Abstract
Screening for in vitro susceptibility to pyrimethamine and sequencing of the pfmdr2 and pfdhfr genes were performed in 140 Plasmodium falciparum isolates. The risk of in vitro resistance to pyrimethamine was analyzed with a logistic regression model. The mutation F423Y in pfmdr2 (odds ratio [OR] = 2.12 [confidence interval {CI}, 1.02 to 4.59]; P = 0.0489) and the mutation N51I, C59R, or S108N in pfdhfr (OR = 42.34 [CI, 5.52 to 324.61]; P = 0.0003) were independently associated with in vitro resistance to pyrimethamine.
TEXT
Sulfadoxine and pyrimethamine (Pyr) (SP) were widely used as first-line drugs for the treatment of malaria until the spread of resistance in Plasmodium falciparum isolates all over the world. SP is still recommended in combination with artesunate or chloroquine as a second-line therapy for uncomplicated P. falciparum and P. vivax malaria (7, 17, 19). SP is also still widely used in Africa for the intermittent preventive treatment (IPT) of malaria in infants (6), children (3), and pregnant women (8). SP resistance has been well characterized at the molecular level. Mutations involving residues 51, 59, 108, and 164 of P. falciparum dihydrofolate reductase conferred cross-resistance to the antifolates pyrimethamine and cycloguanil, whereas a mutation in residue 16 specifically conferred resistance to cycloguanil (16). The accumulation of mutations in the enzyme dihydropteroate synthase (in which Gly437 proved to be a key residue) was associated with sulfadoxine resistance in P. falciparum (18). Mutations involving other residues in dihydrofolate reductase and dihydropteroate synthase have also been linked to SP resistance in P. vivax isolates (1, 9). More recently, other mechanisms of resistance to SP have been proposed. Dahlström et al. (4) have suggested that mutations (specifically, I876V and K1466R) in multidrug resistance protein 1 may mediate an alternative mechanism of SP resistance in P. falciparum, and Martinelli et al. (10) have identified a mutation (K392Q) in the mdr2 gene in a P. chabaudi clone that was genetically linked to sulfadoxine resistance.
pfmdr2 is an ABC transporter gene that encodes a P-glycoprotein similar to that encoded by pfmdr1 (13). Although Ekong et al. (5) reported increased transcription of pfmdr2 in chloroquine-resistant parasites, other studies have demonstrated neither amplification nor overexpression of pfmdr2 (15, 21). To date, there appears to be no evidence to suggest that pfmdr2 is involved in drug resistance in P. falciparum.
In this paper, we assessed the association between single nucleotide polymorphisms in the sequence of pfmdr2 and in vitro resistance to pyrimethamine (Pyr) in P. falciparum isolates.
Between 2004 and 2010, 140 P. falciparum isolates (55 from Comoros, 18 from Cameroon, 18 from Republic of Ivory Coast, 11 from Senegal, 7 from Burkina Faso, 2 from Madagascar, 1 from French Guyana, 1 from Mayotte, and 27 from 13 other African countries) were collected from patients with uncomplicated malaria who were hospitalized in different French hospitals, and the isolates were sent to the French National Reference Center for Malaria. In vitro susceptibility to Pyr was evaluated as described in reference 11, and a threshold of 2,000 nM was applied to determine the in vitro resistance of P. falciparum isolates as defined by Basco et al. (2). A 562-bp coding region of pfdhfr was amplified by PCR and sequenced as described in reference 12; three mutations, including a Ser→Asn change at codon 108, an Asn→Ile change at codon 51 and a Cys→Arg change at codon 59, were analyzed. Copy numbers and single nucleotide polymorphisms in the pfmdr2 gene were evaluated as described in reference 20. All statistical analyses were performed using the R software package, version 2.10.1.
