine-, pyrimethamine-, and mefloquine-resistant Dd2 strain (IC50s, 40 and 70 ng/ml, respectively). The in vitro activity of MK-4815 against an expanded panel of geographically diverse, drug-resistant clinical isolates of P. falciparum is illustrated in Table 1. Like artesunate, MK-4815 has broad-spectrum and potent activity against the entire panel. Of particular significance are the potent MK-4815 IC50s for the K1, V1/S, and FCB strains of parasite, which clearly demonstrate reduced susceptibility to chloroquine.
Table 1.
In vitro efficacy of MK-4815 versus drug-resistant P. falciparum
| Drug | IC50 (μg/ml) for P. falciparum isolate: |
|||||
|---|---|---|---|---|---|---|
| 3D7a | K1b | FCBc | V1/Sd | FCR-3e | TM90f | |
| MK-4815 | 0.030 | 0.004 | 0.010 | 0.007 | 0.010 | 0.03 |
| Artesunate | 0.004 | 0.001 | 0.002 | 0.001 | 0.002 | < 0.01 |
| Chloroquine | 0.008 | 0.484 | 0.221 | 0.370 | 0.17 | |
Clone of the NF54 isolate (unknown origin); drug sensitive.
From Thailand; CQ-R, PYR-R, and CYC-R.
From Colombia; CQ-R.
From Vietnam; CQ-R, PYR-R, and CYC-R.
From The Gambia; CQ-R.
From Thailand; CQ-R, PYR-R, and CYC-R.
Dose-response study with P. berghei-infected mice.
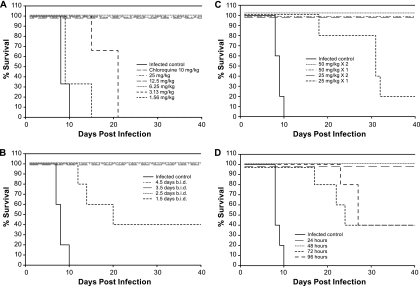
In order to determine whether MK-4815 is orally active, we treated infected mice once on the day of infection and then twice daily for an additional 4 days (a total of nine treatments). In this study, control infected mice developed an acute infection by day 10, while 100% survival was observed in mice receiving 25, 12.5, or 6.25 mg/kg MK-4815 or 10 mg/kg chloroquine (once a day for 5 days, Fig. 2A). Mice were completely clear of parasites (determined by microscopic examination of blood smears) for the duration of the study (74 days). No mice in the 3.13-mg/kg MK-4815 treatment group survived for the duration of the study, although there was an approximately 10-day increase in survival versus that of infected control mice. No significant efficacy was seen at 1.56 mg/kg MK-4815.
Fig 2.
Oral administration of MK-4815 is efficacious in a mouse model of P. berghei infection. In each panel, BALB/c mice were infected by i.p. injection of 106 P. berghei-infected RBCs. MK-4815 formulated in 10% DMSO was dosed orally as described below. Sham-treated animals were dosed with vehicle. (A) Efficacy of MK-4815 in a dose-titration study. P. berghei-infected animals were treated with a single dose of compound on the day of infection (2 h postinfection) and then twice daily (b.i.d.) for the following 4 days (a total of nine treatments). MK-4815 was given at 25, 12.5, 6.25, 3.13, or 1.56 mg/kg (three mice per treatment group). Chloroquine was included as a positive control, using a once-a-day dose of 10 mg/kg for 5 days beginning on the day of infection. (B) Number of MK-4815 treatments, at a dose of 12.5 mg/kg, required for in vivo efficacy. Parasite-infected animals (five per group) were treated with 12.5 mg/kg MK-4815 once on the day of infection (2 h postinfection) and then twice daily for 1 (1.5 doses, a total of three treatments) to as many as 4 subsequent days (4.5 doses, a total of nine treatments). An additional treatment group received MK-4815 at 12.5 mg/kg once daily for 4 days beginning on the day of infection. (C) A single p.o. MK-4815 treatment at 50 mg/kg is sufficient for efficacy in the animal model. P. berghei-infected mice (five per group) were treated with either a single oral dose of MK-4815 (administered at 2 h postinfection) or two doses administered 24 h apart. Treatment groups included MK-4815 at 50, 25, or 12.5 mg/kg. (D) Efficacy of MK-4815 in a delayed-therapy study. Parasite-infected mice (five per group) were treated with a single 50-mg/kg oral dose of MK-4815 at 2, 24, 48, 72, or 96 h postinfection.
