Abstract
Voriconazole prophylaxis is common following lung transplantation, but the value of therapeutic drug monitoring is unknown. A prospective, observational study of lung transplant recipients (n = 93) receiving voriconazole prophylaxis was performed. Serum voriconazole troughs (n = 331) were measured by high-pressure liquid chromatography. The median initial and subsequent troughs were 1.91 and 1.46 μg/ml, respectively. The age of the patient directly correlated with initial troughs (P = 0.005). Patients that were ≥60 years old and cystic fibrosis patients were significantly more likely to have higher and lower initial troughs, respectively. In 95% (88/93) of patients, ≥2 troughs were measured. In 28% (25/88) and 32% (28/88) of these patients, all troughs were ≤1.5 μg/ml or >1.5 μg/ml, respectively. Ten percent (10/93) and 27% (25/93) of the patients developed invasive fungal infection (tracheobronchitis) and fungal colonization, respectively. The median troughs at the times of positive and negative fungal cultures were 0.92 and 1.72 μg/ml (P = 0.07). Invasive fungal infections or colonization were more likely with troughs of ≤1.5 μg/ml (P = 0.01) and among patients with no trough of >1.5 μg/ml (P = 0.007). Other cutoff troughs correlated less strongly with microbiologic outcomes. Troughs correlated directly with aspartate transferase levels (P = 0.003), but not with other liver enzymes. Voriconazole was discontinued due to suspected toxicity in 27% (25/93) of the patients. The troughs did not differ at the times of suspected drug-induced hepatotoxicity, central nervous system (CNS) toxicity, or nausea/vomiting and in the absence of toxicity. Voriconazole prophylaxis was most effective at troughs of >1.5 μg/ml. A cutoff for toxicity was not identified, but troughs of >4 μg/ml were rare. The data support a target range of >1.5 to 4 μg/ml.
INTRODUCTION
Invasive fungal infections (IFIs) account for 15 to 35% of infections following lung transplantation, and mortality rates approach 60% (33). The two most common IFIs among lung transplant recipients are pneumonia, which is caused most frequently by Aspergillus fumigatus and other molds, and anastomic site tracheobronchitis, which is caused by molds or yeasts. Fifty-nine percent of lung transplant programs worldwide employ universal antifungal prophylaxis (22, 25). Voriconazole is effective in preventing IFIs among lung transplant recipients (17), and it has emerged as the preferred prophylactic agent (25). Drug intolerance is the main indication for switching from voriconazole to alternative regimens (25).
Voriconazole therapeutic drug monitoring (TDM) has been advocated during treatment of IFIs to optimize efficacy and minimize toxicity and intolerance (2). Indeed, voriconazole is ideally suited to TDM. There is significant interpatient variability in drug exposure, reflecting nonlinear saturable pharmacokinetics, drug-drug interactions, CYP2C19 genetic polymorphisms, and physiological conditions associated with underlying diseases (2, 28, 35). Voriconazole serum troughs are good measures of drug exposure (14, 15). Low troughs are associated with therapeutic failure among patients with IFIs, and dose adjustments for undetectable concentrations are reported to improve treatment efficacy (10, 13, 21, 24, 28, 32, 36, 37, 39). In some studies, elevated troughs are linked to increased toxicity, including central nervous system (CNS) events and elevated liver function tests (5, 10, 16, 19, 21, 24, 28, 34). In general, a target trough range of 1 to 4 μg/ml is recommended for treatment of IFIs (2, 7).
Voriconazole TDM is not well characterized during prophylaxis, and associations between serum troughs and outcomes are not established. The mean bioavailability of voriconazole among lung transplant recipients ranges from 24% to 64% compared to 96% in populations of individuals who did not receive a lung transplant (15). Nevertheless, only 26% of lung transplantation programs employ TDM (25). In late 2008, we instituted routine TDM of voriconazole troughs to ensure that our lung transplant recipients absorbed the drug and to help minimize toxicity. We performed a prospective, observational study of TDM to determine the distribution of voriconazole troughs and their associations with efficacy and toxicity during prophylaxis.
MATERIALS AND METHODS
Patients.
Consecutive patients (>18 years old) who underwent lung transplantation at the University of Pittsburgh Medical Center in 2009, received voriconazole prophylaxis, underwent TDM, and received our standard immunosuppressive regimen (alemtuzumab as induction and a calcineurin inhibitor, mycophenolate mofetil, and low-dose prednisone [5 mg] as maintenance) were eligible. Patients were excluded if they had received phenytoin, carbamezepime, or rifampin, had previous IFIs or fungal colonization, or were receiving voriconazole for treatment of an IFI. The patients were monitored while they received voriconazole or until they developed an IFI through 1 July 2010. The study was approved by the University of Pittsburgh Institutional Review Board (PRO09050022).
Protocols.
Voriconazole was administered immediately after the transplant in 2 intravenous doses (6 mg/kg of body weight) (the two doses were given 12 h apart), and then 200 mg orally or per nasogastric tube was administered twice daily. The recommended duration of voriconazole prophylaxis was ≥3 months. Patients also received valganciclovir and life-long trimethoprim-sulfamethoxazole. During the study period, it was recommended that clinicians measure voriconazole troughs weekly on inpatients starting at ≥6 days after the transplant and at the time of hospital readmission. There were no formal recommendations for trough measurements during outpatient clinic visits, and such testing was ordered at the discretion of the patients' physicians. At the time of the study, there were no institutional protocols for voriconazole dose adjustments based on troughs. Given the lack of data to support a cutoff for effective prophylaxis, doses generally were not adjusted unless troughs were >4 μg/ml. For patients who suffered side effects such as elevated liver function tests, nausea, or vomiting, and central nervous system (CNS) events, a trough was obtained, and the dose of voriconazole was either reduced or voriconazole treatment was stopped. In the latter event, antifungal prophylaxis was substituted with another triazole or inhaled amphotericin B. All troughs were measured in-house using a high-pressure liquid chromatography method (20).
