Abstract
This study was conducted to evaluate the evolution of the antimicrobial susceptibility of Neisseria meningitidis causing invasive diseases in Belgium in the period of January 2000 to December 2010. A total of 1,933 cases of N. meningitidis from invasive infections were analyzed by antimicrobial susceptibility testing at the Belgian Meningococcal Reference Centre. The majority of strains were susceptible to antibiotics that are currently used for the treatment and prophylaxis of meningococcal disease, but the prevalence of clinical isolates with reduced susceptibility to penicillin increased over the years. The phenotyping, genotyping, and determination of MICs of penicillin G were performed. The systematic shift of the curves toward higher penicillin MICs in the susceptible population indicated that this population became less sensitive to penicillin in this period. A 402-bp DNA fragment in the 3′ end of penA was sequenced for the 296 nonsusceptible meningococcal strains isolated between 2000 and 2010 to examine the genetic diversity and evolution of their penA gene. In conclusion, the data obtained in our study support the statement that the position of penicillin G as a first choice in the treatment of invasive meningococcal diseases in Belgium should be reexamined. Despite an important number of isolates displaying a reduced susceptibility to penicillin, at present the expanded-spectrum cephalosporins, such as ceftriaxone, are not affected. The follow-up of the evolutionary changes in antimicrobial resistance has also proved to be essential for the recommendation of an appropriate antimicrobial treatment for invasive meningococcal diseases.
INTRODUCTION
Neisseria meningitidis, a human pathogen, colonizes the nasopharynx of individuals and can cross the epithelial barrier of the nasopharynx, causing septicemia and/or meningitis (8, 9, 12). Despite appropriate treatment, the case fatality rate varies from 5 to 15% (8, 9) due to fulminant septicemia, frequently resulting in sequelae.
In Europe and North America, the overall incidence of meningococcal disease is 1 to 3 per 100,000 inhabitants, and the infections are mostly sporadic cases among infants (8, 9).
Serogroup B has always been the main cause of meningococcal disease in Belgium (24, 25), but in the late 1990s the incidence of serogroup C disease increased considerably and peaked in 2001 (10). The greatest burden of serogroup C meningococcal disease was observed in young children and in teenagers. Health authorities decided to finance a mass immunization campaign targeting children aged 1 to 5 years and using a conjugate vaccine against serogroup C (10). Vaccination against serogroup C meningococcal infection was incorporated into the childhood immunization program from 2002 onwards, with a single dose of vaccine at the age of 12 to 15 months (10).
Prompt treatment is critical in the management of invasive meningococcal diseases. Penicillin G remains, in several countries, the drug of choice, particularly when a bacteriological diagnosis has been established.
The degree of susceptibility of N. meningitidis to penicillin has decreased during the past 2 decades, and meningococcal isolates showing intermediate resistance to penicillin (Peni) have been widely described in several countries (11).
Decreased susceptibility to penicillin G is linked to alterations in the structure of a penicillin-binding protein (PBP2) encoded by the penA gene (2, 3, 22). The penA gene from penicillin-susceptible meningococcal strains (Pens) appears to be highly conserved. However, those from nonsusceptible strains (Penns) are variable, showing mosaic structures. These variations have been suggested to be due to genetic exchange through transformation between N. meningitidis and nonpathogenic commensal Neisseria species (2, 22, 23). The modification of the PBP2 resulted in a decreased affinity of PBP2 to penicillin G, as well as in modifications in the peptidoglycan structure of the bacterial cell wall (4).
The aim of this study was to evaluate the evolution of the antimicrobial susceptibility of Neisseria meningitidis causing invasive diseases in Belgium in the period of 2000 to 2010 and to examine the genetic diversity and the evolution of their penA genes.
MATERIALS AND METHODS
Bacterial strains and phenotypic characterization.
Meningococcal strains from patients with invasive meningococcal disease are referred to the Belgian Reference Centre for N. meningitidis by clinical laboratories for the confirmation and determination of serogroup, serotype, subtype, and antimicrobial susceptibility pattern. Serogrouping was performed by slide agglutination using commercial antisera (Remel Europe Ltd., Dartford, United Kingdom). Serotypes and subtypes were determined by whole-cell enzyme-linked immunosorbent assay (ELISA) using monoclonal antibodies, including monoclonal antibodies for the detection of P3.1, P2.2a, P2.2b, P3.4, P3.15, and P3.21 antigens (NIBSC, Potters Bar, United Kingdom) (1).