Duration of treatment study.
To identify the minimum oral treatment regimen required for MK-4815 efficacy at a dose of 12.5 mg/kg, P. berghei-infected mice were treated once on the day of infection and then twice daily for 1, 2, 3, or 4 additional days (a total of three to nine treatments at 12.5 mg/kg). Full efficacy (100% survival with no detectable parasitemia for up to 95 days postinfection) was achieved in mice treated for ≥2.5 days (Fig. 2B). Forty percent of the mice treated for 1.5 days remained parasite free throughout the study, while the remaining mice showed an increase in survival relative to that of controls (13, 15, and 21 days postinfection). All control mice succumbed to infection by day 10 postinfection.
Short-term treatment with MK-4815.
In the same P. berghei infection model, single p.o. treatments with MK-4815 at doses of 50 and 100 mg/kg on the day of infection were fully efficacious (data not shown). In order to identify a minimal dose required to achieve this level of protection, P. berghei-infected mice were treated p.o. with either a single dose of MK-4815 (50, 25, or 12.5 mg/kg) or two doses of the compound (25 or 12.5 mg/kg) administered 24 h apart. One hundred percent survival was observed in the groups treated with a single dose of 50 mg/kg or two doses of 25 mg/kg (Fig. 2C), and no parasites were detected in these mice at any time during the course of the study (out to 107 days postinfection). Only one mouse in the 25-mg/kg single-dose group showed a complete cure (1/5). All control infected mice succumbed to the infection by day 11 postinfection.
Single-dose delayed-treatment study.
The impact of a delay in treatment following parasite infection was tested using a single 50-mg/kg oral dose of MK-4815. Treatment groups in this study included mice dosed at 24, 48, 72, or 96 h after i.p. infection with P. berghei. A single 50-mg/kg dose of MK-4815 was fully effective in animals treated following a 24- or 48-h delay (Fig. 2D). Forty percent of the mice in the 72- and 96-h delayed-treatment groups were parasite free at the conclusion of the experiment (107 days postinfection); the remaining three mice in each group showed a significant increase in survival (>10 days) compared to that of sham-treated animals.
Differential effect of MK-4815 on early- and late-stage P. falciparum parasites in vitro.
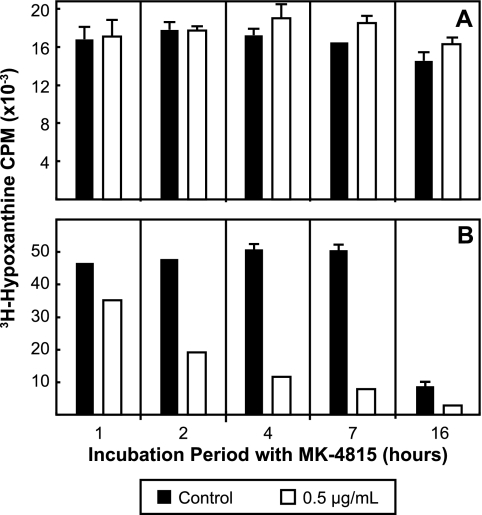
Cultures of the Dd2 strain of P. falciparum parasites were enriched for ring stage parasites using d-sorbitol and exposed to MK-4815 for various lengths of time. The viability of ring stage-enriched parasites using this assay format was not affected by exposure to MK-4815 (Fig. 3A). When ring stage parasites were allowed to progress in culture to a population enriched for trophozoite/schizont developmental stages (22 h in culture following release from synchrony) and then treated, MK-4815 had a substantial effect (Fig. 3B). In contrast, when late-stage trophozoites and schizonts were exposed to MK-4815, there was a 25% reduction in subsequent parasite growth after only 1 h of exposure (Fig. 3B). Longer exposures had an even greater effect, with almost 85% inhibition after 7 h of exposure to MK-4815.