Definitions.
Voriconazole troughs were considered to be at the same time as tests or events if they were performed within 48 h. IFIs were defined by modified European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) criteria (11), recognizing that these criteria have not been validated in the lung transplant recipient population. We defined fungal anastomotic tracheobronchitis as the presence of ulceration, necrosis, pseudomembrane cartilage invasion in the airways and either positive biopsy culture for fungus or fungal invasion seen on tracheobronchial biopsy (18). Colonization was defined as recovery of a fungus without evidence of IFI. Hepatotoxicity was graded according to National Cancer Institute criteria (11a). For a given patient, only the first episode of each type of intolerance/toxicity was considered. Patients that were ≥60 years of age were considered elderly, and this age cutoff was used for comparison of voriconazole troughs.
Statistics.
Instat software (Graphpad Software Inc., San Diego, CA) was used. Dichotomous variables were compared using chi-squared or Fisher's exact test. Continuous variables were reported as medians, and the differences between groups were calculated using Mann-Whitney U test. Correlations between voriconazole levels and age or liver function tests were assessed using Spearman rank correlation. P values of <0.05 were significant. Since the breakpoint for protective voriconazole levels during prophylaxis is currently unknown, we performed the analyses in this exploratory study at breakpoints suggested by data from treatment of IFIs (0.5 to 2 μg/ml at intervals of 0.5 mg/liter).
RESULTS
A total of 331 serum voriconazole troughs were measured in 93 lung transplant recipients (Fig. 1 and Table 1). Ninety-eight percent (324/331) of the troughs were from hospitalized patients. The troughs were first measured at a median time of 11 days after the transplant (interquartile range [IQR], 8 to 20 days). In 95% (88/93) of patients, ≥2 troughs were measured (median, 4; IQR, 3 to 5). In 23% (20/88) and 48% (42/88) of these patients, all troughs were ≤1 μg/ml or >1 μg/ml, respectively. In 28% (25/88) and 32% (28/88) of the patients, all troughs were ≤1.5 μg/ml or >1.5 μg/ml, respectively. A single patient (a 68-year-old Caucasian man) had troughs that were consistently >4 μg/ml. There were no significant differences in the numbers of troughs measured among patients with IFIs, colonization, or negative fungal cultures (data not shown). In 7 patients, voriconazole dosages were adjusted in response to the recovery of a fungus, suspected toxicity, or troughs of >4 μg/ml (details summarized in Table 2).
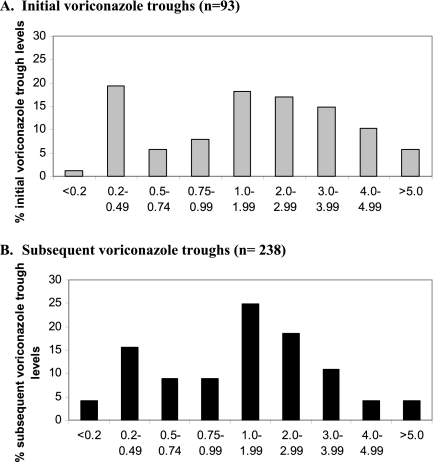
Fig 1.
Distribution of voriconazole troughs. Voriconazole troughs were measured in 93 patients (A), and subsequent troughs were measured in 95% (88/93) of these patients (B). The median initial and subsequent troughs were 1.91 μg/ml (IQR, 0.64 to 3.42 μg/ml) and 1.46 μg/ml (IQR, 0.63 to 2.56 μg/ml), respectively. One percent (1/93) and 4% (10/238) of initial and subsequent troughs, respectively, were undetectable (≤0.20 μg/ml). Thirty-two percent (30/93) and 37% (89/238) of the troughs were ≤1 μg/ml, respectively (not significant [NS]). Forty percent (37/93) and 51% (121/238) were ≤1.5 μg/ml (P values for each cutoff were NS). Fifteen percent (14/93) and 8% (20/238) were >4 μg/ml.
Table 1.
Patient characteristics
| Characteristic | Valuea |
|---|---|
| Age, yr [median (range)]b | 60 (20–74) |
| Gender, male | 54 (50/93) |
| Race | |
| White | 91 (85/93) |
| African-American | 8 (7/93) |
| Middle Eastern | 1 (1/93) |
| BMI ≥ 30b | 24 (22/93) |
| Body wt ≥ 100 kgb | 14 (13/93) |
| Underlying lung diseases | |
| Chronic obstructive pulmonary disease | 39 (36/93) |
| Idiopathic pulmonary fibrosis | 27 (25/93) |
| Cystic fibrosis | 13 (12/93) |
| Pulmonary fibrosis (nonidiopathic) | 5 (5/93) |
| Sarcoidosis | 3 (3/93) |
| Scleroderma | 3 (3/93) |
| Primary pulmonary hypertension | 2 (2/93) |
| Miscellaneousc | 8 (7/93) |
| Underlying liver diseaseb,d | 3 (3/93) |
| Type of transplant | |
| Double lung | 80 (74/93) |
| Single lung | 19 (18/93) |
| Heart-lung | 1 (1/93) |
| Route of voriconazole administratione | |
| Oral | 74 (69/93) |
| Nasogastric tube (suspension) | 26 (24/93) |
All values except for age are the percentages of patients (number of patients/total number of patients) with the characteristic.