The mean 50% inhibitory concentration (IC50) of Pyr for the 140 isolates was 3,093 nM (95% confidence interval [CI], 2,527 to 3,660; minimum and maximum, 9.43 and 19,654 nM). Of these isolates, 69 had a Pyr IC50 > 2,000 nM (mean Pyr IC50 = 5,687 nM; 95% CI, 4,941 to 6,432) and were considered resistant; 71 isolates had a Pyr IC50 < 2,000 nM (mean Pyr IC50 = 573.3 nM; 95% CI, 425.2 to 721.4) and were considered susceptible to Pyr. Regarding the pfdhfr gene polymorphism, 74% (104/140) of the isolates had a point mutation at codon 51 (N51I); 73% (102/140) had a mutation at codon 59 (C59R), and 79% (110/140) had a mutation at codon 108 (S108N). Further, 69% (97/140) of the isolates had a triple mutation in pfdhfr. All 140 isolates possessed only one copy of the pfmdr2 gene. The analysis of the sequence of pfmdr2 gene revealed three points of mutation: S208N, F423Y, and K924E. There was no significant association between the S208N or K924E mutation and in vitro resistance to Pyr (P = 1; Fisher's exact test). The difference between the median of the Pyr IC50 (2,598 nM) for isolates harboring the Y substitution at codon 423 (n = 81) and the median of the Pyr IC50 (890 nM) for wild-type isolates (n = 59) was statistically significant (P < 0.02; Mann-Whitney test).
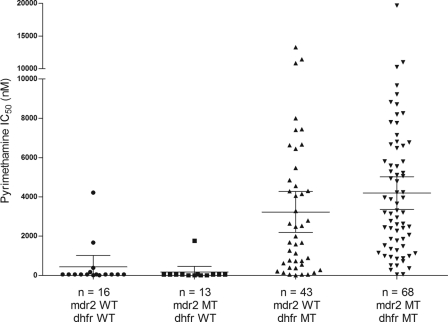
There was no statistically significant correlation between the F423Y mutation in pfmdr2 and the mutation in pfdhfr (P = 0.14; Fisher's exact test). As illustrated in Fig. 1, 16 isolates were wild type for both the pfmdr2 and pfdhfr genes; the mean Pyr IC50 for these isolates was 434.6 nM (95% CI, 0 to 1,014; minimum and maximum, 10 and 4,214 nM). A total of 13 isolates had the F423Y mutation in pfmdr2 and were wild type for pfdhfr; the mean Pyr IC50 for these isolates was 170.1 nM (95% CI, 0 to 459.3; minimum and maximum, 10 and 1,762 nM). A total of 43 isolates were wild type for pfmdr2 and mutant type for pfdhfr (with at least one of the mutations N51I, C59R, and S108N); the mean Pyr IC50 for these isolates was 3,229 nM (95% CI, 2,186 to 4,272; minimum and maximum, 9.43 and 13,294 nM). A total of 68 isolates were mutant type for both the pfmdr2 and pfdhfr genes; the mean Pyr IC50 for these isolates was 4,192 nM (95% CI, 3,362 to 5,022; minimum and maximum, 50 and 19,654 nM). There was no statistically significant association between the median of the Pyr IC50 (50 nM) for isolates with both pfmdr2 and the pfdhfr wild type and the median of the Pyr IC50 (50 nM) for isolates with the mutant type for pfmdr2 and the wild type for pfdhfr (P = 0.25; Mann-Whitney test). The difference between the median of the Pyr IC50 (1,993 nM) of pfmdr2 wild-type and pfdhfr mutant-type isolates and the median of the Pyr IC50 (3,255 nM) for isolates harboring the Y substitution at codon 423 and a pfdhfr mutation was statistically significant (P = 0.0438; Mann-Whitney test).
Fig 1.
Pyrimethamine IC50s (in nanomoles) of the 140 Plasmodium falciparum isolates according to their genotypes.
The in vitro resistance to Pyr (Pyr IC50 > 2,000 nM) was analyzed using a logistic regression model (Table 1). The mutation F423Y in pfmdr2 (OR = 2.12 [95% CI, 1.02 to 4.59]; P = 0.0489) and the mutation N51I, C59R, or S108N in pfdhfr (OR = 42.34 [95% CI, 5.52 to 324.61]; P = 0.0003) (Hosmer-Lemeshow goodness-of-fit test; P = 0.77) were independently associated with in vitro resistance to Pyr.
Table 1.
