Abstract
For drug-compliant patients, poor responses to tuberculosis (TB) treatment might be attributable to subtherapeutic drug concentrations. An impaired absorption of rifampin was previously reported for patients with diabetes mellitus (DM) or HIV. The objectives of this study were to determine whether TB drug pharmacokinetics differed in Peruvian TB patients with DM or HIV. In this cross-sectional study, TB patients, recruited from health centers in Lima, Peru, had blood samples taken at 2 and 6 h after directly observed TB drug ingestion, to determine plasma concentrations of rifampin. Of 105 patients, 50 had TB without a comorbidity, 26 had coexistent DM, and 29 had coexistent HIV. Unexpectedly, the overall median 2- and 6-h levels of rifampin were 1.6 and 3.2 mg/liter, respectively, and the time to the peak concentration was 6 h (slow absorber) instead of 2 h (fast absorber) for 61 patients (62.2%). The geometric mean peak concentration of drug in serum (Cmax) was significantly higher in fast absorbers than in slow absorbers (5.0 versus 3.8 mg/liter; P = 0.05). The rifampin Cmax was significantly lower in male patients than in female patients (3.3 versus 6.3 mg/liter; P < 0.001). Neither slow nor fast absorbers with comorbidities (DM or HIV) had significantly different Cmax results compared to those of TB patients without comorbidities. An analysis of variance regression analysis showed that female gender (P < 0.001) and the time to maximum concentration of drug in serum (Tmax) at 2 h (P = 0.012) were independently correlated with increased exposure to rifampin. Most of this Peruvian study population exhibited rifampin pharmacokinetics different from those conventionally reported, with delayed absorption and low plasma concentrations, independent of the presence of an HIV or DM comorbidity.
INTRODUCTION
Over the last few decades, noncommunicable diseases (NCDs) have exhibited an increasing trend in developing countries (4), and the disease burden falls predominantly in these regions in terms of health and economic impacts (14). Diabetes mellitus (DM) is one of the major NCDs, and global statistics estimate an increase from 194 million people with this disease in 2003 to 330 million in 2030 (42). A substantial proportion (75%) will be living in developing countries (32), where concurrently, many communicable diseases, such as tuberculosis (TB), remain highly prevalent, particularly in countries with high rates of HIV infection (40).
The increased risk of active TB in diabetic patients is now well recognized (2, 31), and the danger of the convergence of these two global pandemics is clear, with 8 of the 10 countries with the highest incidences of DM in the world also presenting a major burden of TB (41).
Although less prevalent than DM, HIV is a more potent risk factor for TB and is a major cause of morbidity and mortality in HIV patients in low-income countries (9).
Most TB patients treated with standardized-dosing drug regimens exhibit a high cure rate with few side effects (19). Poor compliance with treatment is the major cause of treatment failure and relapse. Nevertheless, recently reported in vitro and animal model data suggest that the pharmacokinetic variability of antituberculosis drugs might be another factor to be considered (35). Therefore, an unknown percentage of cases with a poor response to treatment (defined by clinical failure or relapse) may be attributable to low drug concentrations (6, 15, 22, 27, 36). Therapeutic drug monitoring (TDM) has been proposed as a potential approach for patients failing therapy despite appropriately executed directly observed therapy (DOT) (27). Although TB drug dosing is based upon patient weight banding, antituberculosis drug pharmacokinetics (PK) may be altered by several factors, including age, gender, ethnicity, drug formulations, drug interactions, and gastroenteritis (6). Impaired absorption was previously suggested to occur in some patients with DM, HIV/AIDS, or cystic fibrosis (7, 12, 23, 33).
Culture conversion may be delayed in treated TB patients with DM; the only (small) study to evaluate PK in diabetic TB patients suggested that serum drug concentrations may be lower than those in TB patients without DM (23). For patients with HIV, conflicting results have been reported (7, 8, 11, 25, 29, 33, 37), although it was proposed that impaired pharmacokinetics leading to low drug concentrations could contribute to acquired drug resistance in fully adherent patients (39). This operational research study was undertaken to determine whether TB drug PK differed in Peruvian TB patients with a comorbidity of DM or HIV after controlling for potential confounders such as body weight (and, thus, the dose received in mg/kg of body weight) and intestinal parasitic infection.
MATERIALS AND METHODS
Study design, participants, and setting.
This cross-sectional observational study was conducted in Lima, Peru, from July to December 2009. TB patients who had received at least 15 days of treatment were recruited from health centers in Lima where directly observed therapy (6 days per week during the intensive phase and twice a week during the maintenance phase) is provided through the DOTS program of the Peruvian National Tuberculosis Program (NTP). Patients unwilling or unable to give informed consent were excluded. Patients with known HIV disease or DM were particularly sought, with the aim of recruiting at least 25 individuals for each subgroup.
Field methods.
At the interview, a semistructured questionnaire was administered to all participants. Particular emphasis was put on detecting previous gastrointestinal surgery, ongoing chronic diarrhea (three or more unformed stools per day for >15 days), and other factors that could contribute to malabsorption. Gender, age, height (cm), and weight (kg) were recorded, and the body mass index (BMI) was calculated.
