Abstract
Ischemic postconditioning is a concept originally defined to contrast with that of ischemic preconditioning. While both preconditioning and postconditioning confer a neuroprotective effect on brain ischemia, preconditioning is a sublethal insult performed in advance of brain ischemia, and postconditioning, which conventionally refers to a series of brief occlusions and reperfusions of the blood vessels, is conducted after ischemia/reperfusion. In this article, we first briefly review the history of preconditioning, including the experimentation that initially uncovered its neuroprotective effects and later revealed its underlying mechanisms-of-action. We then discuss how preconditioning research evolved into that of postconditioning – a concept that now represents a broad range of stimuli or triggers, including delayed postconditioning, pharmacological postconditioning, remote postconditioning – and its underlying protective mechanisms involving the Akt, MAPK, PKC and KATP channel cell-signaling pathways. Because the concept of postconditioning is so closely associated with that of preconditioning, and both share some common protective mechanisms, we also discuss whether a combination of preconditioning and postconditioning offers greater protection than preconditioning or postconditioning alone.
Keywords: postconditioning, preconditioning, stroke, cerebral ischemia, focal ischemia, neuroprotection
INTRODUCTION
In this article we will review the protective effects and underlying mechanisms of both ischemic preconditioning and postconditioning against stroke. While postconditioning is performed after ischemia/reperfusion[1, 2], preconditioning is conducted before ischemic onset[3], and the former is considered to be derived from the latter[2]. We will first review the literature about ischemic preconditioning, and discuss how the concept of preconditioning research evolved into that of postconditioning. Thereafter, we will discuss various in vivo and in vitro models of postconditioning and the potential protective mechanisms present in these approaches, and finally, we will discuss whether the combination of preconditioning and postconditioning offers greater protection than preconditioning or postconditioning alone.
THE PROTECTIVE EFFECTS OF ISCHEMIC PRECONDITIONING IN BOTH HEART AND BRAIN ISCHEMIA
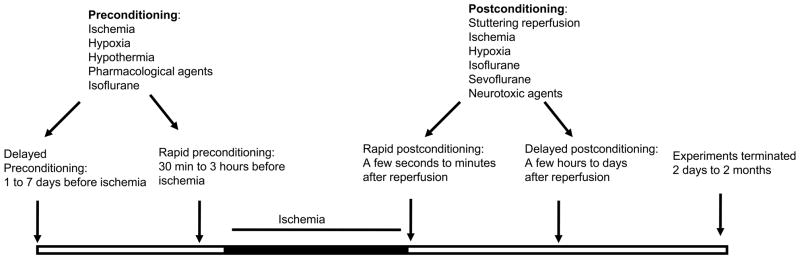
Preconditioning in the brain is a phenomenon in which the brain protects itself against future injury by adapting to low doses of noxious insults [4–7](Fig.1). Investigators from diverse backgrounds have used different approaches to show that stimuli such as anesthetic agents, hypothermia/hyperthermia, hypoxia/ischemia, as well as low doses of certain toxins can promote preconditioning-dependent protective responses via the activation of endogenous protective mechanisms, and thus potentially lessen the impact of a subsequent and more severe attack [4].
Fig. 1.
Preconditioning and postconditioning time lines and their stimulus types. While preconditioning is conducted before ischemia onset, postconditioning is induced post ischemia/reperfusion. Both preconditioning and postconditioning can be performed with a number of stimuli, and consist of both rapid and delayed time windows.