At the time of the transplant. BMI, body mass index.
One patient each had eosinophilic granuloma, primary ciliary dyskinesia, pneumoconiosis, lymphangioleiomyomatosis, alpha-1 anti-trypsin deficiency syndrome, congenital heart disease, and another transplant.
All three patients had mild liver disease. One patient had liver disease resulting from cystic fibrosis, one had liver disease resulting from congenital heart disease, right heart failure and liver congestion, and one had liver disease resulting from hepatitis C virus infection and alcohol abuse.
At the time of the initial level.
Table 2.
Clinical data for patients whose voriconazole dosages were changed
| Patient | Demographic and clinical dataa | Initial voriconazole trough(s) (μg/ml) | Voriconazole dose adjustmentb | Reason(s) | Subsequent voriconazole troughs (μg/ml)c |
|---|---|---|---|---|---|
| 1 | 20 yrs old, W, F, cystic fibrosis | <0.20 | From 200 mg BID to 300 mg BID | Pathogenic molds recovered from BAL specimend | 0.63, 0.65, 1.24 |
| 2 | 72 yrs old, W, M, idiopathic pulmonary fibrosis | 1.13 | From 200 mg BID to 200 mg daily | Drug intolerance with vision problem and hallucination | 0.5, 0.6, 0.76, 0.87 |
| 3 | 53 yrs old, W, F, COPD | 2.31, 1.93 | From 200 mg BID to 200 mg daily | Nausea, vomiting | <0.2 (twice) |
| 4 | 42 yrs old, W, F, pulmonary fibrosis | 3.72, 4.11 | From 200 mg BID to 200 mg daily | Nausea, vomiting | (No follow-up levels) |
| 5 | 63 yrs old, W, M, idiopathic pulmonary fibrosis | 4.8 | From 200 mg BID to 200 mg daily | High level of voriconazole | 0.87, 1.01, 1.18, 1.25, 1.42 |
| 6 | 70 yrs old, W, M, idiopathic pulmonary fibrosis | 4.93 | From 200 mg BID to 200 mg daily | High level of voriconazole | 1.94, 1.97, 2.16, 2.17 |
| 7 | 60 yrs old, W, M, COPD | 5.21 | From 200 mg BID to 200 mg daily | Nausea, vomiting, and high level | <0.2, 0.82 |
The age, race (white [W]), gender (female [F] and male [M]), and underlying lung disease (chronic pulmonary obstructive disease [COPD]) are shown for each patient.
The original dose is shown first and then the final dosage that each patient received until discharge from the hospital. BID, twice a day.
The troughs are listed in increasing order.
BAL, bronchoalveolar lavage.
There was a direct correlation between the age of the patient and initial trough (P = 0.005) (Fig. 2). Initial troughs of >1 μg/ml, >1.5 μg/ml, or >4 μg/ml were significantly more likely among patients that were ≥60 years old (P = 0.004, 0.03, and 0.02, respectively) (Table 3). Cystic fibrosis (CF) was significantly associated with initial troughs of ≤1 μg/ml and ≤1.5 μg/ml (P = 0.02 and 0.01, respectively) compared to other underlying lung diseases (Table 3). There were no significant relationships between initial troughs and other patient characteristics listed in Table 1 (data not shown). Age was the only factor independently associated with initial troughs of ≤1 μg/ml by multivariate analysis (P = 0.02).
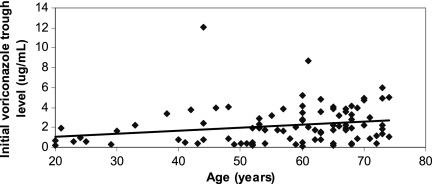
Fig 2.
Distribution of initial voriconazole troughs by the age of the patient at the time of the transplant. There was a direct correlation between the age of the patient and initial voriconazole trough (P = 0.005 by Spearman rank correlation). The median age of patients with initial troughs of ≤1 μg/ml was 52 years (IQR, 42 to 64 years) compared to 63 years (IQR, 55 to 68 years) for patients with initial troughs of >1 μg/ml (P = 0.002). The median age of patients with initial troughs of >4 μg/ml was 66 years (IQR, 60 to 71 years) compared to 60 years (IQR, 50 to 67 years) for patients with initial troughs of ≤4 μg/ml (P = 0.02).
Table 3.
Patient characteristics associated with specific initial voriconazole troughsa
| Patient characteristic | % of patients with an initial voriconazole trough concnb of: |
P value | % of patients with an initial voriconazole trough concn of: |
P value | % of patients with an initial voriconazole trough concn of: |
P value | |||
|---|---|---|---|---|---|---|---|---|---|
| ≤1 μg/ml (n = 33) | >1 μg/ml (n = 60) | ≤1.5 μg/ml (n = 37) | >1.5 μg/ml (n = 56) | ≤4 μg/ml (n = 79) | >4 μg/ml (n = 14) | ||||
| Age ≥ 60 yrs (n = 53) | 23 (12/53) | 77 (41/53) | 0.004 | 30 (16/53) | 70 (37/53) | 0.03 | 77 (41/53) | 23 (12/53) | 0.02 |
| Age < 60 yrs (n = 40) | 52.5 (21/40) | 47.5 (19/40) | 52.5 (21/40) | 47.5 (19/40) | 95 (38/40) | 5 (2/40) | |||
| Cystic fibrosis (n = 12) | 67 (8/12) | 33 (4/12) | 0.02 | 75 (9/12) | 25 (3/12) | 0.01 | 100 (12/12) | 0 (0/12) | 0.20 |
| Other disease (n = 81) | 31 (25/81) | 69 (56/81) | 35 (28/81) | 65 (53/81) | 83 (67/81) | 17 (14/81) | |||
There were no significant associations between initial voriconazole troughs and patients' sex, race, body mass index, lung diseases other than cystic fibrosis, presence of underlying liver disease, route of voriconazole administration, or receipt of concomitant acid suppressive therapy (data not shown). The P values result from a comparison of values for the patients with the lower trough concentration compared to the patients with the higher trough concentration.
