Abstract
GSK2248761 is a novel, once-daily (QD), next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI) with activity against efavirenz-resistant strains. Two phase I/IIa, double-blind, randomized, placebo-controlled studies investigated the antiviral activity, safety, and pharmacokinetics (PK) of several doses of GSK2248761 monotherapy in treatment-naive HIV-infected subjects. In the initial study, 10 subjects (8 active and 2 placebo) per dose received sequentially descending GSK2248761 monotherapy regimens of 800, 400, 200, and 100 mg QD for 7 days. Because a dose-response relationship was not identified, a second study examined a lower, 30-mg QD dose in 8 subjects (6 active and 2 placebo). Adverse events, viral load (VL), PK, and reverse transcriptase mutations were assessed and combined for analysis. Treatment with GSK2248761 for 7 days was well tolerated with no serious adverse events or discontinuations. The mean VL reductions from baseline on day 8 were 0.97, 1.87, 1.84, 1.81, and 1.78 log10 copies/ml for GSK2248761 doses of 30, 100, 200, 400, and 800 mg QD, respectively. GSK2248761 PK (maximum drug concentration in serum [Cmax], area under the plasma concentration-time curve from 0 h to the end of the dosing interval [AUC0-τ], and concentration at the end of the dosing interval [Cτ]) increased proportionally over the dose range of 30 to 800 mg QD. The relationship between short-term VL change and GSK2248761 PK was best described by a maximum-effect (Emax) model using Cτ (Emax = 2.0; 50% effective concentration [EC50] = 36.9 ng/ml). No NNRTI resistance mutations emerged during the study. GSK2248761 at 100 to 800 mg QD for 7 days was well tolerated, demonstrated potent antiviral activity in treatment-naive HIV-infected subjects, and had favorable PK and resistance profiles. GSK2248761 is no longer in clinical development.
INTRODUCTION
Current guidelines from the World Health Organization and the U.S. Department of Health and Human Services recommend the inclusion of nonnucleoside reverse transcriptase inhibitors (NNRTIs) in initial combination-therapy regimens for the treatment of antiretroviral therapy (ART)-naive, human immunodeficiency virus type 1 (HIV-1)-infected individuals (1, 3). NNRTIs, such as efavirenz (EFV) and nevirapine, although highly potent and durable in their antiviral activity, have low genetic barriers to HIV-1 resistance, with only a single mutation necessary to confer resistance to the whole class (1). Of note, transmission of viral variants with the K103N mutation has been shown to significantly diminish viral response to an initial treatment with EFV and 2 analogue nucleosides (2). In addition, not unlike other classes of antiretrovirals, NNRTIs have treatment limitations, such as adverse events (AEs) or restricted use in certain patient populations. For example, EFV, the only NNRTI listed in the Department of Health and Human Services HIV treatment guidelines (1) as part of a preferred regimen for the initial treatment of HIV, is not recommended for use in pregnant women because of potential teratogenic effects. Thus, there is a need for next-generation NNRTIs with different resistance profiles and improved safety and tolerability.
GSK2248761 (formerly IDX899) is a novel NNRTI that has shown potent antiviral activity (50% inhibitory concentration [IC50], 1.2 nM against HIV-1 replication) with an advantageous resistance profile (4). Specifically, GSK2248761 has been shown to be highly active against common NNRTI-resistant mutants containing the canonical K103N and Y181C single-point mutations and double mutations in vitro (50% effective concentration [EC50], ≤2 nM), as well as clinical isolates with documented EFV resistance-conferring mutations (4; D. D. Richman et al., presented at the 15th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 3 to 6 February 2008). In vitro cross-resistance studies suggested that GSK2248761 has a different resistance profile than EFV and remains active against EFV-resistant virus pools containing up to 4 mutations. Furthermore, the pathway to developing GSK2248761-resistant mutants was slower and more robust than that for EFV-resistant mutants (Richman et al., presented at the 15th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 3–6 February 2008). Resistant HIV-1 pools bearing up to 3 GSK2248761 mutations remained susceptible to EFV, suggesting the potential for rescue therapy in case of emergence of EFV resistance during the course of therapy.