The concept of ischemic preconditioning was first described in ischemic hearts by Murry et al. in 1986 [3]. The cardioprotective effect was observed in a canine experimental model by preconditioning 4 cycles of a 5 minute ischemia interspersed with a 5 minutes reperfusion before a sustained 40 minute circumflex artery occlusion. This effect disappeared when the myocardium was subjected to a 3 hour ischemia, indicating the beneficial effects were limited to short-term ischemia [3, 8]. Nevertheless, this phenomenon indicates that the heart has an important endogenous response to resist a cardiac ischemia-reperfusion injury. In 1993, beneficial effects of delayed preconditioning (late preconditioning) were shown by Kuzuya and Marber [9, 10]. In their experiments a significant myocardial protective effect was found when sustained ischemia was conducted 24 hours after the initial preconditioning stimulus, including reduced infarct size and decreased endothelial injury. The “second window of protection” appeared relatively long and lasted several days [9, 10]. For the last decade, studies by many independent groups have demonstrated, in several species tested with different protocols, the robust cardioprotective effect of myocardial preconditioning, which had in common brief episodes of sublethal ischemia [11].
In addition to myocardium, the phenomenon of ischemic preconditioning for acute ischemia–reperfusion injury has been reproduced in other organs including the liver[12], kidney [13]and brain[4]. Similar to cardiac preconditioning, the development of a tolerant brain appears to result from the activation of endogenous mechanisms of protection against brain injuries. In early studies of ischemic/hypoxic brains, several papers documented increased survival times in rats and hippocampal CA1 neuron protection during anoxia when the brains were exposed earlier to brief anoxia [14, 15], but these findings were not recognized at that time as a type of ischemic preconditioning.
The concept of cerebral ischemic tolerance was first introduced in the early 1990s. Kitagawa et al. reported the neuroprotective effects against neuronal cell death when adding 2 minutes of transient ischemia 24 hours before global cerebral ischemia in rats [16, 17]. After that, the preconditioning-dependent protective responses have been found in many species, including gerbils [18–20], rats [21–23], and mice [24], and also in brain slices [25], as well as in murine cell culture [26]. In addition, preconditioning can be induced not only by a sublethal ischemia or hypoxia, but also by a number of pharmacological agents, including Ginkgolide B[27], resveratrol[28], adenosine[29], isoflurane[30], estrogen[31], Erythropoietin[32].
Rapid and delayed preconditioning in both the heart and the brain have different mechanisms. Generally, early preconditioning is related to a rapid response such as changes in ion channel permeability and post-translational modifications of proteins, while late preconditioning involves gene activation and protein synthesis [33–36]. In most experiments, the protective effects on the brain need hours and sometimes days to fully manifest; thus, delayed preconditioning has been studied more often. To date, studies on the mechanisms of both cardiac and cerebral preconditioning at the molecular, cellular and tissue levels span more than 20 years [37, 38]. These studies have shown that the neuroprotective mechanisms of preconditioning involve a series of molecular regulatory pathways. The major signaling pathways that participate in the brain in ischemic/hypoxic preconditioning are briefly summarized as below:
Activation of the N-methyl-D-aspartate (NMDA): One of the important neuroprotective mechanisms of brain ischemic preconditioning is through mild activation of NMDA receptors [39]. Consistent exposure of cortical cells to low levels of glutamate or NMDA to induce NMDA-receptor activation has a preconditioning effect [26].
Regulation of protein kinase cell signaling transduction pathways such as mitogen-activated protein kinases (MAPK), and Akt and protein kinase C (PKC) pathways. The MAPK signaling pathway plays a significant role in cerebral ischemic injury. Two MAPK pathway kinases, extracellular signal-regulated kinase (ERK) and c-Jun N-terminal protein kinase (JNK) are generally known to promote cell survival and proliferation (ERK) or induce apoptosis (JNK). The roles of MAPK cascades in regulating neuronal death and survival depend on the types of cells and the magnitude and timing of insults. Neuronal apoptosis and cerebral ischemia both induce the robust activation of MAPK cascades. However, preconditioning-induced neuroprotection against ischemia is mediated by MAPK-activated protein kinases including ERK, JNK and p38 [40, 41]. Akt activation also contributes to ischemic tolerance. Akt is transiently activated in non-preconditioned rats after ischemia/reperfusion, but the activation is long-lasting in the preconditioned animals, which contributes to the inhibition of neuronal apoptosis and prevention of infarction enlargement induced by preconditioning [42]. Furthermore, the neuronal protection of hypoxic/ischemic preconditioning requires sequential activation of the vascular endothelial growth factor (VEGF) receptor and Akt phosphorylation [43, 44]. In addition to the MAPK and Akt pathways, PKC pathways also play critical roles in ischemic tolerance, as ischemic preconditioning increases adenosine levels and initiates a series of intracellular signaling events via G-protein coupled receptor signaling, leading to activation of phospholipases, production of diacylglycerol, calcium influx and PKC activation [45]. Further studies report that preconditioning is associated with subcellular translocation of specific subtypes of PKC, suggesting εPKC is essential for the induction of ischemic tolerance [25, 46]. εPKC is found to target mitochondrial KATP channel, and treatment with the KATP channel openers diazoxide or pinacidil before global ischemia attenuates hippocampal neuronal injury [47].