The values in parentheses are the number of patients with the initial voriconazole trough concentration to the total number of patients.
Voriconazole troughs and fungal infections.
During voriconazole prophylaxis, 10% (10/93) and 27% (25/93) of patients developed IFIs or had fungi colonize their respiratory tracts, respectively. All IFIs were anastomotic tracheobronchitis due to yeasts (6 patients) or molds (4 patients). The median time to diagnosis of IFI was 6 weeks after the transplant (IQR, 4 to 10 weeks). There were 43 episodes of fungal colonization among 25 patients, including 18 and 25 episodes due to yeasts and molds, respectively. Of note, Aspergillus fumigatus was not isolated from any patient.
Overall, 157 voriconazole troughs were measured at the time of cultures (IFI, n = 10; colonization, n = 43; negative cultures, n = 104). The median troughs associated with positive and negative cultures were 0.92 μg/ml (IQR, 0.55 to 2.62) and 1.72 μg/ml (IQR, 0.72 to 3.12), respectively (P = 0.07). Among positive cultures, median troughs associated with IFI and colonization were 0.99 μg/ml (IQR, 0.74 to 2.40) and 0.92 μg/ml (IQR, 0.52 to 2.70), respectively (P = 0.44). A positive culture was significantly more likely with a trough of ≤1.5 μg/ml (P = 0.01) (Table 4). Indeed, 60% (6/10) and 70% (30/43) of the IFI and colonization instances, respectively, occurred at troughs of ≤1.5 μg/ml compared to 46% (48/104) of negative cultures.
Table 4.
Respiratory tract culture results, stratified by voriconazole troughs at the time of culture
| Voriconazole trough | % of positive cultures (n = 53)a |
% of total positive cultures (n = 53)a | % of negative cultures (n = 104)a | P valued | |
|---|---|---|---|---|---|
| IFIb (n = 10) | Colonizationc (n = 43) | ||||
| ≤0.5 μg/ml | 10 (1/10) | 23 (10/43) | 21 (11/53) | 22 (23/104) | 1.0 |
| >0.5 μg/ml | 90 (9/10) | 77 (33/43) | 79 (42/53) | 78 (81/104) | |
| ≤1 μg/ml | 50 (5/10) | 51 (22/43) | 51 (27/53) | 35 (36/104) | 0.06 |
| >1 μg/ml | 50 (5/10) | 49 (21/43) | 49 (26/53) | 65 (68/104) | |
| ≤1.5 μg/ml | 60 (6/10) | 70 (30/43) | 68 (36/53) | 46 (48/104) | 0.01 |
| >1.5 μg/ml | 40 (4/10) | 30 (13/43) | 32 (17/53) | 54 (56/104) | |
| ≤2 μg/ml | 70 (7/10) | 72 (31/43) | 72 (38/53) | 58 (60/104) | NS (0.12) |
| >2 μg/ml | 30 (3/10) | 28 (12/43) | 28 (15/53) | 42 (44/104) | |
The values in parentheses are the number of positive or negative cultures to the total number of cultures.
Anastomotic tracheobronchits was caused by Candida glabrata (n = 3), Candida albicans (n = 2), a yeast species that was not identified to the species level (n = 1), Penicillium (n = 1), Aspergillus niger (n = 1), and an unidentified mold (n = 2).
Colonization was caused by 19 Candida isolates (9 C. glabrata isolates, 6 C. albicans isolates, 2 C. krusei isolates, and one isolate each of C. tropicalis and C. parapsilosis, and 24 mold isolates (9 Penicillium isolates, 6 Aspergillus niger isolates, 2 Fusarium isolates, 2 Rhizopus isolates, 1 Scedosporium isolate, 1 Lecythofora isolate, and 3 unidentified molds). Seven patients were colonized for a single episode, and 18 patients were colonized for two episodes at unique times.
The P values result from a comparison of values for the 53 positive cultures compared to the values for the 104 negative cultures at their respective troughs. NS, not significant.
Patients who failed to achieve at least one trough of >1.5 μg/ml or >1 μg/ml were significantly more likely to have a positive fungal culture (P = 0.007 and 0.01, respectively) (Table 5). There were no associations between troughs and recovery of particular fungal species, and the initial and maximum troughs did not correlate with microbiology results (data not shown).
Table 5.
Respiratory tract culture results among 93 patients, stratified by achievement of voriconazole cutoff troughs
| Voriconazole troughs (n = 93) | % of patients with the following microbiology resulta: |
P valueb | |
|---|---|---|---|
| Positive cultures (n = 35) | Negative cultures (n = 58) | ||
| No trough > 1 μg/ml (n = 20) | 65 (13/20) | 35 (7/20) | 0.01 |
| At least one trough > 1 μg/ml (n = 73) | 30 (22/73) | 70 (51/73) | |
| No trough > 1.5 μg/ml (n = 24) | 62.5 (15/24) | 37.5 (9/24) | 0.007 |
| At least one trough > 1.5 μg/ml (n = 69) | 29 (20/69) | 71 (49/69) | |
The values in parentheses are the number of patients with a positive or negative result to the total number of patients.