A recent phase I study assessing the safety, tolerability, and pharmacokinetics (PK) of GSK2248761 showed that single rising doses of up to 1,200 mg once daily (QD), as well as multiple doses up of 400 mg twice daily and 800 mg QD, for 7 days in healthy volunteers were safe and well tolerated; no relationship between AEs and dose or signs of liver or kidney toxicity were observed (4). On the basis of the favorable safety and in vitro resistance profiles for GSK2248761, 2 trials were conducted to assess the antiviral activity, safety, and tolerability of GSK2248761 as monotherapy in treatment-naive HIV-1-infected subjects. The initial study evaluated doses of 100, 200, 400, and 800 mg or placebo administered QD for 7 days (C. Zala et al., presented at the 17th International AIDS Conference, Mexico City, Mexico, 3 to 8 August 2008). A follow-up, low-dose extension study was similarly conducted with 30 mg to allow better characterization of dose- and concentration-response relationships (S. White et al., presented at the 50th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, 12 to 15 September 2010). The combined results from both studies are presented here.
(These data were presented in part at the 17th International AIDS Conference, Mexico City, Mexico, August 2008, and at the 50th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, September 2010.)
MATERIALS AND METHODS
Study design.
Two phase I/IIa, single-center, double-blind, randomized, sequential-cohort, placebo-controlled studies evaluated several doses of GSK2248761 in treatment-naive HIV-1-infected subjects. All trials were conducted according to the protocols of and in compliance with Good Clinical Practice, the ethical principles stated in the Declaration of Helsinki, sponsor standard operating procedures, and other applicable regulatory requirements, including the archiving of essential documents. All subjects or their legal guardians provided written informed consent prior to treatment. The studies took place in a research unit at Hospital Privado Modelo, Buenos Aires, Argentina. The subjects remained confined to the unit for the duration of the dosing period.
Methodology.
The proof-of-concept initial study (iPOC) was a phase I/IIa study that evaluated the safety, tolerability, antiretroviral activity, PK, and pharmacodynamics (PD) of GSK2248761 administered as monotherapy for 7 days in treatment-naive HIV-1-infected subjects. Initially, a total of 10 eligible subjects were randomized to receive 800 mg of GSK2248761 or placebo. After near-maximum antiviral activity was demonstrated at 800 mg QD, additional dose groups (400, 200, and 100 mg QD) of 10 eligible subjects each were explored sequentially because significant antiviral activity was demonstrated at each dose. Subsequently, a phase IIa, low-dose, proof-of-concept extension study (ePOC) (NCT00945282) was conducted with a single 30-mg dose or placebo in 8 eligible subjects for 7 days to better characterize the dose- and concentration-response relationships of GSK2248761.
At each dose level, 6 (ePOC) or 8 (iPOC) subjects received GSK2248761 and 2 subjects received placebo under standardized fed conditions for the duration of the study. Immediately after 7 days of GSK2248761 monotherapy, the study subjects were placed on highly active antiretroviral treatment (HAART). Those subjects who did not meet the criteria for HAART or who were not willing to receive HAART were placed on 28 days of lopinavir/ritonavir monotherapy. The short treatment duration of 7 days was chosen to minimize the risk of selecting for NNRTI-resistant HIV-1 while still demonstrating antiviral activity.
Inclusion criteria.
The main criteria for inclusion in the trial were male or female of nonchildbearing potential, 21 to 65 years of age, plasma HIV-1 RNA value of ≥5,000 copies/ml, CD4+ count of ≥200 cells/mm3, antiretroviral treatment naive, and agreement not to start antiretroviral therapy before clinic check-in (day −1). Subjects were also required to have had no history of AIDS-defining illness, no preexisting major NNRTI drug resistance based on genotyping at screening, and no coinfection with chronic hepatitis B or active hepatitis C.
Assessments.
For both trials, the primary objectives were to evaluate the safety, tolerability, PK, and antiviral activity of GSK2248761 and to explore the relationship between drug exposure and change in plasma HIV-1 RNA. The primary efficacy endpoint was change in HIV-1 RNA log10 copies/ml from baseline to day 8. GSK2248761 PK parameters included the area under the plasma concentration-time curve from 0 h to infinity (AUC0-∞), from 0 to 24 h (AUC0-24), and from 0 h to the end of the dosing interval (AUC0-τ); predose concentration; maximum drug concentration in serum (Cmax); time to maximum drug concentration; and concentration at the end of the dosing interval (Cτ). Safety and tolerability parameters, including AEs, clinical laboratory evaluations, electrocardiograms, and vital signs, were also monitored.