Fig 3.
Differential in vitro activity of MK-4815 on synchronized early- and late-stage P. falciparum parasites. Synchronized P. falciparum parasite populations (ring stage- and trophozoite/schizont-enriched parasites; see Materials and Methods) were exposed to MK-4815 at 0.5 μg/ml for various amounts of time (1 to 16 h). Following exposure to the compound, the parasite preparations were washed and placed in fresh medium containing [3H]hypoxanthine to quantitate the impact of MK-4815 on viability. (A) Results for ring stage parasites. (B) Results for parasites enriched in trophozoite/schizont stages. Data are expressed as the percent inhibition of [3H]hypoxanthine incorporation relative to that of untreated cultures.
Selective uptake of MK-4815 into infected RBCs.
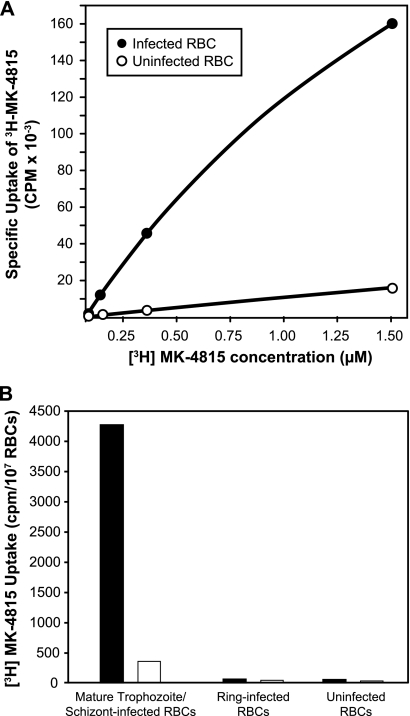
The uptake of MK-4815 into P. berghei-infected mouse RBCs was measured using a tritium-labeled analog of the compound. Infected RBCs enriched for mature trophozoite and schizont developmental stages of the parasite (prepared by Percoll gradient centrifugation) accumulate [3H]MK-4815 in a dose-dependent manner, while only minimal compound is associated with uninfected RBCs (Fig. 4A). The association of the compound with these P. berghei-infected RBCs was not saturated even at a concentration of 1.5 μM, the highest level tested.
Fig 4.
MK-4815 is preferentially localized in infected RBCs. (A) Preparations of Percoll-purified RBCs from P. berghei-infected mice (enriched for mature trophozoite/schizont developmental stages), as well as normal mouse RBCs, were incubated with various concentrations of [3H]MK-4815 for 20 min at room temperature. After several washes, the cell-associated [3H]MK-4815 in parasite-infected RBCs and normal mouse RBCs was quantitated by scintillation counting. (B) Preferential uptake of MK-4815 into mature developmental stages of P. falciparum. Synchronized P. falciparum parasite populations (RBCs infected with ring stage- and trophozoite/schizont-enriched parasites; see Materials and Methods) were incubated with [3H]MK-4815 for 20 min at room temperature either in the absence (black bars) or in the presence (white bars) of a 1,000-fold molar excess of unlabeled MK-4815. RBCs infected with parasites enriched for ring stages and uninfected human RBCs were handled in a parallel fashion. After several washes, cell-associated [3H]MK-4815 was quantitated by scintillation counting.
The uptake of [3H]MK-4815 was also measured in P. falciparum-infected RBC preparations. In a representative experiment (Fig. 4B), a considerable amount of MK-4815 is detected in the Percoll gradient fraction enriched for mature trophozoite/schizont parasites. Association of [3H]MK-4815 with RBCs infected with this developmental stage of P. falciparum can be competed with excess unlabeled compound. In a manner that parallels the developmental-stage-specific sensitivity to MK-4815 (Fig. 3), very little compound uptake is measured in ring stage parasites. The amount of [3H]MK-4815 in uninfected human RBCs is also negligible. Both compound uptake and the impact of MK-4815 on growth are much more profound in the metabolically active trophozoite/schizont developmental stages of parasites.