Regulation of lipid raft/caveolae signaling. Recent progress in membrane biology has proposed roles for the calveolae that reside within specialized membrane microdomains known as lipid rafts in many cellular events including apoptotic cell death. Several isoforms of proteins, caveolins-1, 2, 3 with size ~ 22 kDa, are found to reside in the flask-shaped caveolae. The responses of caveolin-1 and -2 to ischemic stimulation appear to be dependent on ischemic duration during the cerebral ischemic injury. The expression of caveolin-1 in the ischemic penumbral and core areas is significantly down-regulated after rats are exposed to 1 hour of focal cerebral ischemia [48]. However, a remarkable increase of caveolin-1 and caveolin-2 was reported in the vascular endothelial cells at 48hours, 1-week, and 2-weeks after 3 hours of ischemia [49]. Caveolin-1 plays a role in protecting neuronal cells from oxidative injury by modulating nitric oxide (NO) production; Cav-1 KO ischemic brains showed impaired angiogenesis and increased apoptotic cell death in an experimental ischemic stroke model [49]. It appears to participate in the hypoxic tolerance in neuroblastoma cells [50]. Caveolins are also involved in signal transduction by regulating the functions of caveolae-associated signaling molecules such as PKC and tyrosine kinase-associated receptors, as well as endothelial nitric oxide synthase (eNOS) [51–55]. In ischemic preconditioning of the heart, caveolin-1 and caveolin-3 appear to interact with proapoptotic p38MAPKα and anti-apoptotic p38MAPKβ, respectively, and such interactions function as a molecular switch for the conversion of an ischemia-reperfusion–induced cell death signal into a preconditioning-induced survival signal [56].
Activation of transcription factors. Currently several transcription factors are known to be sensitive to regulation by hypoxia/ischemia and participate in ischemic/hypoxic preconditioning, including activating protein 1 (AP1) [57], cAMP response element-binding protein (CREB) [58, 59], nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) [60], hypoxia-inducible factors (HIF) [61, 62], early growth response 1 and the redox-regulated transcriptional activator SP1 [63]. Among the transcription factors, HIF isoforms have garnered the most experimental support in the transcriptional regulation of ischemic/hypoxic preconditioning so far [64], therefore, a more detailed discussion follows. HIF is a heterodimer with an unstable α-subunit (HIFα) and a stable β-subunit (HIFβ). The post-translational hydroxylation of HIFα is oxygen-dependent. Under typical oxygen conditions, HIFα becomes hydroxylated at 2 prolyl residues by members of the prolyl-4 hydroxylase domain family. Under hypoxic conditions, HIFα cannot degrade and subsequently accumulates and transactivates about 100 genes [65]. These genes encode proteins involved in oxygen transport (erythropoietin), angiogenesis (VEGF and angiopoietin-2), vasomotor control (adrenomedullin and β-adrenergic receptors), cell survival (erythropoietin and VEGF), pH regulation (carbonic anhydrases), and energy metabolism (glucose transporters and glycolytic enzymes). HIF1 has an important role in the complex transcriptional response to ischemic/hypoxic brain damage. Ischemic preconditioning activates HIF1 and its target genes. HIF1 activation is neuroprotective [66], as a neuron specific HIFα deletion exacerbates ischemic brain injury [67]. However, HIF1α knockdown mice maintain their ability to develop ischemic tolerance as a result of ischemic preconditioning, suggesting that HIF1α might not be essential for conferring robust neuroprotection [67]. The precise mechanisms of transcriptional regulation of HIF remain to be further clarified.