Blood was drawn at the health center by dedicated study staff into 10-ml lithium heparin tubes at two time points, 2 and 6 h, after directly observed TB drug ingestion, and a fecal sample was collected for parasitological analysis.
Laboratory methods.
Blood samples were refrigerated, kept in the dark, and transported to the Universidad Peruana Cayetano Heredia (UPCH), where serum was separated (by centrifugation at 2,000 rpm for 10 min) between 2 and 5 h after sample collection. Aliquots were stored at −70°C until they were batched and transported to the pharmacokinetics laboratory of the Liverpool School of Tropical Medicine (LSTM), where rifampin (RIF) levels were measured by using a validated high-performance liquid chromatography (HPLC) technique. All sample preparations were carried out in a darkened room. Two hundred microliters of each sample was assayed alongside a plasma calibration curve (0 to 32 μg/ml) and quality control samples with a low concentration of 1.5 μg/ml, a medium concentration of 17 μg/ml, and a high concentration of 26 μg/ml. Plasma was precipitated by using 50 μl of an ethanol-containing internal standard (butyl 4-hydroxybenzoate at 16 μg/ml), followed by the addition of 1 ml of methanol, and vortexed for 20 s. Tubes were incubated at 4°C for 1 h and then centrifuged at 2,000 × g for 10 min. One milliliter of the upper solvent layer was transferred into a clean glass soda tube, evaporated to dryness under a stream of nitrogen at 30°C, and reconstituted in 120 μl methanol, and 100 μl of sample was then injected onto a Shimadzu LC 2010 HT HPLC system, with detection at 254 nm. Data acquisition was performed by using Chromeleon (Dionex). The compounds were separated on a Luna C8 150- by 4.6-mm 5-μm column (Phenomenex Inc.) protected by a LiChrosphere Si 60 5-μm column (VWR) using a gradient system, with the mobile phase containing solvent A (35% acetonitrile and 65% 50 mM ammonium formate [pH 5], adjusted with formic acid) and solvent B (70% acetonitrile and 30% 50 mM ammonium formate [pH 5], adjusted with formic acid). The column temperature was set at 30°C.
The assay was linear (r2 > 0.99) in the concentration range of 0 to 32 μg/ml, with intra- and interday precisions with a <13% coefficient of variation (CV) and a <11% CV, respectively. The lower limit of quantification (LLOQ) (0.5 μg/ml) has been accepted as the lowest point on the standard curve, with a relative standard deviation of less than 10% and a 5:1 signal-to-noise ratio. The determination of RIF stability following three freeze-thaw cycles showed that for all quality control (QC) samples, there was a <9% CV in RIF concentrations. RIF stability after heat inactivation showed a <11% CV in RIF concentrations.
Serum glucose levels were measured for all patients, and glycosylated hemoglobin levels (HbA1c) were measured in diabetic patients. Fecal samples were concentrated by the Formol ether method and examined for the presence of ova, cysts, and parasites within 24 h of collection.
Measurements of pharmacokinetic outcomes.
Two- and six-hour plasma concentrations were determined, and for each patient, the maximum serum drug concentration (Cmax) was estimated as the higher of the two measured concentrations; the Tmax for each patient was the time point at which the Cmax occurred.
Cmax values were also categorized as normal (>8 mg/liter), low (4 to 8 mg/liter), or very low (<4 mg/liter), in accordance with data reported previously (28, 38).
Statistical analysis.
Data were double entered into an EpiData database; checked with EpiData software, version 3.1; and analyzed with STATA, version 10.
Demographic and general characteristics of the three patient groups (TB, TB-HIV, and TB-DM) were compared by using the chi-square (χ2) test for the comparison of proportions and the Student t test for continuous and normally distributed variables. Non-normally distributed data were analyzed by using the Mann-Whitney test. The primary comparison of interest was between-group serum rifampin levels at 2 and 6 h postdosing and were analyzed with the independent-sample t test on the natural-logarithm-transformed pharmacokinetic data. Patients with TB and no other comorbidity (“TB”) were compared with (i) patients with TB and DM (“TB-DM”) and (ii) patients with TB and HIV (“TB-HIV”). A stratified analysis was performed to assess the effects of gender, age, BMI, and the dose of rifampin administered. The dose of rifampin was calculated by dividing the total milligrams of rifampin received by the weight of patients in kg.
A multivariate analysis of variance was performed to assess the variation in rifampin pharmacokinetics (Cmax) attributable to the presence of a comorbidity (HIV or DM), gender, BMI, and other variables that emerged from the univariate analyses.
Ethics and institutional review.
The study protocol and consent form were approved by the ethics committee of the London School of Hygiene and Tropical Medicine (LSHTM), the institutional review boards of the UPCH and Dirección de Salud-II (DISA-II) Lima Sur (regional Ministry of Health), and the ethics committee of the Hospital Nacional Dos de Mayo, Lima, Peru.