Antimicrobial susceptibility.
The MICs of penicillin G, cefotaxime, chloramphenicol, rifampin, and ciprofloxacin were determined by Etest (AB Biodisk, Solna, Sweden). Etest was carried out according to the manufacturer's instructions, and the EUCAST breakpoint definitions (www.eucast.org.) were used for the interpretation of antimicrobial susceptibility test results.
Molecular analysis.
Multilocus sequence typing (MLST) was carried out using the method of Taha et al. (20), and isolates were assigned to clonal complexes according to the Neisseria MLST website (http://pubmlst.org/neisseria/) (14).
The amplification and sequencing of the porA and penA genes was carried out as described in Birtles et al. (6) and Taha et al. (22), respectively.
The amplification and sequencing of the fetA gene was performed by following the protocol mentioned on the Neisseria.org website (http://neisseria.org/nm/typing/). The porA variable regions, VR1 and VR2, and porB, penA, and fetA variable regions were assigned using the databases of the Neisseria.org website.
Statistical analysis.
The chi-square test was used to evaluate differences in proportions. Other evaluations (populations and mean MICs) were done by using the Mann-Whitney test and/or the Student's t test. Significance was set at 0.05.
RESULTS
Meningococcal surveillance: 2000 to 2010.
In the period of January 2000 to December 2010, a total of 2,025 cases of invasive meningococcal diseases were reported, with an average annual incidence of 1.8 cases per 100,000 inhabitants (ranging from 0.89 in 2010 to 3.7 in 2001). Overall, 93.2% (1933/2,025) of the isolates remained viable and were included for analysis in the study.
During this 11-year period, antimicrobial susceptibility testing was performed by Etest on 1,933 N. meningitidis strains. All isolates were found to be susceptible to cefotaxime, chloramphenicol, and ciprofloxacin. Only one strain, isolated in 2007, presented resistance against rifampin (MIC, 8 μg/ml). The large majority of isolates (1,637/1,933; 84.7%) were fully susceptible to penicillin (MICs of ≤0.064 μg/ml), 273 strains (14.1%) had reduced susceptibility (Peni), with MICs between 0.125 and 0.25 μg/ml, and 23 isolates presented full resistance to penicillin (MICs of ≥0.5 μg/ml) (Penr). One hundred four of these 296 Penns isolates belonged to serogroup C, 159 to serogroup B, 28 to serogroup W135, and 4 to serogroup Y, while one isolate could not be classified in a particular group. The prevalence of Penns isolates was higher in serogroup W135 (56.3%) and in serogroup C (21.7%) than in serogroup B (11.7%).
The proportion of Peni isolates fluctuated between 1.2 and 31.8% in serogroup B, between 10.8 and 75% in serogroup C, and between 4.8 and 33.0% in the total population of isolates. All Penns isolates were tested for ampicillin susceptibility. The MICs of ampicillin ranged from 0.032 to 1 μg/ml, and 26.0% of the isolates presented a reduced susceptibility to ampicillin (MIC, ≥0.12 μg/ml).
Table 1 shows the evolution of the MICs at which 50% of the tested isolates are inhibited (MIC50) and the geometric mean MICs (expressed in μg/ml) of Pens and Penns isolates.
Table 1.