In summary, ischemic/hypoxic preconditioning is modulated by a complex cellular regulatory process that involves multiple cellular signaling pathways and leads to enhanced tolerance to ischemia/hypoxia. The changes associated with protein kinase cell signaling pathways and transcriptional and translational pathways adapt the brain to a relative homeostatic state that allows it to be refractory to lethal ischemic insults. Despite the fact that ischemic pre- and postconditioning are conducted at distinct time courses, both share some common protective mechanisms; therefore, the elucidation of the cell signaling pathways in ischemic preconditioning may shed light on those of ischemic postconditioning.
THE EVOLUTION OF ISCHEMIC PRECONDITIONING TO RAPID POSTCONDITIONING IN HEART ISCHEMIA
As discussed above, preconditioning refers to a brief ischemia that does not cause injury to the ischemic organ but prevents ischemic injury caused by a subsequent, prolonged ischemia [3, 68]. It has served as a powerful tool in understanding the endogenous mechanisms by which the ischemic organs are protected [68].
Unlike ischemic preconditioning, which has been studied for decades, ischemic postconditioning is a relatively novel concept [2] (Fig.1). The term ischemic postconditioning was originally adopted to contrast with that of ischemic preconditioning [69, 70]. It was also believed to be derived from the concept of partial or controlled reperfusion [70]. However, more than 50 years ago, Sewell and colleagues have reported that intermittent reperfusion, which equates to the current concept of ischemic postconditioning, abolished fibrillation [71], and, such protection was once observed in a clinical case reported in 1994 in which the patient was subjected to acute myocardial ischemia [72]. Although this protective phenomenon was repeated by Na and colleagues in 1996 [73], who found that postconditioning was as effective as preconditioning in preventing ventricular fibrillation in cats,, only after Z.Q Zhao and colleagues published their first study on ischemic postconditioning in a myocardial ischemic model have postconditioning studies thrived [2]. The protective effects of postconditioning in myocardial ischemia has been confirmed by many other studies [74] including studies using models of rats [75], mice [76], rabbits [77] and pigs [78], as well as in vitro settings [79]. The intensive research on postconditioning in the heart has led to clinical trials. Statt et al. reported that postconditioning performed by 4 cycles of a 1 minute reperfusion/1 minute occlusion via an angioplasty balloon, reduced acute myocardial injury in patients who had ongoing myocardial infarction [80]. Taken together, the concept of ischemic postconditioning in myocardial research has been well-established.
FROM THE HEART TO THE BRAIN: RAPID ISCHEMIC POSTCONDITIONING PROTECTS AGAINST CEREBRAL ISCHEMIA
As previously discussed, ischemic postconditioning was initially defined in the field of myocardial ischemia research as a series of brief mechanical occlusions and reperfusions [2, 73]. Since then, its protective effects have been tested in other organs with ischemia/reperfusion injury, including cerebral ischemia (Table 1). The mechanisms of ischemic brain injury share many similarities to those of myocardial ischemic injury. For instance, both the brain and the heart are subjected to reperfusion injury after ischemia and free radical products play a critical role. In addition, both apoptosis and necrosis occur in the ischemic brain and heart [81–83] and similar cell-signaling pathways contribute to cell death. These pathways include the Akt/PKB survival pathways [84], the MAPK pathways [85], the PKC pathway [86, 87], cytochrome c/caspase-mediated apoptotic pathways [88], and calpain-mediated necrotic pathways [89]. Furthermore, ischemic preconditioning reduces ischemic damage in both the brain and the heart [3, 68]. Based on these similarities, testing whether postconditioning also protects against cerebral ischemia was a logical and intriguing idea. We have summarized the major studies of postconditioing against cerebral ischemia in Table-1.