The secondary objectives were to study the immunologic effects of GSK2248761 administration, to evaluate possible changes in viral resistance, and to assess the dose proportionality and time invariance of GSK2248761 PK parameters after repeat-dose administration. The secondary endpoints assessed were change from baseline to day 8 in reverse transcriptase (RT) sequences of HIV-1 and in CD4+ and CD8+ T lymphocyte counts and percentages. In addition, AUC, Cmax, and Cτ accumulation ratios, as well as the time invariance, steady state, and dose proportionality, of GSK2248761 after repeat administration were determined. Individual reverse transcriptase sequences from each subject were obtained by extraction of viral RNA using the NucliSens easyMag system (bioMérieux, Inc., Durham, NC), followed by amplification using the TruGene HIV-1 Genotyping Assay (Siemens Healthcare Diagnostics, Inc., Deerfield, IL). Samples were analyzed on the TruGene gel system.
Statistical analyses.
The antiviral efficacy of GSK2248761 in the intention-to-treat population was assessed using the HIV-1 RNA COBAS Amplicor (F. Hoffmann-La Roche Ltd., Basel, Switzerland) assay. The changes from baseline of plasma HIV-1 RNA to day 8 were compared between active treatment and placebo using an analysis of covariance, with treatment fitted as a fixed effect and baseline plasma HIV-1 RNA as a covariate. The corresponding point and 2-sided 95% confidence interval (CI) estimates of the differences and the t test P values are presented.
Pharmacokinetic parameters were obtained using noncompartmental analysis and were summarized using descriptive statistics in the iPOC trial. For PK analysis in the ePOC 30-mg QD study, an analysis of variance (ANOVA) with term of subject as a random effect and day as a fixed effect was performed by dose on the loge-transformed PK parameters. Day was treated as a class variable in the model. The accumulation ratio of GSK2248761 and the corresponding 90% CI for each dose was estimated. Plasma GSK2248761 PK parameters on day 1 were considered to be the reference phase in the analysis and those on day 7 the test phase. An ANOVA was used on the loge-transformed predose concentrations collected from day 4 to day 7 to assess steady state by calculating the point estimate and 90% CI of the slope. For dose proportionality, PK parameter estimates from both trials for all doses were combined. The dose proportionality of repeat-dose plasma GSK2248761 AUC0-τ, Cmax, and Cτ was assessed using the power model. The power model was fitted by restricted maximum likelihood using SAS PROC MIXED (SAS Institute Inc., Cary, NC), in which the loge-transformed PK parameter was regressed on the loge-transformed dose, with dose fitted as a continuous fixed effect. Secondary analysis of dose proportionality was performed using a pairwise ANOVA for (i) GSK2248761 regimens of 100, 200, and 400 mg QD with PK parameters dose normalized to 100 mg and (ii) GSK2248761 regimens of 30, 100, 400, and 800 mg QD with PK parameters dose normalized to 100 mg using the SAS mixed-models procedure.
For PK/PD analyses, plasma GSK2248761 PK and plasma HIV-1 RNA data from the QD treatments from both trials were pooled. The plasma HIV-1 RNA change from baseline to day 8 and the plasma HIV-1 RNA rate of decline from baseline to day 8 were both assessed for correlations with repeat-dose plasma GSK2248761 PK parameters (untransformed and log transformed) using Pearson's correlation. Relationships between the plasma HIV-1 RNA change from baseline to day 8 and the repeat-dose plasma GSK2248761 PK parameters were also explored with various maximum-effect (Emax) and linear models using SAS PROC NLMIXED.
RESULTS
Subjects.
A total of 48 treatment-naive subjects were randomized and treated in both studies (Table 1), with 38 receiving the study drug, GSK2248761, and 10 receiving placebo. All 48 subjects who were randomized completed the study, with no premature withdrawals or discontinuations. The mean age of the subjects in both studies ranged from 30 to 35 years, with a majority (>92%) being male (Table 1). Most subjects were either white or Hispanic/Latino.