Evolution of MIC50 and geometric mean MIC (expressed in μg/ml) in Pens and Penns isolates of N. meningitidis
| Year | MIC50a values and no. of isolates at indicated concn according to resistance category |
Geometric mean MIC |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pens |
Penns |
||||||||||||||
| Peni |
Penr |
||||||||||||||
| ≤0.012 | 0.016 | 0.023 | 0.032 | 0.047 | 0.064 | 0.094 | 0.125 | 0.19 | 0.25 | 0.38 | 0.5 | Total | Pens | Penns | |
| 2000 | 33 | 22 | 49 | 72 | 41 | 23 | 3 | 4 | 1 | 4 | 0.029 | 0.027 | 0.152 | ||
| 2001 | 46 | 65 | 96 | 78 | 24 | 20 | 7 | 15 | 4 | 3 | 0.027 | 0.023 | 0.133 | ||
| 2002 | 12 | 59 | 77 | 46 | 17 | 6 | 8 | 10 | 7 | 3 | 0.029 | 0.023 | 0.138 | ||
| 2003 | 11 | 23 | 61 | 58 | 21 | 6 | 4 | 12 | 5 | 2 | 1 | 0.032 | 0.026 | 0.144 | |
| 2004 | 3 | 8 | 31 | 37 | 26 | 14 | 7 | 7 | 5 | 3 | 1 | 1 | 0.042 | 0.032 | 0.152 |
| 2005 | 8 | 13 | 48 | 43 | 13 | 6 | 10 | 10 | 7 | 3 | 1 | 0.037 | 0.027 | 0.140 | |
| 2006 | 1 | 4 | 26 | 32 | 24 | 9 | 3 | 17 | 6 | 2 | 4 | 1 | 0.050 | 0.033 | 0.164 |
| 2007 | 5 | 8 | 22 | 57 | 22 | 18 | 2 | 6 | 7 | 3 | 2 | 0.041 | 0.033 | 0.174 | |
| 2008 | 1 | 3 | 7 | 17 | 21 | 16 | 8 | 8 | 10 | 8 | 3 | 0.067 | 0.040 | 0.167 | |
| 2009 | 1 | 3 | 10 | 21 | 22 | 19 | 2 | 7 | 3 | 8 | 2 | 0.055 | 0.039 | 0.184 | |
| 2010 | 0 | 0 | 6 | 9 | 22 | 15 | 10 | 7 | 3 | 9 | 7 | 0.078 | 0.044 | 0.177 | |
MIC50, MIC at which 50% of the tested isolates are inhibited. MIC50 values are shaded.
Between 2000 and 2003, there was no significant change in mean penicillin MICs for the total population. In 2004, a significantly higher mean MIC was found compared to the period of 2000 to 2003 (P < 0.001). In the period of 2004 to 2007, the mean MIC remained stable, albeit at a significantly higher value than that of the period of 2000 to 2003. From 2007 to 2010, a significant increase was noted again (0.01 > P > 0.001). The increase in mean MIC in the Pens population showed a comparable evolution, while such a systematic evolution was not found in the Penns population (Table 1).
During the period of 2000 to 2010, a significant increase in the rates of nonsusceptibility to penicillin was observed (from 0.01 > P > 0.001 to P < 0.001). The nonsusceptibility rate increased from 4.8% in 2000 to 16.8% in 2004 and 25.6% in 2006. In 2007, this rate dropped to 13.1% but increased again to 36.3% in 2008. In 2009, we noticed again a drop to 20.4%. Finally, the 2010 rate was 40.9%. For the Penr isolates, the first significant increase was seen in 2006 (3.9%; it was 0% in 2000; 0.01 > P > 0.001), and no further significant increase was seen between 2006 and 2010 (7.9%). Overall, analyses for linear trends in proportions showed that there was a significant increase in Penns and Penr isolates during the period of 2000 to 2010 (P < 0.001). The prevalence of the Penns isolates increased significantly over the years in all of the serogroups.
Characterization of penA: correlation between penA allele and MIC and allelic evolution from 2000 to 2010.
The sequence of a 402-bp DNA fragment of the 3′ part of penA was obtained for 56 randomly selected Pens isolates and for the 296 Penns isolates. Fifty-four of 56 Pens isolates harbored the penA1 allele or an allele with an identical deduced amino acid sequence (only silent, synonymous DNA polymorphisms for the corresponding 402-bp fragment). The penA12 allele was found in only 2 susceptible isolates (with a MIC of 0.064).
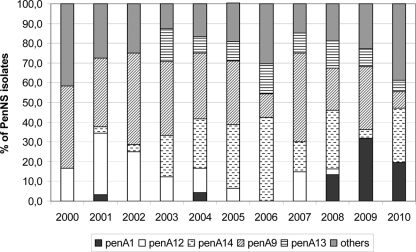
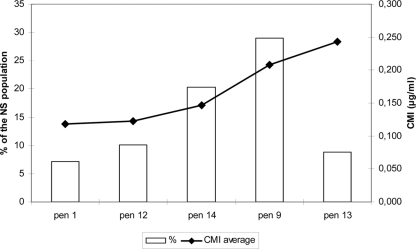
Forty-one different alleles of penA were identified among the 296 PenNS isolates. The most frequent alleles in the Penns population were penA9 (29.1%), penA14 (20.3%), penA12 (10.1%), penA13 (8.8%), and penA1 (7.1%) (see Fig. 2). Most of these alleles (21 out of 41) had an identical deduced amino acid sequence. The alleles penA14, pen13, and penA9 were most frequent among isolates, presenting a high mean MIC of 0.139, 0.189, and 0.231, respectively. These mean MIC values differed significantly from each other (0.05 > P > 0.02 for penA9 versus penA13 and P < 0.001 for penA14 versus penA9 and penA1). On the other hand, penA1 and penA12 were most frequent in isolates presenting a lower MIC (mean MIC of 0.115 and 0.118, respectively). There was no significant difference between the mean MIC values of penA1 and penA12, while their values were significantly lower than those for the other three types (0.05 > P > 0.02 compared to penA14 and P < 0.001 for penA9 and penA13) (Fig. 1).