Table 1.
Summary of major postconditioning studies in cerebral ischemia
| Reference | Species | Model | Postconditioning | Comments on Mechanisms |
|---|---|---|---|---|
| [1] | Rat | Focal | Rapid: stuttering reperfusion | Inhibit Free radical generation and apoptosis stained by TUNEL |
| [90] | Rat | Focal | Rapid: intermittent reperfusion, or a single period of ischemia. | Akt, but not ERK and P38 activity, contributes to postconditioning. However, these pathways contribute to preconditioning’s protection. Combination of postconditioning plus preconditioning offers no synergistic effect. |
| Rat | Culture OGD | Rapid: a single period of OGD | ||
| [146] | Rat | Focal | Rapid: stuttering reperfusion | It improved neurological and enhanced SOD and Bcl-2 expression, but inhibitd cytochrome c release, caspase-3 activity, DNA fragmentation attenuated. |
| [91] | Rat | Focal | Rapid: stuttering reperfusion | Improved CBF at 30 min after CCA release. Combination of preconditioning with postconditioning offered no additional protection. |
| [131] | Rat | Focal | Rapid: stuttering reperfusion | Akt pathway contributed to its protection; both MAPK and PKC pathways are involved in the protective mechanisms. |
| [95] | Rat | Global | Rapid: stuttering reperfusion | Spatial learning and memory was improved; It also improved CBF and blocked cytochrome c release. |
| [94] | Mouse | Global | Rapid: stuttering reperfusion | Improved behavioral tests. Wortmannin, an Akt inhibitor, attenuated the beneficial effect of postconditioning. |
| [147] | Rat | Global | Rapid: stuttering reperfusion | Enhanced glutamate transporter-1expression |
| [148] | Rat | Global | Rapid: stuttering reperfusion | Upregulated Glutamine synthetase |
| [149] | Rat | Focal | Rapid: stuttering reperfusion | Suppressed ER stress-induced apoptosis and enhanced Akt activity |
| [150] | Rat | Focal | Rapid: stuttering reperfusion | Mitochondrial KATP channel is invovled |
| [151] | Rat | Focal | Rapid: 10 min ischemia | Na+/Ca2+ exchanger (NCX) 3 is essential |
| [96] | Rat Hipp slice | 30 min OGD | Rapid g: A single period of OGD or DHPG application | mGlu1 and mGlu5 antagonists and PI3K/Akt inhibitor abolish postconditioning’s protection. Combination of pre and postconditioning has no additive protection. |
| [105] | Rat | Focal | Rapid sevoflurane postconditioning | Increased Akt, Bcl-2, inhibited p53 and Bax |
| [139] | Rat cortical culture | OGD model | Rapid: OGD | Akt activity is essential |
| [106] | SH-SY5Y cells | OGD | Rapid: Isoflurane, sevoflurane or desflurane | Increased GSK3beta phosphorylation |
| [103] | Rat cortical slices | 15 min OGD | Rapid: isoflurane | KATP channel blockers inhibited postconditioning’s protection. |
| Rat | Focal | Rapid: isoflurane | ||
| [104] | Rat | Focal | Rapid: Sevoflurane | Mitochondrial KATP channel is essential for the protection. |
| [126] | Rat | Focal | Remote: lime ischemia/reperfusion | Limb postconditioning blocks ischemic injury by modulating protein synthesis and nerve activity. |
| [128] | Rat (pups) | Hypoxia/ischemia | Remote: lime ischemia/reperfusion | Promoted Akt phosphorylation and inhibited Bax expression |
| [127] | Rat | Focal | Remote: limb ischemia/reperfusion | Inhibited ROS and deltaPKC activity |
| [107] | Rat | Global | Delayed: 3-NP and s-NE, or a single period of ischemia, performed 2 days after ischemia | Cycloheximide, inhibitor of protein synthesis, blocks the protective effect of postconditioning. |
| [152] | Rat | Global | Delayed: bradykinin applied 2 days post-ischemia | SOD activity increased 5h after postconditioning. |
| [153] | Rat | Global | Delayed: bradykinin applied 2 days post-ischemia | Ischemia caused increases in MnSOD activity and decreases in CuZnSOD activity; postconditioning inhibits these changes. |