RESULTS
Participant characteristics.
Participant characteristics are shown in Table 1. Of 113 patients recruited into the study, PK data were available for 105 (mean age, 36.9 years; 63.8% male patients); for 5 of these patients, only one measurement was available, because either the sample was insufficient or the 6-h sampling time was missed. Sixty-one patients were in the intensive phase and 44 were in the continuation phase of their treatment; 70.5% of cases were new cases. The majority of patients (87%) had pulmonary TB; 77 had microbiologically confirmed TB, while 28 were receiving treatment based on clinicoepidemiological and radiological grounds. Fifty patients had TB without a comorbidity (TB, the baseline group for comparison), 26 had coexistent DM (TB-DM), and 29 had coexistent HIV (HIV-TB). Diabetic TB patients were significantly older and had significantly higher BMIs and significantly higher blood glucose levels than TB patients without a comorbidity (P < 0.001 for all). The median HbA1c level for diabetic patients was 8.3% (range, 4.6 to 12.8%; data were available for only 23/26 patients).
Table 1.
Participant characteristics by subgroupa
| Parameter | Value for group |
|||
|---|---|---|---|---|
| TB (n = 50) | DM-TB (n = 26) | HIV-TB (n = 29) | All (n = 105) | |
| % male patients | 48.0 | 65.4 | 90.0*** | 63.8 |
| Mean age (yr) (range) | 31.1 (18–65) | 51.3 (29–79)*** | 34.1 (24–48) | 36.9 (18–79) |
| No. of patients with microbiologically confirmed TB (%) | 38 (76.0) | 22 (84.6) | 17 (58.6) | 77 (73.3) |
| Mean BMI (kg/m2) (range) | 23.3 (18.4–29.6) | 27.5*** (21.9–36.3) | 22.8 (15.8–34.8) | 24.2 (15.8–36.3) |
| No. of patients with diarrhea <15 days (%) | 1 (2.0) | 3 (11.5) | 2 (6.9) | 6 (5.7) |
| No. of patients with chronic diarrhea (%) | 0 (0) | 0 (0) | 2 (6.9) | 2 (1.9) |
| No. of patients with intestinal surgery (%) | 4 (8.0) | 4 (15.4) | 2 (6.9) | 10 (9.5) |
| Mean blood glucose level (mg/dl) (range)b | 92.7 (40–159) | 178.8 (85–441)*** | 92.7 (61–123) | 114.2 (40–441) |
| No. of patients with pathogenic parasites (%)c,d | 4 (8.0) | 1 (3.9) | 4 (14.8) | 9 (8.7) |
| No. of patients with nonpathogenic parasites (%)c | 16 (32.0) | 12 (46.2) | 7 (25.9) | 35 (34.0) |
| No. of patients with intensive-phase treatment (%) | 31 (62.0) | 13 (50.0) | 17 (58.6) | 61 (58.1) |
| Median dose of rifampin received (mg/kg) (range) | 10.1 (7.4–12.6) | 8.8 (5.7–10.9)*** | 10.3 (6.7–15.8) | 9.8 (5.7–15.8) |
Proportions are expressed as cases/total number of patients (percent). Numerical values are expressed as means (ranges). All the tests compared the DM-TB or HIV-TB group to the TB (non-HIV, non-DM) group. Continuous variables were analyzed by using an independent t test, and categorical variables were analyzed with a Pearson χ2 test. BMI, body mass index. ***, P < 0.001, compared with the TB group without a comorbidity.
Results were not available for one TB patient (no comorbidity), one TB-DM patient, and two TB-HIV patients.
Results were not available for 2 HIV patients.
Two HIV patients had asymptomatic giardiasis, 1 patient had Cyclospora cayetanensis, 1 patient had Cryptosporidium parvum, and 1 diabetic patient also had giardiasis; in the TB group, all 4 patients had giardiasis.
A significantly higher proportion of HIV-TB patients were male, but otherwise, this group did not differ significantly from TB patients without a comorbidity. The mean CD4 count, available for only 14 patients, was 298 cells/μl. Eight HIV patients were receiving antiretroviral therapy, none with protease inhibitors.
The dose of rifampin (provided by IQ-farma and Infarmasa-Corporación, both from Peru) recommended by the Peruvian NTP is 10 mg/kg/day to a maximum of 600 mg per day for both the intensive and maintenance phases. The calculated dosage received was significantly lower in the DM-TB group (Table 1) and was significantly lower overall in males (9.5 mg/kg; range, 8.3 to 10.5 mg/kg) than in females (10.5 mg/kg; range, 9.3 to 11.0 mg/kg) (P = 0.004 by a Welch t test).
Pharmacokinetic analysis. (i) Rate of rifampin absorption (Tmax).
Unexpectedly, the Tmax was at 6 h instead of at 2 h for 61 patients (62.2%). For further analysis, subjects were therefore divided into 2 groups, (i) fast absorbers, with Tmax at 2 h, and (ii) slow absorbers, with Tmax at 6 h. Being a slow absorber (with a delayed Tmax) was not associated with gender, age group, DM or HIV comorbidity, phase of treatment, intestinal parasitic infection, or dose received (data not shown).