Fig 2.
Distribution of the most frequent penA alleles (penA1, penA12, penA9, penA14, and penA13) in the Penns isolates between 2000 and 2010.
Fig 1.
Distribution of the penA allele among the nonsusceptible population and relationship between the penA allele and the average MIC value.
The prevalence of penA14 increased systematically beginning in 2003 and reached a prevalence of 42.2% in the Penns isolates in 2006 (P < 0.001). The penA13 allele was detected for the first time in 2003, and its prevalence remained relatively stable over the years (between 5.6 and 16.7% of the nonsusceptible population). The prevalence of the penA9 allele fluctuated over the years. The lowest prevalences were found in 2006 (12.0%) and 2010 (8.3%). In the other years its prevalence varied from 21.6% (2008) to 46.4% (2002) (Fig. 2). The proportion of penA1 allele increased significantly from 2007 (0%) to 2009 (31.8%) and 2010 (19.4%) (0.02 > P > 0.01). The prevalence of penA12 decreased drastically during the period of 2001 (31.0%) to 2008 (2.7%) (0.01 > P > 0.001), while it was not present in 2009 and 2010.
Distribution of penA alleles in the different serogroups.
The association between serogroups and the alteration of PBP2 was analyzed for the 296 Penns isolates. A significant association was observed between isolates of serogroup B and the pen13 allele and between serogroup C and the pen12 allele. Indeed, 24 isolates out of 26 harboring penA13 belonged to serogroup B, with the majority (n = 23) of them belonging to serotype 4 and presenting the phenotype B:P1.4:F1-5:cc-41/44 (P < 0.001). Twenty-six out of 30 isolates harboring penA12 belonged to serogroup C. In this group, the majority (n = 24) of the isolates belonged to serotype 2b with the phenotype C:P1.1,7:F5-8:cc-8 or C:P1.2,5:F5-8:cc-8 (P < 0.001). Among the isolates presenting the pen14 allele, 37 belonged to the B serogroup (the majority belonged to the phenotype B:P1.4:F1-5:cc-41/44 complex/Lineage 3) and 24 to serogroup W135 (the majority belonged to the phenotype W135:P1.3,6:F4-1:cc-22).
DISCUSSION
Since the emergence of resistance is correlated with the outcome of treatment and the successful prophylaxis of close contacts, the surveillance of antimicrobial resistance is particularly important for Neisseria meningitidis (18). Due to the high risks of morbidity and death, immediate treatment with effective antibiotics is essential when a patient is suspected of having meningococcal diseases. In Belgium, as in other countries, rifampin and ciprofloxacin are recommended to eliminate the pathogen from the nasopharynx and are used to avoid transmission to close contacts (15).
Our data show that meningococcal strains isolated in Belgium during the last 11 years remained highly susceptible to the antibiotics currently used to treat patients with meningococcal diseases and their close contacts. Only one case presenting resistance to rifampin was detected in 1,933 tested strains. The resistance was due to a point mutation of the rpoB gene (rpoB allele 36) (21). As previously described, resistance to rifampin is only occasionally observed and can occur following chemoprophylaxis (18, 19).
No resistance or intermediate resistance to fluoroquinolones was observed on the 1,933 Belgian isolates. To date, reduced susceptibility or resistance to fluoroquinolones in Neisseria meningitidis remains rare (13). Nevertheless, sporadic cases have been reported in Europe, India, and North and South America (16, 17, 26). In Belgium, the use of quinolones to treat meningococcal diseases is limited because quinolones are not approved for routine pediatric use (15).
Another important issue shown in the present study is the systematic and significant shift of the curves toward higher penicillin MICs. This shift can be attributed to a shift in the susceptible population, indicating that the susceptible population became less sensitive to penicillin in this period.
As described in other European countries, our study shows that the degree of susceptibility of N. meningitidis to penicillin has decreased in the last decade. In the last 3 years, the meningococcal population displayed a high degree of reduced susceptibility to penicillin, and this has led clinicians to use expanded-spectrum cephalosporins for initial treatment. Interestingly, in our study, none of the isolates presented resistance or a reduced susceptibility to expanded-spectrum cephalosporins.