The P values result from a comparison of values for the positive cultures compared to the values for the negative cultures.
Only one patient with IFI had antecedent acute cellular rejection requiring augmented immunosuppression, and no patients had prior evidence of active cytomegalovirus infection.
Voriconazole troughs and drug toxicity.
Voriconazole was discontinued in 27% (25/93) of patients due to suspected drug toxicity, including nausea/vomiting (14% [13/93]), hepatotoxicity (11% [10/93]), and central nervous system (CNS) events (3% [3/93]). CNS events included visual disturbances (n = 2) and delirium (n = 1). One patient had nausea/vomiting and a visual disturbance. The median voriconazole troughs at the time of suspected drug-induced nausea/vomiting, hepatoxicity, and CNS toxicity were not significantly different from the median troughs among patients who did not exhibit these symptoms or toxicity (Fig. 3). Similarly, initial or maximum voriconazole troughs did not correlate with suspected toxicity (data not shown). Overall, 209 voriconazole troughs were measured at the time of liver function tests. Troughs correlated with serum aspartate transferase (AST) levels (P = 0.003) (Fig. 4), but not alanine transaminase (ALT), alkaline phosphatase, or total bilirubin levels (P = 0.13, 0.55, and 0.62, respectively).
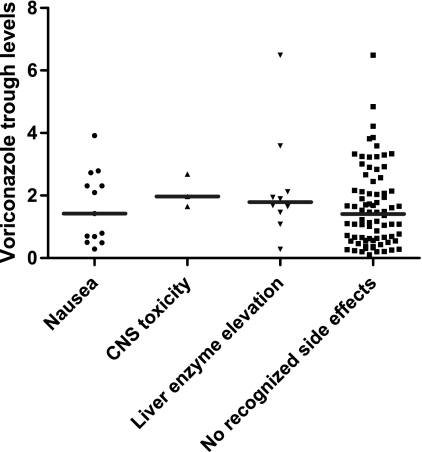
Fig 3.
Distribution of voriconazole troughs by type of suspected toxicity. There were no significant differences in the median troughs among patients at the time of suspected voriconazole-related nausea/vomiting, CNS toxicity, or hepatotoxicity (liver enzyme elevations) (symbols) and the troughs among patients without suspected toxicity (horizontal lines).
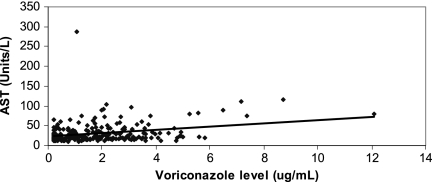
Fig 4.
Correlation between voriconazole troughs and aspartate transaminase (AST) values. The data include 209 voriconazole troughs drawn at the time of liver function tests. Voriconazole troughs correlated with AST values (P = 0.003 by Spearman rank correlation), but not with ALT, alkaline phosphatase, or total bilirubin values (data not shown).
Nausea and vomiting were definitively attributed to voriconazole in only 23% (3/13) of suspected cases, as the symptoms significantly improved within 48 h of discontinuing the drug. In the remaining cases, other drugs were discontinued simultaneously (mycophenolate, 9 patients; multiple medications, 1 patient). Within 4 months, four of these patients were rechallenged with voriconazole with no recurrence of nausea/vomiting. Elevated transaminase or alkaline phosphatase levels were encountered in 56% (52/93) of patients during prophylaxis (Table 6), but grade 3 hepatotoxicity was evident in only 5% (5/93). Grade 3 hepatotoxicity was ascribed to voriconazole in three patients in whom alkaline phosphatase levels improved within 1 week of discontinuing the drug; levels did not return to baseline until a mean of 7 weeks later. Among the patients for whom hepatotoxicity was not ascribed to voriconazole, an alternative etiology was identified (sepsis and cirrhosis [1 patient each]) or liver function test results normalized during continued prophylaxis. The three patients with suspected CNS toxicity were 53, 67, and 68 years of age, and each was also receiving tacrolimus. Magnetic resonance images did not show evidence of posterior reversible encephalopathy syndrome, and discontinuation of tacrolimus did not reverse the findings. Symptoms resolved within 8 days of discontinuing voriconazole.
Table 6.
Liver enzyme abnormalities during voriconazole prophylaxis
| Grade of toxicity | % of patients with the following liver enzyme toxicitya: |
||
|---|---|---|---|
| AST | ALT | ALP | |
| 1 | 18 (17/93) | 9 (8/93) | 25 (23/93)b |
| 2 | 2 (2/93) | 2 (2/93) | 15 (14/93)c |
| 3 | 1 (1/93) | 0 | 4 (4/93)d |
| 4 | 0 | 0 | 0 |
For aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP), liver enzyme toxicity was defined as follows: grade 1, upper limit of normal (ULN) to 2.5× ULN; grade 2, >2.5× to 5× ULN; grade 3, >5× to 20× ULN; grade 4, >20× ULN. At the University of Pittsburgh Medical Center (UPMC), the respective ULN values for AST, ALT, and ALP, are 41 U/liter, 63 U/liter, and 126 U/liter, respectively. The values in parentheses are the number of patients with toxicity to the total number of patients.
One patient also had transaminase toxicity.
Three patients also had transaminase toxicity.
Five patients also had transaminase toxicity.