Pharmacokinetics of MK-4815.
The pharmacokinetic properties of MK-4815 in the mouse and rhesus monkey were examined (Table 2). The oral bioavailability of MK-4815 is 61% in rhesus monkeys and 99% in mice. While the volumes of distribution at steady state (Vss) were similar in the two species, the drug has a longer half-life (t1/2) and lower clearance in rhesus monkeys, suggesting that the pharmacokinetic parameters of MK-4815 are superior in the higher species.
Table 2.
Pharmacokinetic parameters of MK-4815 in mice and monkeys
| Parametera | Mouse | Monkey |
|---|---|---|
| Dose (i.v./p.o., mg/kg) | 1/2 | 1/2 |
| CLp (ml/min/kg) | 21 | 6.7 |
| Vss (liters/kg) | 7.2 | 8.0 |
| t1/2 (h) | 4.5 | 14 |
| Oral Cmax (μM) | 0.85 | 0.44 |
| Oral Tmax (h) | 2 | 7 |
| Oral AUCnorm(0-∞) (μM.h/mg/kg) | 3.4 | 6.5 |
| F (%) | 99 | 61 |
The i.v. and p.o. dose formulation was ethanol-PEG 400-water at 20:25:55 (vol/vol/vol) for monkeys and at 5:15:80 for mice. Cmax, maximum concentration of drug in serum; CLp, clearance from plasma; Tmax, time to Cmax; AUCnorm(0-∞), AUC divided by dose per kilogram of body weight (from 0 h to infinity); F, bioavailability.
DISCUSSION
MK-4815, a compound identified by screening of the Merck Chemical collection, is considered a Mannich base, a class of compounds previously reported to have various degrees of antimalarial activity (1, 2, 3, 6, 9, 17). SN 7.744 (2, 3, 6) and WR-194,965 (17) are Mannich bases which have reported antimalarial activity. WR-194,965 was active against multidrug-resistant parasites and cured four of six patients of an experimental P. falciparum infection. Since there is limited information available, it is unclear why this compound was not pursued further. In order to understand the efficacy of MK-4815, a series of studies was conducted to determine the optimal dosing regimen in the P. berghei mouse model. The P. berghei model used in these studies was designed to achieve 100% lethality of control infected mice at approximately 10 to 12 days postinfection and to demonstrate a 100% cure rate with chloroquine as a positive control. Several parameters were examined in these experiments, including dose, duration of dosing, and timing of the first dose postinfection. The results of these studies indicated that a 100% cure rate can be achieved in this model with MK-4815 at a minimal oral dose of 6.25 mg/kg twice daily for 4.5 days. Higher doses can be used to achieve a 100% cure rate with a shorter dosing period, such as 12.5 mg/kg twice daily for 2.5 days, 25 mg/kg once daily for 2 days, or a single dose of 50 mg/kg given only once at 2 h postinfection. The total dose of MK-4815 required for full efficacy translates to total cumulative doses of 56.25, 62.5, 50, and 50 mg/kg, respectively. An additional mouse study demonstrated that a single oral dose of MK-4815 at 50 mg/kg provides a 100% cure rate when given up to 48 h postinfection, while intervals of 72 and 96 h between infection and treatment were much less effective. These results have an interesting parallel in in vivo data reported for WR-194,965 in owl monkeys, which suggested that the total cumulative dose in mg/kg determines efficacy (17).
MK-4815 has good oral bioavailability in mice and rhesus monkeys and has lower clearance, a greater area under the concentration-time curve (AUC), and a longer t1/2 in the monkey, confirming good pharmacokinetic properties in a second species and suggesting that a lower dose might be required to treat malaria infections in higher species. Further studies are required to determine the key pharmacokinetic parameter(s) that best predicts the efficacy of MK-4815.