(ii) Peak rifampin level (Cmax).
Data are expressed as geometric means since the analysis was performed with the natural logarithm of the Cmax. Overall, median 2- and 6-h levels of rifampin were 1.6 mg/liter (range, 0 to 18 mg/liter) and 3.2 mg/liter (range, 0 to 16 mg/liter), respectively. The magnitude of the geometric mean peak rifampin absorption (Cmax) was 4.2 mg/liter (range, 0.5 to 18 mg/liter). The geometric mean Cmax of fast absorbers was significantly higher (5.0 mg/liter) than that of slow absorbers (3.8 mg/liter) (P = 0.05).
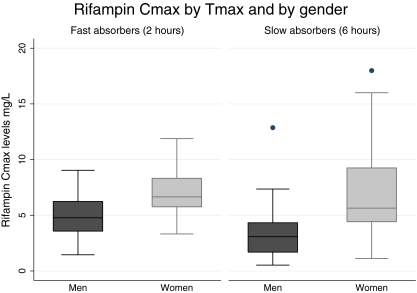
The rifampin Cmax was significantly lower in male than in female patients (3.3 versus 6.3 mg/liter; P < 0.001), and this effect was consistent in both fast (4.4 versus 6.7 mg/liter; P = 0.009) and slow (2.7 versus 6.1 mg/liter; P < 0.001) absorbers (Fig. 1).
Fig 1.
The rifampin Cmax is lower in males regardless of the Tmax.
Neither slow nor fast absorbers with comorbidities had results that were significantly different Cmax from those of TB patients without comorbidities (Table 2). The rifampin dosage received had no discernible effect upon Cmax values among either slow or fast absorbers.
Table 2.
Determinants of Cmax in fast and slow absorbers of rifampina
| Determinant | Geometric mean Cmax (mg/liter) for fast absorbers (2 h) (n = 37) | P value for fast absorbers | Geometric mean Cmax (mg/liter) for slow absorbers (6 h) (n = 61) | P value for slow absorbers |
|---|---|---|---|---|
| TB comorbidity | ||||
| DM-TB | 5.1 | 0.99 | 3.4 | 0.29 |
| HIV-TB | 4.8 | 0.76 | 3.1 | 0.10 |
| None | 5.1 | 4.5 | ||
| Sex | ||||
| Male | 4.4 | 0.009 | 2.7 | <0.001 |
| Female | 6.7 | 6.1 | ||
| Phase of treatment | ||||
| Intensive phase | 5.5 | 0.64 | 3.4 | 0.06 |
| Maintenance phase | 4.9 | 4.7 | ||
| Age group | ||||
| 18–30 yr | 5.0 | 0.93 | 4.4 | 0.61 |
| 31–40 yr | 4.9 | 3.5 | ||
| >40 yrs | 5.2 | 3.5 | ||
| Presence of intestinal parasites | ||||
| Yes | 3.9 | 0.40 | 3.0 | 0.49 |
| No | 5.1 | 3.8 | ||
| Overall geometric mean | 5.0 | 3.8 |
Numerical values are expressed as geometric means. Comparisons of groups were made between DM-TB or HIV-TB group and the TB (non-HIV, non-DM) group. All the variables were analyzed by using the independent-sample t test on the natural logarithm-transformed data.
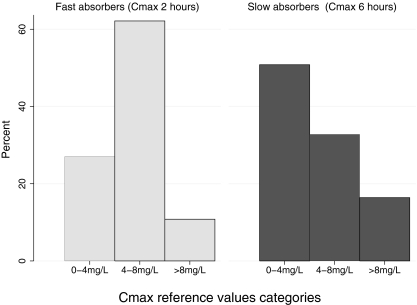
Thirty-six patients (34.3%) had undetectable rifampin levels at 2 h, two of whom also had undetectable levels at 6 h (Fig. 2). In the fast-absorber group, 10 patients (27.0%) had Cmax values of <4 mg/liter, 23 (62.2%) had rifampin levels between 4 and 8 mg/liter, and only 4 (10.8%) had values that are regarded as acceptable normal levels. For slow absorbers, 31 (50.8%) had Cmax values of <4 mg/liter, 20 (32.8%) had levels between 4 and 8 mg/liter, and 10 patients (16.3%) had normal levels (>8 mg/liter) (Fig. 2).
Fig 2.
Frequency distribution of Cmax categories by slow- and fast-absorber subgroups.
A multivariate analysis was performed, where the dependent variable was “the natural logarithm of Cmax.” We built a model to assess the independent effect of the time of absorption, gender, comorbidity (DM or HIV associated with TB), and rifampin dose received. The rifampin dose received was not found to have an effect upon the Cmax. However, males were found to receive lower doses than females. Therefore, it was decided to include the variable rifampin dose received in the multivariate analysis. Other variables, such as age, serum glucose level, or the presence of intestinal parasites, were not significantly associated and were excluded during the building of the model (Table 3). The rifampin dose received did not significantly influence the Cmax in our model. However, males were found to have Cmax levels 1.9 times lower than those of females (confidence interval [CI], 1.42 to 2.56; P value, <0.001), and fast absorbers also differed significantly compared to slow absorbers (0.71; CI, 0.54 to 0.93; P value, 0.012) in our model.