DISCUSSION
In this study, we describe our experience with voriconazole TDM among 93 patients who received prophylaxis following lung transplantation. To our knowledge, this is the first study of routine voriconazole TDM in this setting. Our most important finding is that serum voriconazole troughs correlated with efficacy of prophylaxis. The median trough associated with IFI or fungal colonization was 0.92 μg/ml compared to 1.72 μg/ml for negative respiratory tract cultures. Moreover, IFI and fungal colonization were significantly less likely if the trough at the time was >1.5 μg/ml compared to lower levels. Patients with at least one serum trough of >1.5 μg/ml were significantly less likely to develop IFI or colonization than patients who failed to achieve a trough above this cutoff. A trough of >1 μg/ml was also protective against IFI or colonization, although the association was less robust. Our data suggest that the target trough for optimizing the efficacy of voriconazole prophylaxis in lung transplant recipients is within the range of targets previously advocated for the treatment of IFIs (6, 19, 28, 31, 32).
To date, there are limited data for voriconazole TDM during antifungal prophylaxis. The largest study consisted of 71 allogeneic stem cell transplant recipients, of whom 6 and 4 patients developed breakthrough candidiasis and Mucorales infections, respectively (38). For candidiasis, the most recent voriconazole troughs were <2 μg/ml; for Mucorales infections, the most recent troughs ranged from 1.1 to 5.9 μg/ml (median, 3.8 μg/ml). These data are difficult to interpret, since the troughs were measured 1 to 12 weeks prior to the diagnosis of IFI. Previous experience with itraconazole prophylaxis among neutropenic hosts at high risk for IFIs suggested that target concentrations for effective disease prevention were two- to fourfold lower than for treatment (31). Along these lines, a recent review hypothesized that the voriconazole concentration necessary to prevent IFIs may be lower than needed for treatment (31). Our data do not support this hypothesis. Clearly, further studies are needed to clarify the relationship between voriconazole levels and the efficacy of prophylaxis, recognizing that optimal target concentrations may differ based on the patient population.
Associations between voriconazole troughs and drug toxicity in this study were less clear-cut, and cutoff troughs that identified patients at increased risk could not be assigned. Indeed, voriconazole prophylaxis was safe and, on the whole, well-tolerated. Our 27% (25/93) discontinuation rate was consistent with previous studies in other populations (http://www.rxlist.com/vfend-drug.htm). As in earlier reports, we found a direct correlation between troughs and AST levels (23, 36). Elevated liver enzymes were common, but the vast majority were attributable to etiologies other than voriconazole exposure. Moreover, grade 3 elevations were documented in only 5% (5/93) of patients, and voriconazole was discontinued due to hepatotoxicity in only 11% (10/93) of patients. Our rate of significant hepatotoxicity was lower than generally reported among patients with hematologic malignancies or stem cell transplant recipients, in whom tumor infiltration, fungal infection of the liver, chemotherapeutic agents, or graft-versus-host disease can cause liver injury (1, 9). Nausea and vomiting were the most common indications for discontinuation of voriconazole (14% [13/93]). In most instances, however, other drugs known to cause gastrointestinal side effects like calcineurin inhibitors or mycophenolate were discontinued simultaneously, making it difficult to establish causal links. Finally, our 3% rate of suspected voriconazole-induced CNS toxicity was consistent with the rate previously reported (VFEND patient counseling information [http://www.pfizer.com/files/products/uspi_vfend.pdf]; Pfizer Inc., New York, NY). A narrow therapeutic window between efficacy and CNS events was also observed in a recent study of lung transplant recipients with CF (4). It is possible that the threshold for neurotoxicity in our 3 patients was lowered due to their ages (53, 67, and 68 years) and the receipt of tacrolimus, which has been associated with neurological adverse events in as many as 40 to 60% of patients (3, 40). In the end, our ability to demonstrate associations between voriconazole troughs and toxicity was likely limited by the small number of troughs of >4 μg/ml, which reflected a general practice in our program of reducing dosages at this threshold. In this regard, we feel that we were successful in our initial objective of instituting TDM to minimize serious toxicity.
Based on our data, our present practice is to target voriconazole troughs during prophylaxis to a range of >1.5 to 4 μg/ml. This range encompasses our efficacy cutoff, allows some cushion for variations in levels over time, and acknowledges previous reports of increased toxicity at higher troughs (6, 8). At the same time, our experience highlights the challenges of achieving and maintaining serum troughs of >1.5 μg/ml. Despite a standardized dosing protocol, troughs were consistently ≤1.5 μg/ml in 28% (25/88) of our patients; 40% (35/88) had levels that fluctuated between ≤1.5 and >1.5 μg/ml. Therefore, serial monitoring to ensure that troughs remain within the desired range is essential, even if earlier TDM results suggest that targets have been achieved (39). In conjunction with systematic TDM, evaluation for CYP2C19 genetic polymorphisms, which was not performed in this study, may be useful in identifying patients predisposed to lower voriconazole levels due to increased drug metabolism (31).
Age was the most important demographic or clinical factor that impacted voriconazole levels, correlating directly with troughs. Indeed, older patients (≥60 years of age) were significantly more likely to have initial serum troughs of >1.5 μg/ml. Our findings are in line with pharmacokinetic data from 552 patients in 10 voriconazole clinical trials, which demonstrated that median voriconazole concentrations among patients older than 65 years were 80% to 90% higher than in younger patients (http://www.rxlist.com/vfend-drug.htm). In contrast, CF patients were significantly more likely to have initial troughs of ≤1.5 μg/ml by univariate analysis. A recently published voriconazole pharmacokinetics study among lung transplant recipients at our center identified CF as the most important patient variable associated with poor bioavailability (14, 15) likely due to intestinal mucosal dysfunction and other physiologic changes that accompany the disease (12, 14, 15). In another report, 50% to 70% of voriconazole troughs measured in CF patients were ≤1.5 μg/ml during any given time period following lung transplantation (4). As reported previously (27), we did not find an association between body mass index (BMI) or weight and voriconazole troughs. In patients who receive weight-adjusted parenteral loading doses of voriconazole, therefore, our data suggest that subsequent adjustments of oral doses are not necessary.