The proportion of Peni isolates increased from 2.2% in 1997 to 13.1% in 2002 and was higher in serogroup C than in serogroup B. However, this percentage was lower than the nonsusceptibility rate reported in France (31.2%) in 1999 to 2002 (26). In Belgium, serogroup:serotype C:2a (especially C:2a:P1.5 isolates) was frequently associated with reduced susceptibility to penicillin in 2001 and 2002. The increase of meningococcal diseases caused by C:2a strains contributed to the overall increase in the proportion of Peni isolates in Belgium during this period. As has been described in Spain (5) and in Portugal (7), we also found a strong association between reduced susceptibility to penicillin and serogroup:serotype C:2b and, more specifically, with the phenotypes C:P1.1,7:F5-8:cc-8 and C:P1.2,5:F5-8:cc-8. Moreover, these strains harbored the penA12 allele. Interestingly, in 2006, 2009, and 2010, no cases of invasive meningococcal diseases were caused by a Neisseria isolate belonging to serotype C2b, and the penA12 allele was absent in these years, suggesting a strong association between the C2b serotype and this penA allele.
In the present study, the most common alleles in the nonsusceptible population were penA9 and penA14, representing 29.1 and 20.3% of the isolates, respectively. This is in agreement with an extensive study presenting data on the sequencing of the penA gene of N. meningitidis isolates (n = 1,670) from 22 mainly European countries (22). In that study, penA9 and pen14 were found in 5 and 6% of the total isolates, respectively, but were more frequently isolated in France and Denmark (22). As it was shown in that particular study, the penA9 allele was present in isolates with different phenotypes and genotypes, whereas penA14 was found primarily in ST-22 isolates. In our study, the penA14 allele was detected in both ST-22 and ST-41/44.
In conclusion, our data indicate that N. meningitidis isolates from Belgian patients remained highly susceptible to the antibiotics used for treatment and prophylaxis, with the exception of penicillin. However, the increasing prevalence of Penns strains urges the reexamination of the position of penicillin as a first choice in the treatment of invasive meningococcal diseases in Belgium. The follow-up of the evolutionary changes in antimicrobial resistance has also proved to be essential for the recommendation of an appropriate antimicrobial treatment for invasive meningococcal diseases.
ACKNOWLEDGMENTS
We are very grateful to J. Griselain, N. Weynants, F. Lamranni, M. Thirionet, and G. Dupont for their technical help.
In Belgium, this work was financed in part by the Federal Public Health Service. R.W. is a Research Associate at the FNRS-FRS (Belgium).
This publication made use of the Neisseria multilocus sequence typing website (http://pubmlst.org/neisseria/), which was developed by Keith Jolley and Man-Suen Chan and is sited at the University of Oxford. The development of that website was funded by the Wellcome Trust and European Union. Sequencing reactions were run on an ABI 3130xl at the Platform Biotechnology and Molecular Biology at the Scientific Institute of Public Health.
Footnotes
Published ahead of print 30 January 2012
REFERENCES
- 1. Abdillahi H, Poolman JT. 1988. Definition of meningococcal class 1 OMP subtyping antigens by monoclonal antibodies. FEMS Microbiol. Immunol. 1:139–144 [DOI] [PubMed] [Google Scholar]
- 2. Antignac A, et al. 2003. Correlation between alterations of the penicillin-binding protein 2 and modifications of the peptidoglycan structure in Neisseria meningitidis with reduced susceptibility to penicillin G. J. Biol. Chem. 278:31529–31535 [DOI] [PubMed] [Google Scholar]
- 3. Antignac A, Kriz P, Tzanakaki G, Alonso JM, Taha MK. 2001. Polymorphism of Neisseria meningitidis penA gene associated with reduced susceptibility to penicillin. J. Antimicrob. Chemother. 47:285–296 [DOI] [PubMed] [Google Scholar]
- 4. Arreaza L, et al. 2004. Sequencing of Neisseria meningitidis penA gene: the key to success in defining penicillin G breakpoints. Antimicrob. Agents Chemother. 48:358–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berron S, Vazquez JA. 1994. Increase in moderate penicillin resistance and serogroup C in meningococcal strains isolated in Spain. Is there any relationship? Clin. Infect. Dis. 18:161–165 [DOI] [PubMed] [Google Scholar]