Table 3.
Multivariate regression model of the independent association of various variables with exposure to rifampina
| Variable | Proportional difference | CI | P valued |
|---|---|---|---|
| Tmax (6 h) | 0.71b | 0.55–0.93 | 0.012 |
| Sex (female) | 1.91c | 1.42–2.56 | <0.001 |
| Rifampin dose received | 1.05 | 0.96–1.14 | 0.299 |
| Comorbidity | |||
| HIV | 0.98 | 0.71–1–37 | 0.926 |
| DM | 0.99 | 0.71–1.37 | 0.931 |
The model was considered based on the natural logarithm of the Cmax values. The proportional difference was calculated as the exponential of the coefficient obtained for each variable in the multivariate model.
For interpretations of the proportional difference, Cmax levels in slow absorbers were 29% lower than Cmax levels in fast absorbers.
For interpretations of the proportional difference, Cmax levels in women were 91% higher than those in men.
Boldface indicates significant difference.
Since (unexpectedly) many patients had undetectable levels at 2 h and many others had rifampin levels that were higher at 6 h than at 2 h, the area under the curve could not be calculated.
It is recommended that rifampin be taken on an empty stomach, as a modest reduction in absorption was previously observed when rifampin was taken with food. Information on oral intake was available for only 48 patients, 12 of whom fasted for at least 2 h before taking the treatment. In this small, underpowered subgroup analysis, oral intake was not associated with a delayed Tmax or with a reduced Cmax. Two Peruvian companies provide rifampin to the NTP. Health centers do not necessarily receive only a single brand, and it was possible to reliably identify the manufacturer for only 48 patients. Thirty-eight patients (80.9%) had been treated with drugs from manufacturer “A,” while 9 (19.2%) had been treated with drugs produced by manufacturer “B.” The median plasma rifampin Cmax values achieved did not differ between manufacturers (4.5 mg/liter for manufacturer A [range, 0 to 15.6 mg/liter] versus 4.9 mg/liter [range, 1.5 to 12.9 mg/liter] for manufacturer B; P = 0.7).
The treatment responses of 99 out of the 105 TB patients were evaluated 6 months after the completion of treatment. Ten patients were found to have multidrug-resistant TB (MDR-TB) and changed treatment schema after the PK analysis and were therefore not included in this subanalysis. A further 29 patients either had abandoned treatment and restarted several months later or were lost to follow-up. Of the remaining evaluable 60 patients, 55 were considered completely cured and relapse free 6 months after finishing treatment, and 5 had an unfavorable outcome. Out of these 5 patients, 2 of them were diabetic patients, 2 were HIV positive (1 of the HIV-positive patients had extrapulmonary cerebral TB without improvement despite prolonged therapy), and 1 patient had no comorbidity. Four of these 5 patients had an absorption delay (Tmax at 6 h instead of at 2 h), 3 of them had very low Cmax levels, and the other 2 had low levels (4 to 8 mg/liter). Among the 55 patients with a good outcome, 31 patients (56%) had delayed absorption, 18 patients (33%) had Cmax levels of <4 mg/liter, 26 (47%) had low levels, and 8 (15%) had normal Cmax levels. For 3 of these patients with a good outcome, neither the Tmax nor the Cmax could be calculated because only 1 blood sample was available.
DISCUSSION
The key finding of this research study, carried out under real-life field conditions and not in the controlled environment of a dedicated PK unit, is that measured serum rifampin levels in patients receiving directly observed TB treatment in the Peruvian NTP were highly variable and very frequently well below what is conventionally regarded as a therapeutic range. This study was designed to determine to what extent a DM or HIV comorbidity might impair rifampin pharmacokinetics, but neither of these conditions had any demonstrable effect on either the rate of rifampin absorption (Tmax) or the magnitude of absorption (Cmax). Instead, there was a widespread perturbation of what is considered to be “normal” rifampin pharmacokinetics across all patient groups. Specifically, two-thirds of patients had delayed rifampin absorption, with higher levels at 6 h than at 2 h postdosing, and for only one-quarter of patients was a measured rifampin serum level (whether at 2 or 6 h) above the 8-mg/liter threshold regarded as necessary for therapeutic efficacy.
Although therapeutic drug monitoring (TDM) is neither widely used nor recommended during TB treatment, it is widely held that abnormal TB drug pharmacokinetics may adversely influence outcomes, with potential consequences including treatment failure, relapse or death, prolonged infectiousness, and the development of acquired drug resistance (5, 39). In addition, TDM might contribute not only to the identification patients with low levels of rifampin but also to a shrinking of the duration of treatment (13), since there are many patients in directly observed therapy (DOT) programs who complete the treatment in 1 year rather than in 6 months.