There are several caveats to our findings. First, we assessed patients receiving voriconazole within the first few months of lung transplantation, and the results may not be relevant to other dosing regimens or populations. Second, tracheobronchitis accounted for all cases of IFI. While the data may suggest that voriconazole prophylaxis was protective against pneumonia and other parenchymal diseases, we cannot draw conclusions about the impact of serum troughs on these entities. Third, we recognize that voriconazole concentrations within the lung and/or respiratory tract may be more important determinants of the efficacy of prophylaxis than serum levels. It is important to understand that pulmonary artery circulation is surgically restored during transplantation but that bronchial artery circulation is not. In studies of canine lung transplantation, bronchial artery circulation is slowly regenerated by angiogenesis (29, 30). As such, bronchial anastomosis is susceptible in the early posttransplant period to ischemia (26), which may predispose the patients to IFIs despite voriconazole prophylaxis through attenuated local host defenses and diminished drug delivery. Indeed, we recognize that serum voriconazole troughs are only one of several potential risk factors for IFI. Along these lines, there were no differences in immunosuppressive regimens, acute cellular rejection, or active cytomegalovirus infections among patients with IFIs, colonization, or no positive fungal cultures in this study. Finally, we cannot assess the impact of prophylaxis on the emergence of voriconazole-resistant fungi because susceptibility testing was not available at our center during the study period. Nevertheless, it was striking that Rhizopus spp. or other typically voriconazole-resistant agents of the Mucorales group did not emerge in the face of higher troughs. Furthermore, Candida glabrata and Candida krusei, species for which azole resistance is commonly encountered, were recovered from patients with a wide range of troughs.
In conclusion, the results of this study suggest that routine voriconazole TDM may identify lung transplant recipients who are at increased risk of developing fungal infections during prophylaxis. Dose adjustments based upon TDM results may reduce the occurrence of these infections, as well as minimize drug toxicity. Future studies assessing TDM-directed dose adjustment strategies are warranted.
ACKNOWLEDGMENTS
Funding for this project was provided through an investigator-initiated research grant from Pfizer to C. J. Clancy. Pfizer had no input into the study design, data interpretation, or conclusions. C. J. Clancy has received grant support for unrelated research projects from Pfizer, Merck, and Astellas. M. H. Nguyen has received grant support for unrelated research projects from Pfizer, Merck, and ViraCor-IBT Laboratories. R. K. Shields has received grant support for unrelated research projects from Merck and Astellas. E. J. Kwak has received grant support for unrelated research projects from Pfizer.
We thank Lloyd Clarke for assistance with data retrieval and database management.
Footnotes
Published ahead of print 13 February 2012
REFERENCES
- 1. Amigues I, et al. 2010. Hepatic safety of voriconazole after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 16:46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andes D, Pascual A, Marchetti O. 2009. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob. Agents Chemother. 53:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Appignani BA, et al. 1996. Neuroimaging findings in patients on immunosuppressive therapy: experience with tacrolimus toxicity. AJR Am. J. Roentgenol. 166:683–688 [DOI] [PubMed] [Google Scholar]
- 4. Berge M, et al. 2009. Voriconazole pharmacokinetic variability in cystic fibrosis lung transplant patients. Transpl. Infect. Dis. 11:211–219 [DOI] [PubMed] [Google Scholar]
- 5. Boyd AE, et al. 2004. Adverse reactions to voriconazole. Clin. Infect. Dis. 39:1241–1244 [DOI] [PubMed] [Google Scholar]
- 6. Bruggemann RJ, et al. 2008. Therapeutic drug monitoring of voriconazole in a child with invasive aspergillosis requiring extracorporeal membrane oxygenation. Ther. Drug Monit. 30:643–646 [DOI] [PubMed] [Google Scholar]
- 7. Bruggemann RJ, et al. 2008. Therapeutic drug monitoring of voriconazole. Ther. Drug Monit. 30:403–411 [DOI] [PubMed] [Google Scholar]
- 8. Bruggemann RJ, van der Linden JW, Verweij PE, Burger DM, Warris A. 2011. Impact of therapeutic drug monitoring of voriconazole in a pediatric population. Pediatr. Infect. Dis. J. 30:533–534 [DOI] [PubMed] [Google Scholar]
- 9. Chamilos G, et al. 2007. Effects of liposomal amphotericin B versus an amphotericin B lipid complex on liver histopathology in patients with hematologic malignancies and invasive fungal infections: a retrospective, nonrandomized autopsy study. Clin. Ther. 29:1980–1986 [DOI] [PubMed] [Google Scholar]
- 10. Denning DW, et al. 2002. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin. Infect. Dis. 34:563–571 [DOI] [PubMed] [Google Scholar]
- 11. De Pauw B, et al. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a. Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health December 2004. Division of AIDS table for grading the severity of adult and pediatric adverse events. Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD: www3.niaid.nih.gov/LabsAndResources/resources/DAIDSClinRsrch/PDF/DAIDSAEGradingTable.pdf [Google Scholar]