- 6. Birtles A, et al. 2005. Multilocus sequence typing of Neisseria meningitidis directly from clinical samples and application of the method to the investigation of meningococcal disease case clusters. J. Clin. Microbiol. 43:6007–6014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Canica M, Dias R, Ferreira E. 2004. Neisseria meningitidis C:2b:P1.2,5 with intermediate resistance to penicillin, Portugal. Emerg. Infect. Dis. 10:526–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caugant DA. 2008. Genetics and evolution of Neisseria meningitidis: importance for the epidemiology of meningococcal disease. Infect. Genet. Evol. 8:558–565 [DOI] [PubMed] [Google Scholar]
- 9. Caugant DA, Maiden MC. 2009. Meningococcal carriage and disease–population biology and evolution. Vaccine 27(Suppl. 2):B64–B70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Schrijver K, Maes I. 2003. An outbreak of serogroup C meningococcal disease in the province of Antwerp (Belgium) in 2001–2002. Eur. J. Epidemiol. 18:1073–1077 [DOI] [PubMed] [Google Scholar]
- 11. du Plessis M, von Gottberg A, Cohen C, de Gouveia L, Klugman KP. 2008. Neisseria meningitidis intermediately resistant to penicillin and causing invasive disease in South Africa in 2001 to 2005. J. Clin. Microbiol. 46:3208–3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hill DJ, Griffiths NJ, Borodina E, Virji M. 2010. Cellular and molecular biology of Neisseria meningitidis colonization and invasive disease. Clin. Sci. (London) 118:547–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lapadula G, et al. 2009. Imported ciprofloxacin-resistant Neisseria meningitidis. Emerg. Infect. Dis. 15:1852–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maiden MC. 1998. The Impact of Molecular Techniques on the Study of Meningococcal Disease. Methods Mol. Med. 15:265–291 [DOI] [PubMed] [Google Scholar]
- 15. Sanford JP, et al. 2011. The Sanford guide to antimicrobial therapy 2010–2011. Jeb C. Sanford, Wavre, Belgium [Google Scholar]
- 16. Skoczynska A, Alonso JM, Taha MK. 2008. Ciprofloxacin resistance in Neisseria meningitidis, France. Emerg. Infect. Dis. 14:1322–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skoczynska A, Ruckly C, Hong E, Taha MK. 2009. Molecular characterization of resistance to rifampicin in clinical isolates of Neisseria meningitidis. Clin. Microbiol. Infect. 15:1178–1181 [DOI] [PubMed] [Google Scholar]
- 18. Stefanelli P. 2011. Emerging resistance in Neisseria meningitidis and Neisseria gonorrhoeae. Expert. Rev. Anti. Infect. Ther. 9:237–244 [DOI] [PubMed] [Google Scholar]
- 19. Stefanelli P, et al. 2001. Rifampicin-resistant meningococci causing invasive disease: detection of point mutations in the rpoB gene and molecular characterization of the strains. J. Antimicrob. Chemother. 47:219–222 [DOI] [PubMed] [Google Scholar]
- 20. Taha MK, Giorgini D, Ducos-Galand M, Alonso JM. 2004. Continuing diversification of Neisseria meningitidis W135 as a primary cause of meningococcal disease after emergence of the serogroup in 2000. J. Clin. Microbiol. 42:4158–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taha MK, et al. 2010. Multicenter study for defining the breakpoint for rifampin resistance in Neisseria meningitidis by rpoB sequencing. Antimicrob. Agents Chemother. 54:3651–3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taha MK, et al. 2007. Target gene sequencing to characterize the penicillin G susceptibility of Neisseria meningitidis. Antimicrob. Agents Chemother. 51:2784–2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thulin S, Olcen P, Fredlund H, Unemo M. 2006. Total variation in the penA gene of Neisseria meningitidis: correlation between susceptibility to beta-lactam antibiotics and penA gene heterogeneity. Antimicrob. Agents Chemother. 50:3317–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Looveren M, Carion F, Vandamme P, Goossens H. 1998. Surveillance of meningococcal infections in Belgium. Clin. Microbiol. Infect. 4:224–228 [DOI] [PubMed] [Google Scholar]
- 25. Van Looveren M, et al. 1998. Molecular epidemiology of recent belgian isolates of Neisseria meningitidis serogroup B. J. Clin. Microbiol. 36:2828–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu HM, et al. 2009. Emergence of ciprofloxacin-resistant Neisseria meningitidis in North America. N. Engl. J. Med. 360:886–892 [DOI] [PubMed] [Google Scholar]