We observed very low levels of rifampin at 2 h in 68.6% of patients, which is in accordance with data from other studies (3, 38). Moreover, 36 patients (34.3%) had undetectable levels at 2 h, when the peak concentration after oral administration theoretically occurs (23, 26). Wilkins et al. previously reported considerable interindividual variability in rifampin pharmacokinetics in South Africa and suggested that highly variable rates of absorption could significantly impact the Cmax, since, as further confirmed by our data here, slower absorption leads to lower peak plasma concentrations (43). In our study, 61 patients had higher levels of rifampin at 6 h than at 2 h, which we attributed to a delay in absorption; thus, the Tmax varied significantly. Aside from interindividual variability, we wanted to explore other potential explanations for our startling findings. A strength of this study was its real-life execution in health centers; thus, samples were taken from patients doing what they do every day and not under the tightly controlled conditions of a dedicated PK ward. As such, despite the known effect of food upon the pharmacokinetics of rifampin (and other TB drugs) (5, 34, 44), many patients did not fast before taking treatment; indeed, fasting is not a feature of the Peruvian NTP guidelines. In the small subgroup of patients for whom oral intake data were available (48 of 105), we could not demonstrate any effect of food on the Cmax or Tmax.
Men received a lower mg/kg dosage and had lower maximum rifampin levels than women. However, even after adjusting for the rifampin dosage received in the multivariate analysis, men had significantly lower plasma levels than women, a finding reported previously (21, 38) and likely due to an increased volume of distribution (24).
No association was found between rifampin PK and intestinal parasitosis, which affected almost 10% of participants, which does not support the hypothesis that intestinal parasitosis might affect TB treatment outcomes through a PK-related mechanism (18).
The absorption of orally administered drugs can be modified by the nonspecific adsorption of drugs to excipients in formulations (5); a study in South Africa documented a poor relative bioavailability of rifampin from some fixed-dose combinations (30). It was not clear whether bioequivalence studies had been performed for the two generic preparations of rifampin in use (10); although data were incomplete, we were unable to demonstrate a difference in the PK results for each preparation.
If DM is confirmed to be an independent risk factor for treatment failure (1), our data suggest that this is not related to deranged pharmacokinetics of rifampin, nor is it associated with hyperglycemia, although the limited pharmacokinetic analysis might hamper the assessment of the effect of DM on the pharmacokinetics of rifampin. It remains controversial whether a significant malabsorption of antimycobacterial drugs occurs in HIV patients (9, 11, 29, 33, 37) and, if so, whether this is associated with treatment failure or the emergence of drug resistance. Although a crude analysis revealed marginally reduced Cmax values in HIV patients (data not shown), after stratifying data by the Tmax, no associations remained between the rifampin level and HIV status in either fast or slow absorbers. HIV-related achlorhydria, HIV enteropathy (17), and opportunistic infections of the intestinal tract were reported previously (5, 16) to be risk factors for a poor treatment outcome; we did not explore these risk factors other than in the search for intestinal parasites.
The main limitation of this work was the inability to calculate the 0- to 6-h area under the PK curve (AUC). We sampled only two time points, 2 and 6 h, for pragmatic and logistic reasons and because this approach was previously successful. However, the real Cmax might have occurred between the 2- and 6-h samplings, and furthermore, the unexpected finding of so many measurements below the limit of detection and the fact that a considerable proportion of patients had their Tmax at 6 h rendered AUC calculations futile. While we regarded the conduct of the study under real-life circumstances as being important and a strength of the data, we were unable to adequately control and reliably measure concomitant oral intake for all participants. Gastrointestinal upset is common in TB patients, and many gain relief by taking medicines with food or juice; a future study might usefully examine detailed PK curves for the same patient on consecutive days under different conditions of oral intake. A perennial and inherent problem with TB drug PK analyses is unpicking whether plasma rifampin levels, defined as the Cmax, or (preferentially) total exposure, measured as the AUC, is the crucial determinant of drug efficacy at the cellular level and, if so, what the minimum drug exposure (measured in plasma) needs to be in order to have a high probability of efficacy. Evidence for poorer treatment outcomes associated with impaired pharmacokinetics is, perhaps surprisingly, somewhat thin and less persuasively demonstrated for rifampin (38) than for pyrazinamide (6). Moreover, treatment outcomes of patients in Peru with standardized regimens using these same drugs and dosages are generally good, implying that efficacy, at least with the existing four-drug combination therapy, is adequate. We could evaluate treatment outcome and relapse rates only to 6 months for 60 patients, 5 of whom had an unfavorable outcome. Given the very low levels of rifampin among patients cured of disease, further investigation is needed to ascertain whether a review of the purported normal Cmax level of >8 mg/liter is warranted. However, some studies have demonstrated that an increase in the dose of rifampin led to a better treatment outcome (20). Therefore, if we assume a higher median concentration in those patients who received a higher dose, we could consider that there is a correlation between the concentration and the treatment outcome.