Multivariate logistic regression model
| Genotype | No. (%) of Pyr-susceptible isolates | No. (%) of Pyr-resistant isolates | Crude OR (95% CI)a | P valueb | Adjusted OR (95% CI) | P valuec |
|---|---|---|---|---|---|---|
| pfmdr2 | ||||||
| F423 | 37 (26.4) | 22 (15.7) | 1 (reference) | 1 (reference) | ||
| Y423 | 34 (24.3) | 47 (33.6) | 2.31 (1.11–4.91) | 0.017 | 2.12 (1.02–4.59) | 0.0489 |
| pfdhfr | ||||||
| Wild type | 28 (20) | 1 (0.7) | 1 (reference) | 1 (reference) | ||
| Mutant type | 43 (30.7) | 68 (48.6) | 43.36 (6.67–1,819.1) | <0.0001 | 42.34 (5.52–324.61) | 0.0003 |
OR, odds ratio; CI, 95% confidence interval.
Fisher's exact test.
Multivariate logistic regression model.
MDR2 is associated with heavy metal ion efflux and resistance to cadmium (14). MDR2 may be involved in the transport of organic anions, such as folate or pABA, as has been suggested for MRP1 (4); it may be also implicated in the transport of sulfadoxine, as suggested by Martinelli et al. (10). Interestingly, in the latter study, an association between the in vivo resistance of Plasmodium chabaudi to sulfadoxine and a mutation in the mdr2 gene has been shown. The same investigation performed with Plasmodium falciparum would be really interesting, but no laboratory is able to evaluate the sulfadoxine IC50 with sufficient reproducibility. As MDR2 (like MDR1, which is well known to be an efflux pump involved in Plasmodium falciparum resistance to mefloquine) belongs to the ABC transporter family, the F423Y mutation in MDR2 might be associated with the efflux of pyrimethamine from outside the parasite. This AA modification concerns a cytoplasmic loop of the MDR2 protein and does not represent a significant alteration in terms of size and charge characteristics of the side chain, as phenylalanine and tyrosine are both hydrophobic. But this tyrosine could be a phosphorylation site, and it is known that protein structure and activity can be modulated by phosphorylation of tyrosine residues. The mechanisms of P. falciparum resistance to SP are probably multigenic; it is important for all of these underlying mechanisms to be elucidated, because SP is still used for the IPT of malaria in Africa. The results of the present study are of interest from this point of view. But further investigations would be necessary to confirm these data with field isolates in particular and also to evaluate the potential association between this pfmdr2 mutation and the resistance of P. falciparum to sulfadoxine.
ACKNOWLEDGMENTS
We thank each of the infectious diseases services in the French hospitals that sent Plasmodium falciparum isolates to the French National Reference Center for Malaria.
This work was supported by the Direction Centrale du Service de Santé des Armées.
We have no conflicts of interest concerning the work reported in this paper. We do not own stocks or shares in any company that might be financially affected by the conclusions of this article.
Footnotes
Published ahead of print 6 February 2012
REFERENCES
- 1. Auliff A, et al. 2006. Amino acid mutations in Plasmodium vivax DHFR and DHPS from several geographical regions and susceptibility to antifolate drugs. Am. J. Trop. Med. Hyg. 75:617–621 [PubMed] [Google Scholar]
- 2. Basco LK, Ramiliarisoa O, Le Bras J. 1994. In vitro activity of pyrimethamine, cycloguanil, and other antimalarial drugs against African isolates and clones of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 50:193–199 [DOI] [PubMed] [Google Scholar]
- 3. Bojang KA, et al. 2011. Two strategies for the delivery of IPTc in an area of seasonal malaria transmission in the Gambia: a randomised controlled trial. PLoS Med. 8:e1000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dahlström S, Veiga MI, Martensson A, Bjorkman A, Gil JP. 2009. Polymorphism in PfMRP1 (Plasmodium falciparum multidrug resistance protein 1) amino acid 1466 associated with resistance to sulfadoxine-pyrimethamine treatment. Antimicrob. Agents Chemother. 53:2553–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ekong RM, Robson KJ, Baker DA, Warhurst DC. 1993. Transcripts of the multidrug resistance genes in chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum. Parasitology 106(Pt. 2):107–115 [DOI] [PubMed] [Google Scholar]