With respect to important drug-resistant isolates of P. falciparum, MK-4815 was fully effective in all cases, including multidrug-resistant strains, with IC50s in the low ng/ml concentration range (Table 1). Using synchronized P. falciparum cultures and [3H]hypoxanthine labeling, minimal efficacy was seen against ring and early trophozoite stages exposed to 500 ng/ml MK-4815 for up to 16 h, while a >80% reduction in parasite-specific labeling was seen when the schizont stage was exposed to the same concentration of MK-4815 for 7 h. It is not clear why infected control labeling is so low for the 16-h exposure in the late-stage experiment; a possible explanation is that the PBS washes interfered with the reinfection process since it would be expected that a significant portion of the parasites would be extracellular by this time.
Further confirmation of stage specificity was provided by the use of [3H]MK-4815 labeling of purified parasite stages from P. berghei-infected mouse blood. While minimal labeling was seen in uninfected erythrocytes and in those containing rings and early trophozoites, significant uptake was seen in erythrocytes containing the more mature trophozoites and schizonts (Fig. 4B). Calculations based on label and erythrocyte volume suggest that MK-4815 could reach low millimolar concentrations in schizont-infected erythrocytes. One interpretation of this result is that the mechanism of parasite death may be a consequence of nonspecific effects due to the high concentration of MK-4815 in infected cells, rather than a specific effect on a parasite target. However, the rapid accumulation of MK-4815 to high concentrations in the infected RBC makes determination of the mechanism of action difficult.
In the case of chloroquine, it has been suggested that the mechanism of action is facilitated by its elevated accumulation in mature-stage parasites, which results in the prevention of heme polymerization, an important detoxification process required for survival of the parasite (11, 19). MK-4815 does have an effect on heme polymerization (data not shown). However, it cannot be determined whether efficacy of MK-4815 results from a direct effect on the heme polymerization process or from a more general toxic effect on the parasite, based on the high concentration of MK-4815 found within parasitized RBCs (Fig. 4A). Regardless, if the mechanism of action of MK-4815 is similar to that of chloroquine, in vitro studies with resistant P. falciparum strains indicate that the mechanism of resistance to chloroquine does not result in cross-resistance to MK-4815.
In summary, MK-4815 appears to be a potential new therapy for treating acute malaria due to P. falciparum. The compound has good pharmacokinetic properties in preclinical species, efficacy against multidrug-resistant strains of P. falciparum, and a simple chemical structure that is inexpensive to synthesize. In vivo studies with the mouse P. berghei model demonstrate that a single 50-mg/kg dose of MK-4815 can provide a 100% cure rate in cases of acute infection. The mechanism of action of MK-4815 is not clear, but the data provided here show that short-term exposure (4 to 7 h) can kill mature P. falciparum parasites in vitro and suggest that efficacy may be due to selective accumulation of MK-4815 in late-stage parasites. MK-4815 is undergoing further evaluation by the Medicines for Malaria Venture, where it has been accepted as a preclinical candidate.
ACKNOWLEDGMENTS
We thank Conrad Dorn, Thomas Walsh, and Matthew Wyvratt (Merck and Company) for chemistry support and the Basic Chemistry Analytical Support group, especially James Pivnichny and Gino Salituro, for pharmacokinetic support. Simon Croft's lab evaluated MK-4815 in the drug-resistant strains shown in Table 1 in the [3H]hypoxanthine assay. We thank Helen Profous-Juchelka and Jacqueline Fine for preparing the various agreements necessary for this project and Kim Strohmaier for editorial assistance with the manuscript.