In addition, we report data only on rifampin here, and patients might have been cured despite impaired rifampin pharmacokinetics because of the effect of the combination therapy (isoniazid, ethambutol, or pyrazinamide). For this reason, the 8-mg/liter threshold might be better regarded as desirable rather than necessary.
We have demonstrated markedly deranged rifampin pharmacokinetics in a majority of TB patients taking directly observed therapy in the Peruvian NTP, with delayed absorption and low drug levels, findings not inconsistent with those of previous work in other countries. A clarification of the implications of these findings for clinical outcomes is now essential; if outcomes are unaffected, perhaps further thought needs to be given to our existing paradigm for normal, therapeutically active rifampin pharmacokinetics.
ACKNOWLEDGMENTS
This work was supported by the Societat Catalana de Malalties Infeccioses i Microbiologia Clinica (SCMIMC), which we gratefully acknowledge.
We are indebted to all staff members at the Universidad Peruana Cayetano Heredia and Asociación Benéfica PRISMA, who provided support to this research in various ways, and to the medical staff members at the community clinic and hospital that participated in this study. We further thank Llorenç Quintó (CRESIB) for his useful comments and suggestion in the statistical analysis. We acknowledge contributions from many others, including David Mabey (London School of Hygiene and Tropical Medicine).
Footnotes
Published ahead of print 13 February 2012
REFERENCES
- 1. Alisjahbana B, et al. 2007. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin. Infect. Dis. 45:428–435 [DOI] [PubMed] [Google Scholar]
- 2. Alisjahbana B, et al. 2006. Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int. J. Tuberc. Lung Dis. 10:696–700 [PubMed] [Google Scholar]
- 3. Berning SE, Huitt GA, Iseman MD, Peloquin CA. 1992. Malabsorption of antituberculosis medications by a patient with AIDS. N. Engl. J. Med. 327:1817–1818 [DOI] [PubMed] [Google Scholar]
- 4. Boutayeb A. 2006. The double burden of communicable and non-communicable diseases in developing countries. Trans. R. Soc. Trop. Med. Hyg. 100:191–199 [DOI] [PubMed] [Google Scholar]
- 5. Burman WJ, Gallicano K, Peloquin C. 1999. Therapeutic implications of drug interactions in the treatment of human immunodeficiency virus-related tuberculosis. Clin. Infect. Dis. 28:419–429 [DOI] [PubMed] [Google Scholar]
- 6. Chideya S, et al. 2009. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin. Infect. Dis. 48:1685–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choudhri SH, et al. 1997. Pharmacokinetics of antimycobacterial drugs in patients with tuberculosis, AIDS, and diarrhea. Clin. Infect. Dis. 25:104–111 [DOI] [PubMed] [Google Scholar]
- 8. Conte JE, Jr, et al. 2002. Effects of gender, AIDS, and acetylator status on intrapulmonary concentrations of isoniazid. Antimicrob. Agents Chemother. 46:2358–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corbett EL, et al. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009–1021 [DOI] [PubMed] [Google Scholar]
- 10. EMEA 2001. Note for guidance on the investigation of bioavailability and bioequivalence. EMEA, London, United Kingdom [Google Scholar]
- 11. Gurumurthy P, et al. 2004. Malabsorption of rifampin and isoniazid in HIV-infected patients with and without tuberculosis. Clin. Infect. Dis. 38:280–283 [DOI] [PubMed] [Google Scholar]
- 12. Gwilt PR, Nahhas RR, Tracewell WG. 1991. The effects of diabetes mellitus on pharmacokinetics and pharmacodynamics in humans. Clin. Pharmacokinet. 20:477–490 [DOI] [PubMed] [Google Scholar]
- 13. Heysell SK, Moore JL, Keller SJ, Houpt ER. 2010. Therapeutic drug monitoring for slow response to tuberculosis treatment in a state control program, Virginia, USA. Emerg. Infect. Dis. 16:1546–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horton R. 2005. The neglected epidemic of chronic disease. Lancet 366:1514. [DOI] [PubMed] [Google Scholar]
- 15. Kimerling ME, et al. 1998. Low serum antimycobacterial drug levels in non-HIV-infected tuberculosis patients. Chest 113:1178–1183 [DOI] [PubMed] [Google Scholar]
- 16. Kotler DP, Francisco A, Clayton F, Scholes JV, Orenstein JM. 1990. Small intestinal injury and parasitic diseases in AIDS. Ann. Intern. Med. 113:444–449 [DOI] [PubMed] [Google Scholar]
- 17. Kotler DP, et al. 1995. Chronic bacterial enteropathy in patients with AIDS. J. Infect. Dis. 171:552–558 [DOI] [PubMed] [Google Scholar]
- 18. Kshirsagar NA, Joshi MV, Takle MR, Acharya VN, Satoskar RS. 1988. Effect of ascariasis and its treatment on drug absorption. Eur. J. Clin. Pharmacol. 34:217–219 [DOI] [PubMed] [Google Scholar]