- 12. Eggermont E, De Boeck K. 1991. Small-intestinal abnormalities in cystic fibrosis patients. Eur. J. Pediatr. 150:824–828 [DOI] [PubMed] [Google Scholar]
- 13. Freifeld A, et al. 2007. Relationship of blood level and susceptibility in voriconazole treatment of histoplasmosis. Antimicrob. Agents Chemother. 51:2656–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han K, Bies R, Johnson H, Capitano B, Venkataramanan R. 2011. Population pharmacokinetic evaluation with external validation and Bayesian estimator of voriconazole in liver transplant recipients. Clin. Pharmacokinet. 50:201–214 [DOI] [PubMed] [Google Scholar]
- 15. Han K, et al. 2010. Bioavailability and population pharmacokinetics of voriconazole in lung transplant recipients. Antimicrob. Agents Chemother. 54:4424–4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howard A, Hoffman J, Sheth A. 2008. Clinical application of voriconazole concentrations in the treatment of invasive aspergillosis. Ann. Pharmacother. 42:1859–1864 [DOI] [PubMed] [Google Scholar]
- 17. Husain S, et al. 2006. Voriconazole prophylaxis in lung transplant recipients. Am. J. Transplant. 6:3008–3016 [DOI] [PubMed] [Google Scholar]
- 18. Husain S, et al. 2011. A 2010 working formulation for the standardization of definitions of infections in cardiothoracic transplant recipients. J. Heart Lung Transplant. 30:361–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Imhof A, Schaer DJ, Schanz U, Schwarz U. 2006. Neurological adverse events to voriconazole: evidence for therapeutic drug monitoring. Swiss Med. Wkly. 136:739–742 [DOI] [PubMed] [Google Scholar]
- 20. Johnson HJ, et al. 2010. Voriconazole pharmacokinetics in liver transplant recipients. Antimicrob. Agents Chemother. 54:852–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lazarus HM, Blumer JL, Yanovich S, Schlamm H, Romero A. 2002. Safety and pharmacokinetics of oral voriconazole in patients at risk of fungal infection: a dose escalation study. J. Clin. Pharmacol. 42:395–402 [PubMed] [Google Scholar]
- 22. Levine SM. 2004. A survey of clinical practice of lung transplantation in North America. Chest 125:1224–1238 [DOI] [PubMed] [Google Scholar]
- 23. Lutsar I, Hodges MR, Tomaszewski K, Troke PF, Wood ND. 2003. Safety of voriconazole and dose individualization. Clin. Infect. Dis. 36:1087–1088 [DOI] [PubMed] [Google Scholar]
- 24. Miyakis S, van Hal SJ, Ray J, Marriott D. 2010. Voriconazole concentrations and outcome of invasive fungal infections. Clin. Microbiol. Infect. 16:927–933 [DOI] [PubMed] [Google Scholar]
- 25. Neoh CF, et al. 2011. Antifungal prophylaxis in lung transplantation: a world-wide survey. Am. J. Transplant. 11:361–366 [DOI] [PubMed] [Google Scholar]
- 26. Nicolls MR, Zamora MR. 2010. Bronchial blood supply after lung transplantation without bronchial artery revascularization. Curr. Opin. Organ Transplant. 15:563–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pai MP, Lodisa TP. 2010. Pharmacokinetics (PK) of voriconazole in obese adults. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., abstr A1-044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pascual A, et al. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201–211 [DOI] [PubMed] [Google Scholar]
- 29. Pearson FG, Goldberg M, Stone RM, Colapinto RF. 1970. Bronchial arterial circulation restored after reimplantation of canine lung. Can. J. Surg. 13:243–250 [PubMed] [Google Scholar]
- 30. Siegelman SS, Hagstrom JW, Koerner SK, Veith FJ. 1977. Restoration of bronchial artery circulation after canine lung allotransplantation. J. Thorac. Cardiovasc. Surg. 73:792–795 [PubMed] [Google Scholar]
- 31. Smith J, Andes D. 2008. Therapeutic drug monitoring of antifungals: pharmacokinetic and pharmacodynamic considerations. Ther. Drug Monit. 30:167–172 [DOI] [PubMed] [Google Scholar]
- 32. Smith J, et al. 2006. Voriconazole therapeutic drug monitoring. Antimicrob. Agents Chemother. 50:1570–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sole A, Morant P, Salavert M, Peman J, Morales P. 2005. Aspergillus infections in lung transplant recipients: risk factors and outcome. Clin. Microbiol. Infect. 11:359–365 [DOI] [PubMed] [Google Scholar]
- 34. Tan K, Brayshaw N, Tomaszewski K, Troke P, Wood N. 2006. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J. Clin. Pharmacol. 46:235–243 [DOI] [PubMed] [Google Scholar]
- 35. Theuretzbacher U, Ihle F, Derendorf H. 2006. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin. Pharmacokinet. 45:649–663 [DOI] [PubMed] [Google Scholar]
- 36. Trifilio S, et al. 2005. Voriconazole therapeutic drug monitoring in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 35:509–513 [DOI] [PubMed] [Google Scholar]
- 37. Trifilio S, et al. 2007. Monitoring plasma voriconazole levels may be necessary to avoid subtherapeutic levels in hematopoietic stem cell transplant recipients. Cancer 109:1532–1535 [DOI] [PubMed] [Google Scholar]
- 38. Trifilio S, et al. 2007. Breakthrough fungal infections after allogeneic hematopoietic stem cell transplantation in patients on prophylactic voriconazole. Bone Marrow Transplant. 40:451–456 [DOI] [PubMed] [Google Scholar]
- 39. Trifilio SM, et al. 2009. Serial plasma voriconazole concentrations after allogeneic hematopoietic stem cell transplantation. Antimicrob. Agents Chemother. 53:1793–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vadnerkar A, et al. 2011. Age-specific complications among lung transplant recipients 60 years and older. J. Heart Lung Transplant 30:273–281 [DOI] [PubMed] [Google Scholar]