Table 1.
Pooled demographics and baseline characteristics
| Characteristic | Value for: |
|||||
|---|---|---|---|---|---|---|
| Placebo (n = 10) | GSK2248761 dose (mg) |
|||||
| 30 (n = 6) | 100 (n = 8) | 200 (n = 8) | 400 (n = 8) | 800 (n = 8) | ||
| Mean age (yr) (SD) | 34.0 (6.8) | 35.0 (7.1) | 33.3 (10.2) | 29.9 (7.1) | 33.9 (10.1) | 32.4 (9.1) |
| Sex [n (%)] | ||||||
| Female | 1 (12.5) | 0 | 0 | 0 | 0 | 2 (25) |
| Male | 9 (87.5) | 6 (100) | 8 (100) | 8 (100) | 8 (100) | 6 (75) |
| Race [n (%)] | ||||||
| White | 0 | 5 (83) | 0 | 0 | 1 (12) | 0 |
| Hispanic/Latino | 10 (100) | 5 (83) | 8 (100) | 8 (100) | 7 (88) | 8 (100) |
| Plasma HIV-1 RNA [mean (SD) log10 copies/ml] | 4.58 (0.45) | 4.85 (0.31) | 4.81 (0.23) | 4.74 (0.34) | 5.09 (0.71) | 4.36 (0.44) |
| CD4+ cell count [mean (SD) cells/mm3] | 550.9 (627.1) | 474.8 (130.1) | 491.4 (202.4) | 432.4 (190.0) | 466.1 (178.3) | 436.8 (130.6) |
Efficacy.
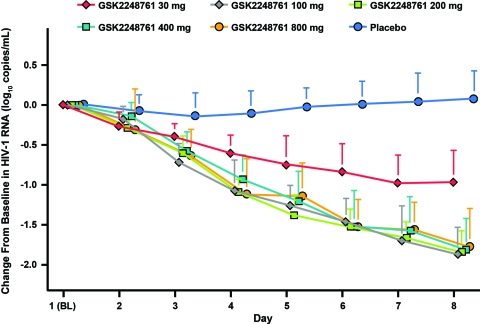
The primary efficacy endpoint was change from baseline through day 8 in plasma HIV-1 RNA. The GSK2248761 100- to 800-mg QD treatment data from the iPOC study and the 30-mg QD treatment data from the ePOC study were plotted together with pooled placebo data from both trials (Fig. 1). At day 8, all GSK2248761 doses demonstrated statistically significant mean reductions in HIV-1 RNA compared with placebo. In the iPOC trial, all study drug doses demonstrated a mean reduction in HIV-1 RNA of at least 1.78 log10 copies/ml compared with an increase of 0.10 log10 copies/ml in the placebo arm (P ≤ 0.001). However, no dose-dependent effect was observed. In the ePOC trial, there was a statistically significant decrease in mean plasma HIV-1 RNA from baseline in the GSK2248761 30-mg group (−0.97 log10 copies/ml) compared with the placebo group (−0.04 log10 copies/ml; P = 0.042) on day 8 of treatment.
Fig 1.
Mean change from baseline (BL) to day 8 in HIV-1 RNA in treatment-naive HIV-1-infected subjects treated with GSK2248761. The error bars represent standard deviations.
Assessment of the lymphocyte subsets for the secondary efficacy variable showed minimal changes in CD4+ or CD8+ cell counts from baseline on day 8 (data not shown). However, increased CD4+ counts were observed in the iPOC trial with all doses, with statistical significance achieved in the 200-mg dose group.
Pharmacokinetics.
In both trials, GSK2248761 showed time-dependent PK; the geometric mean AUC0-τ on day 7 was ∼30% higher than the geometric mean AUC0-∞ on day 1 (Table 2). Steady-state GSK2248761 concentrations were achieved by day 5 of dosing. Dose proportionality was assessed with the combined PK parameter estimates from both trials. The increase in GSK2248761 PK was dose proportional between 100 and 400 mg QD (AUC slope value, ∼1.0). However, the changes in PK were greater than dose proportional when GSK2248761 at 100 mg QD was compared with 30 mg QD (AUC slope = 1.20) and less than dose proportional when 800 mg QD was compared with 400 mg QD (AUC slope = 0.54) (data not shown).