- 6. Griffin JT, et al. 2010. Protective efficacy of intermittent preventive treatment of malaria in infants (IPTi) using sulfadoxine-pyrimethamine and parasite resistance. PLoS One 5:e12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hawkins VN, Joshi H, Rungsihirunrat K, Na-Bangchang K, Sibley CH. 2007. Antifolates can have a role in the treatment of Plasmodium vivax. Trends Parasitol. 23:213–222 [DOI] [PubMed] [Google Scholar]
- 8. Le Port A, et al. 2011. Prevention of malaria during pregnancy: assessing the effect of the distribution of IPTp through the national policy in Benin. Am. J. Trop. Med. Hyg. 84:270–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu F, et al. 2010. Mutations in the antifolate-resistance-associated genes dihydrofolate reductase and dihydropteroate synthase in Plasmodium vivax isolates from malaria-endemic countries. Am. J. Trop. Med. Hyg. 83:474–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martinelli A, Henriques G, Cravo P, Hunt P. 2011. Whole genome re-sequencing identifies a mutation in an ABC transporter (mdr2) in a Plasmodium chabaudi clone with altered susceptibility to antifolate drugs. Int. J. Parasitol. 41:165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parola P, et al. 2007. Antimalarial drug susceptibility and point mutations associated with drug resistance in 248 Plasmodium falciparum isolates imported from Comoros to Marseille, France in 2004 2006. Am. J. Trop. Med. Hyg. 77:431–437 [PubMed] [Google Scholar]
- 12. Parzy D, et al. 1997. Proguanil resistance in Plasmodium falciparum African isolates: assessment by mutation-specific polymerase chain reaction and in vitro susceptibility testing. Am. J. Trop. Med. Hyg. 57:646–650 [DOI] [PubMed] [Google Scholar]
- 13. Peel SA. 2001. The ABC transporter genes of Plasmodium falciparum and drug resistance. Drug Resist. Updat. 4:66–74 [DOI] [PubMed] [Google Scholar]
- 14. Rosenberg E, et al. 2006. pfmdr2 confers heavy metal resistance to Plasmodium falciparum. J. Biol. Chem. 281:27039–27045 [DOI] [PubMed] [Google Scholar]
- 15. Rubio JP, Cowman AF. 1994. Plasmodium falciparum: the pfmdr2 protein is not overexpressed in chloroquine-resistant isolates of the malaria parasite. Exp. Parasitol. 79:137–147 [DOI] [PubMed] [Google Scholar]
- 16. Sirawaraporn W, Sathitkul T, Sirawaraporn R, Yuthavong Y, Santi DV. 1997. Antifolate-resistant mutants of Plasmodium falciparum dihydrofolate reductase. Proc. Natl. Acad. Sci. U. S. A. 94:1124–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tjitra E, Baker J, Suprianto S, Cheng Q, Anstey NM. 2002. Therapeutic efficacies of artesunate-sulfadoxine-pyrimethamine and chloroquine-sulfadoxine-pyrimethamine in vivax malaria pilot studies: relationship to Plasmodium vivax dhfr mutations. Antimicrob. Agents Chemother. 46:3947–3953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Triglia T, Wang P, Sims PF, Hyde JE, Cowman AF. 1998. Allelic exchange at the endogenous genomic locus in Plasmodium falciparum proves the role of dihydropteroate synthase in sulfadoxine-resistant malaria. EMBO J. 17:3807–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization 2006, posting date WHO guidelines for the treatment of malaria in travellers, chapters 7 (section 9.4) and 8. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf [Google Scholar]
- 20. Wurtz N, et al. 2010. Synergy of mefloquine activity with atorvastatin, but not chloroquine and monodesethylamodiaquine, and association with the pfmdr1 gene. J. Antimicrob. Chemother. 65:1387–1394 [DOI] [PubMed] [Google Scholar]
- 21. Zalis MG, Wilson CM, Zhang Y, Wirth DF. 1994. Characterization of the pfmdr2 gene for Plasmodium falciparum. Mol. Biochem. Parasitol. 63:311. [DOI] [PubMed] [Google Scholar]