- 19. Li J, et al. 2004. Use of therapeutic drug monitoring for multidrug-resistant tuberculosis patients. Chest 126:1770–1776 [DOI] [PubMed] [Google Scholar]
- 20. Long MW, Snider DE, Jr, Farer LS. 1979. U.S. Public Health Service cooperative trial of three rifampin-isoniazid regimens in treatment of pulmonary tuberculosis. Am. Rev. Respir. Dis. 119:879–894 [DOI] [PubMed] [Google Scholar]
- 21. McIlleron H, et al. 2006. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob. Agents Chemother. 50:1170–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mehta JB, et al. 2001. Utility of rifampin blood levels in the treatment and follow-up of active pulmonary tuberculosis in patients who were slow to respond to routine directly observed therapy. Chest 120:1520–1524 [DOI] [PubMed] [Google Scholar]
- 23. Nijland HM, et al. 2006. Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes. Clin. Infect. Dis. 43:848–854 [DOI] [PubMed] [Google Scholar]
- 24. Nyakutira C, et al. 2008. High prevalence of the CYP2B6 516G→T(*6) variant and effect on the population pharmacokinetics of efavirenz in HIV/AIDS outpatients in Zimbabwe. Eur. J. Clin. Pharmacol. 64:357–365 [DOI] [PubMed] [Google Scholar]
- 25. Patel KB, Belmonte R, Crowe HM. 1995. Drug malabsorption and resistant tuberculosis in HIV-infected patients. N. Engl. J. Med. 332:336–337 [DOI] [PubMed] [Google Scholar]
- 26. Peloquin CA. 2002. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 62:2169–2183 [DOI] [PubMed] [Google Scholar]
- 27. Peloquin CA. 2001. Tuberculosis drug serum levels. Clin. Infect. Dis. 33:584–585 [DOI] [PubMed] [Google Scholar]
- 28. Peloquin CA. 1997. Using therapeutic drug monitoring to dose the antimycobacterial drugs. Clin. Chest Med. 18:79–87 [DOI] [PubMed] [Google Scholar]
- 29. Peloquin CA, MacPhee AA, Berning SE. 1993. Malabsorption of antimycobacterial medications. N. Engl. J. Med. 329:1122–1123 [DOI] [PubMed] [Google Scholar]
- 30. Pillai G, et al. 1999. Recent bioequivalence studies on fixed-dose combination anti-tuberculosis drug formulations available on the global market. Int. J. Tuberc. Lung Dis. 3:S309–S16; discussion, S317–S321 [PubMed] [Google Scholar]
- 31. Restrepo BI. 2007. Convergence of the tuberculosis and diabetes epidemics: renewal of old acquaintances. Clin. Infect. Dis. 45:436–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruder K. 2007. Fighting the epidemic. A United Nations resolution on diabetes. Diabetes Forecast 60:50–51 [PubMed] [Google Scholar]
- 33. Sahai J, et al. 1997. Reduced plasma concentrations of antituberculosis drugs in patients with HIV infection. Ann. Intern. Med. 127:289–293 [DOI] [PubMed] [Google Scholar]
- 34. Siegler DI, Bryant M, Burley DM, Citron KM, Standen SM. 1974. Effect of meals on rifampicin absorption. Lancet ii:197–198 [DOI] [PubMed] [Google Scholar]
- 35. Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. 2011. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J. Infect. Dis. 204:1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tappero JW, et al. 2005. Serum concentrations of antimycobacterial drugs in patients with pulmonary tuberculosis in Botswana. Clin. Infect. Dis. 41:461–469 [DOI] [PubMed] [Google Scholar]
- 37. Taylor B, Smith PJ. 1998. Does AIDS impair the absorption of antituberculosis agents? Int. J. Tuberc. Lung Dis. 2:670–675 [PubMed] [Google Scholar]
- 38. van Crevel R, et al. 2002. Low plasma concentrations of rifampicin in tuberculosis patients in Indonesia. Int. J. Tuberc. Lung Dis. 6:497–502 [DOI] [PubMed] [Google Scholar]
- 39. Weiner M, et al. 2005. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin. Infect. Dis. 40:1481–1491 [DOI] [PubMed] [Google Scholar]
- 40. WHO 2009. Global tuberculosis control: epidemiology, strategy, financing. WHO report 2009. WHO, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 41. WHO 2007. Global tuberculosis control: surveillance, planning, financing. WHO report 2007. WHO, Geneva, Switzerland [Google Scholar]
- 42. Wild S, Roglic G, Green A, Sicree R, King H. 2004. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 43. Wilkins JJ, et al. 2008. Population pharmacokinetics of rifampin in pulmonary tuberculosis patients, including a semimechanistic model to describe variable absorption. Antimicrob. Agents Chemother. 52:2138–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zent C, Smith P. 1995. Study of the effect of concomitant food on the bioavailability of rifampicin, isoniazid and pyrazinamide. Tuber. Lung Dis. 76:109–113 [DOI] [PubMed] [Google Scholar]