Table 2.
Pharmacokinetic parameters for single-day (day 1) and repeat (day 7) dosing of GSK2248761
| Plasma GSK2248761 PK parametera | Value for GSK2248761 dose (mg) ofb: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30 |
100 |
200 |
400 |
800 |
||||||
| Day 1 | Day 7 | Day 1 | Day 7 | Day 1 | Day 7 | Day 1 | Day 7 | Day 1 | Day 7 | |
| AUC (ng · h/ml) | 2,404 (1,108) | 3,244 (1,738) | 10,102 (2,128) | 12,276 (3,808) | 26,504 (12,396) | 30,427 (14,442) | 37,757 (22,013) | 51,139 (13,577) | 47,863 (38,947) | 66,123 (47,837) |
| Cmax (ng/ml) | 186 (70) | 228 (94) | 831 (268) | 984 (223) | 1,735 (428) | 2,281 (820) | 2,746 (939) | 4,223 (909) | 3,539 (1,108) | 5,905 (2,738) |
| Cτ (ng/ml) | 36 (20) | 66 (50) | 138 (52) | 229 (111) | 400 (244) | 602 (380) | 528 (429) | 962 (456) | 672 (832) | 1,016 (1,267) |
| Tmax (h) | 4 (3–6) | 4 (3–6) | 4 (3–6) | 5 (3–6) | 4 (3–6) | 5 (4–6) | 3 (3–6) | 4 (3–6) | 4.5 (3–8) | 5 (2–8) |
AUC represents AUC0-∞ for day 1 and AUC0-τ for day 7. Tmax, time to the maximum observed concentration.
Values are reported as mean (standard deviation), except for Tmax where medians (ranges) are reported.
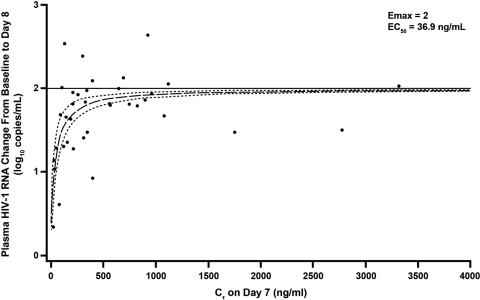
In the initial iPOC study, steady-state trough concentrations exceeded the in vitro protein-binding adjusted EC50 of GSK2248761 by approximately 3- to 9-, 5- to 50-, 7- to 66-, and 6- to 144-fold for the 100-, 200-, 400-, and 800-mg QD doses, respectively (data not shown). Figure 2 shows the relationship of GSK2248761 Cτ and the decrease in viral load from baseline as described by an Emax model based on combined data from the iPOC and ePOC studies. An Emax fixed at 2.0 and gamma fixed at 1 resulted in an estimated (standard error) in vivo EC50 for Cτ of 36.9 (8.56) ng/ml; this is similar to the in vitro protein-binding adjusted EC50 of 23 ng/ml (unpublished data).
Fig 2.
Relationship of GSK2248761 Cτ on day 7 and reduction of plasma HIV-1 RNA from baseline to day 8 with the Emax model. The points indicate individual plasma HIV-1 RNA responses, and the lines represent the best-fit line (long dashes) and 95% confidence interval (short dashes).
Viral resistance analysis.
At baseline, viral genotypes were produced for all 38 subjects who received the study drug. Most subjects (n = 28) had no substitutions at RT resistance codons, whereas 10 showed substitutions, including K103R, V179I, V106I, K101R, and V179D/E. However, none of these substitutions altered the HIV-1 RNA response in the subjects harboring the mutations.
Analysis of RT sequences on day 8 for 36 of the 38 subjects treated with GSK2248761 showed that a majority (35/38) had no treatment-emergent RT substitutions. One subject receiving the 200-mg dose displayed an L100V treatment-emergent substitution. This is not a recognized NNRTI resistance mutation, and it was fully susceptible to treatment with GSK2248761 (IC50 = 0.08 nM).
Safety.