Footnotes
Published ahead of print 6 February 2012
REFERENCES
- 1. Berliner RW, Butler TC. 1946. In Wiselogle FY. (ed), A survey of antimalarial drugs 1941-1945, vol 1 J. W. Edwards, Ann Arbor, MI [Google Scholar]
- 2. Burckhalter JH, Tendick FH, Jones EM, Holcomb WF, Rawlins AL. 1946. Aminoalkylphenols as antimalarials. I. Simply substituted α-aminocresols. Am. Chem. Soc. 68:1894–1901 [DOI] [PubMed] [Google Scholar]
- 3. Burckhalter JH, Tendick FH. 1948. Aminoalkylphenols as antimalarials (heterocyclicamino)-alpha-amino-o-cresols; the synthesis of camoquin. Am. Chem. Soc. 70:1363–1373 [DOI] [PubMed] [Google Scholar]
- 4. Cameron A, et al. 2004. Identification and activity of a series of azole-based compounds with lactate dehydrogenase-directed antimalarial activity. J. Biol. Chem. 279:31429–31439 [DOI] [PubMed] [Google Scholar]
- 5. Chulay JD, Haynes JD, Diggs CL. 1983. Plasmodium falciparum: assessment of in vitro growth by [3H]hypoxanthine incorporation. Exp. Parasitol. 55:138–146 [DOI] [PubMed] [Google Scholar]
- 6. Coatney GR, Cooper WC, Eddy NB, Greenberg J. 1953. Survey of antimalarial agents. Public Health Monogr. 9:129. [PubMed] [Google Scholar]
- 7. Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dluzewski AR, Ling IT, Rangarchari K, Bates PA, Wilson RJ. 1984. A simple method for isolating viable mature parasites of Plasmodium falciparum from cultures. Trans. R. Soc. Trop. Med. Hyg. 78:622–624 [DOI] [PubMed] [Google Scholar]
- 9. Duncan WG, Henry DW. 1969. 2-(Ω-Aminoalkyl)-4-t-butyl-6-phenylphenols as antimalarial agents. J. Med. Chem. 12:711–712 [DOI] [PubMed] [Google Scholar]
- 10. Fleck SL, et al. 2003. Suramin and suramin analogues inhibit merozoite surface protein-1 secondary processing and erythrocyte invasion by the malaria parasite Plasmodium falciparum. J. Biol. Chem. 278:47670–47677 [DOI] [PubMed] [Google Scholar]
- 11. Goldberg DE, Slater AF, Cerami A, Henderson GB. 1990. Hemoglobin degradation in the malaria parasite Plasmodium falciparum: an ordered process in a unique organelle. Proc. Natl. Acad. Sci. U. S. A. 87:2931–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koram KA, Abuaku B, Duah N, Quashie N. 2005. Comparative efficacy of antimalarial drugs including ACTs in the treatment of uncomplicated malaria among children under 5 years in Ghana. Acta Trop. 95:194–203 [DOI] [PubMed] [Google Scholar]
- 13. Lambros C, Vanderberg JP. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418–420 [PubMed] [Google Scholar]
- 14. Lin JT, Juliano JJ, Wongsrichanalai C. 2010. Drug-resistant malaria: the era of ACT. Curr. Infect. Dis. Rep. 12:165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Medicines for Malaria Venture 2010. New medicines for malaria control and eradication, target product profiles (TPPs) for 2010. Medicines for Malaria Venture, Geneva, Switzerland: http://www.mmv.org/research-development/essential-information-scientists/target-product-profiles [Google Scholar]
- 16. Mutabingwa TK. 2005. Artemisinin-based combination therapies (ACTs): best hope for malaria treatment but inaccessible to the needy! Acta Trop. 95:305–315 [DOI] [PubMed] [Google Scholar]
- 17. Schmidt LH, Crosby R. 1978. Antimalarial activities of WR-194,965, an alpha-amino-o-cresol derivative. Antimicrob. Agents Chemother. 14:672–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. 2009. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst. Rev. 8(3):CD007483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sullivan DJ, Jr, Gluzman IY, Russell DG, Goldberg DE. 1996. On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. U. S. A. 93:11865–11870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–676 [DOI] [PubMed] [Google Scholar]
- 21. Warhurst DC, Williams JE. 2004. Laboratory procedures for diagnosis of malaria, p 1–16 In Abdalla SH, Geoffrey P. (ed), Tropical medicine: science and practice, vol 4 Imperial College Press, London, United Kingdom [Google Scholar]
- 22. World Health Organization 2010. Fact sheet no. 94, malaria. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs094/en/ [Google Scholar]
- 23. World Health Organization 2010. World malaria report 2010. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/9789241564106/en/ [Google Scholar]