Treatment with GSK2248761 was generally well tolerated (Table 3). All AEs were mild to moderate in intensity. The most frequently reported AEs were headache, dyspepsia, and nausea. No specific pattern of AEs in relation to GSK2248761 or placebo was observed. No serious AEs, deaths, or AEs leading to withdrawal were reported. No grade 3 or grade 4 laboratory abnormalities or discernible patterns in vital signs or electrocardiographic parameters were reported.
Table 3.
Adverse events occurring in >2 subjects
| AE | No. (%) of subjects |
||||||
|---|---|---|---|---|---|---|---|
| Placebo (n = 10) | GSK2248761 once-daily dose (mg) |
Total (n = 48) | |||||
| 30 (n = 6) | 100 (n = 8) | 200 (n = 8) | 400 (n = 8) | 800 (n = 8) | |||
| Any AE | 7 (70) | 4 (67) | 7 (88) | 5 (63) | 7 (88) | 2 (25) | 32 (67) |
| Headache | 5 (50) | 0 | 4 (50) | 2 (25) | 3 (38) | 2 (25) | 16 (33) |
| Dyspepsia | 3 (30) | 0 | 3 (38) | 1 (13) | 2 (25) | 0 | 9 (19) |
| Nausea | 2 (20) | 0 | 0 | 1 (13) | 1 (13) | 1 (13) | 5 (10) |
| Diarrhea | 1 (10) | 2 (33) | 0 | 0 | 1 (13) | 0 | 4 (8) |
| Back pain | 0 | 0 | 1 (13) | 0 | 1 (13) | 1 (13) | 3 (6) |
| Toothache | 0 | 0 | 0 | 2 (25) | 0 | 1 (13) | 3 (6) |
| Anxiety | 0 | 1 (17) | 0 | 0 | 1 (13) | 1 (13) | 3 (6) |
| Nasopharyngitis | 1 (10) | 1 (17) | 0 | 0 | 1 (13) | 0 | 3 (6) |
DISCUSSION
The outlook for patients infected with HIV-1 has greatly improved since the advent of HAART, with more than 20 approved antiretrovirals in 6 mechanistic classes with which to design combination regimens. Typical first-line regimens for newly diagnosed patients include the use of NNRTIs. However, 2 major disadvantages exist regarding the use of NNRTIs: low genetic barriers to developing resistance and the prevalence of NNRTI-resistant viral strains in treatment-naive patients (1). Thus, developing novel NNRTIs that can be potent against resistant strains with increased safety and tolerability is an ongoing effort, given the lifelong duration of HIV therapy and the longer life span of patients living with HIV-1. GSK2248761 exhibited an advantageous resistance profile and potent activity against common NNRTI-resistant strains with favorable safety and tolerability in healthy volunteers (4; Richman et al., presented at the 15th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 3–6 February 2008) and therefore was deemed appropriate for further investigation in treatment-naive HIV-1-infected subjects.
GSK2248761 displayed highly potent reductions in HIV-1 viral load in both trials, ranging from a −0.97 log10 copies/ml decrease with the 30-mg QD dose to an approximately −1.80 log10 copies/ml mean decrease with the 100-, 200-, 400-, and 800-mg QD doses. Reduction in the viral load was rapid, with most doses in the iPOC trial achieving a decrease of 1.1 log10 copies/ml by day 4. In the iPOC trial, no dose-response relationship was observed, as the trough plasma concentrations of the tested doses exceeded the in vitro protein-binding adjusted EC50 (23 ng/ml) by severalfold, even at the lowest dose (i.e., 100 mg). A smaller decrease in plasma HIV-1 RNA was observed in the ePOC study, in which a much lower dose was investigated than in the iPOC study. No subject in the extension trial reached an HIV-1 RNA viral load of <400 copies/ml (data not shown). Changes in CD4+ and CD8+ counts and percentages were not expected to be statistically significant because of the short duration and the small patient populations in both studies.
Evaluations of GSK2248761 dose- and concentration-response relationships were possible upon inclusion of the 30-mg QD cohort. GSK2248761 consistently exhibited time-dependent PK in both trials; the mean AUC0-τ on day 7 was higher than the AUC0-∞ on day 1 in both studies. Although dose-proportional increases were observed in the plasma drug AUC0-∞ throughout the 30- to 800-mg QD range, dose proportionality was more apparent in the 100- to 400-mg QD range than at the low (30-mg QD) or high (800-mg QD) end of the dose range. Subsequent analysis further confirmed the 100- to 400-mg QD dose proportionality of GSK2248761. The Emax model describing the PK/PD relationship between the change in the HIV-1 load and the plasma GSK2248761 concentration revealed that the in vivo EC50 values for Cτ are similar to the in vitro protein-binding adjusted EC50; this was apparent only after inclusion of the low-dose, 30-mg QD data. Based on the Emax model, Cτ values of ≥211 ng/ml predict a 70% probability of achieving a decrease of 1.5 log10 copies/ml; this level of antiviral activity was achieved at all the QD doses of ≥100 mg, further supporting the notion that the likely therapeutic range of GSK2248761 is within 100 to 400 mg QD.
Previous in vitro studies showed that GSK2248761-resistant strains were slower to develop and required more mutations than EFV-resistant strains (Richman et al., presented at the 15th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 3–6 February 2008). In these trials, the presence of RT substitutions at baseline, highlighting the common issue of preexisting NNRTI resistance within the treatment-naive patient population, did not affect the response to GSK2248761. The absence of NNRTI resistance mutations during the 7 days of GSK2248761 monotherapy in these studies is consistent with the robust in vitro resistance profile of GSK2248761 (Richman et al., presented at the 15th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 3–6 February 2008); however, longer- term studies with larger patient populations are necessary to gain a better understanding of the resistance profile in vivo.
Treatment with GSK2248761 was well tolerated in both studies, with no discontinuations. Adverse events were mild to moderate in intensity, and subjects did not develop any HIV-associated conditions or disease progression during the study. Specifically, AEs such as severe rash, hepatic abnormalities, and central nervous system effects commonly associated with currently existing NNRTIs were not reported during the 7-day period of the 2 studies (1). Longer-term studies are necessary to definitively establish a safety profile for GSK2248761 in the treatment-naive patient population.
In summary, GSK2248761 QD exhibited potent antiviral activity in treatment-naive subjects infected with HIV-1 and was found to be well tolerated without emergence of NNRTI-resistant strains in two 7-day monotherapy studies. Recently, the development of GSK2248761 was placed on clinical hold by the U.S. Food and Drug Administration (http://phx.corporate-ir.net/phoenix.zhtml?c=131556&p=irol-newsArticle&ID=1527182&highlight=) pending further evaluation of 5 reports of seizures in other studies of GSK2248761 in treatment-experienced patients. No seizures were reported in either of the 2 studies presented here. The seizures observed in the phase IIb program will be discussed in a subsequent report.
ACKNOWLEDGMENTS
Funding for the iPOC study was provided by Idenix Pharmaceuticals, Inc. Funding for the ePOC study was provided by GlaxoSmithKline.
All authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. We also acknowledge Todd Parker for his editorial assistance during the development of the manuscript.
Marty St. Clair, Kathleen Dudas, Joseph Kim, Yu Lou, Scott White, Steve Piscitelli, and Etienne Dumont are employees of GlaxoSmithKline and may receive company stock as part of their incentive packages. Keith Pietropaolo, Xiao-Jian Zhou, and Douglas Mayers are employees and shareholders of Idenix Pharmaceuticals, Inc.
Footnotes
Published ahead of print 6 February 2012
REFERENCES
- 1. Department of Health and Human Services 10 January 2011. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf [Google Scholar]
- 2. Goodman DD, et al. 2011. Low level of the K103N HIV-1 above a threshold is associated with virological failure in treatment-naive individuals undergoing efavirenz-containing therapy. AIDS 25:325–333 [DOI] [PubMed] [Google Scholar]
- 3. WHO 2006. Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach, 2006 revision. World Health Organization, Geneva, Switzerland: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf [PubMed] [Google Scholar]
- 4. Zhou X-J, et al. 2009. Single-dose escalation and multiple-dose safety, tolerability, and pharmacokinetics of IDX899, a candidate human immunodeficiency virus type 1 nonnucleoside reverse transcriptase inhibitor, in healthy subjects. Antimicrob. Agents Chemother. 53:1739–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]