| [98] | Rat | Global | Delayed: 3min ischemia 2 days later | Inhibited cytochrome c release, Bax translocation, and promoted Akt |
| [99] | Rat | Global | Delayed: kanate 48h post- ischemia | Not investigated |
| [135] | Rat | Global | Delayed: 5 min ischemia 2 days later | Inhibited Bax but enhanced SOD |
| [101] | Tree shrew | Focal | Delayed: 3 cycles of 5 min ischemia at 4 h post-stroke | Inhibited TLR4 expression |
| [100] | Rat | Focal | Delayed: performed 3 or 6 h post-stroke | Improved CBF and metabolisms |
| [102] | Mouse | Focal | Delayed: hypoxia conducted at 1 and 5 days post-stroke | Enhanced hypoxia-inducible factor-1α and its target genes, erythropoietin and adrenomedullin |
Rapid postconditioning is induced immediately or a few minutes after reperfusion, and it is the main form of postconditioning in heart research. Therefore, we and others sought to determine whether rapid postconditioning is effective against focal cerebral ischemia [1, 90]. Our initial study reported that the magnitude of the reduction in infarct size after rapid postconditioning is a function of ischemic severity –meaning, it is less effective with longer periods of ischemia [1, 69]. We found that rapid postconditioning reduced infarct size by ~80%, ~51%, and ~17%, respectively, after a 15, 30 or 60 minute CCA occlusion combined with permanent dMCA occlusion. Following this study we compared the impact of cycle number and duration of reperfusion/occlusion on the protective effect of rapid postconditioning using the ischemic model of a 30 minute bCCA occlusion combined with permanent dMCA occlusion [91]. Our results showed that rapid postconditioning conducted 10 to 30 seconds after reperfusion reduces infarct size, but not when initiated at 3 minutes after reperfusion. Taken together, this study suggests that the protective effects of rapid postconditioning depend on the number and duration of cycles of reperfusion and occlusion and the onset time of postconditioning [91].
In our experiments robust protection by rapid postconditioning is observed only in moderate or mild brain ischemia in which the bilateral CCA occlusion time was 15 or 30 minutes [1], while protection is mild in more severe ischemia (60 min of CCA occlusion). Xing and colleagues also found that rapid postconditioning reduces infarction by only 16% and 12%, 1 and 3 days after stroke, respectively, in a MCA suture occlusion model [92]. Therefore, in our studies rapid postconditioning does not appear to generate the same level of protection in the ischemic brain as has been shown in the ischemic heart [2]. However, we have not tested if optimized conditions as defined in our recent study [91] afford better protection in a longer period of ischemia, and Xing and colleagues did not compare the protective effect with different postconditioning parameters. Therefore, we cannot exclude the possibility that the relatively weak protection observed is due to the use of sub-optimal parameters.
In contrast to our finding, Pignataro and colleagues demonstrated very strong protection with postconditioning in a severe focal ischemic model [90, 93] where the MCA was occluded for 100 minutes. In their study postconditioning with 10 minutes of occlusion started after 30 minutes reperfusion offered no protection, however, postconditioning with 3 cycles of 5 minute reperfusion/5 minute occlusion reduced infarction by 38%, and 1 cycle of 10 minute occlusion initiated after 10 minutes of reperfusion reduced infarct size by ~70 % compared to rats subjected to control ischemia. Again, this study suggests that onset time of postconditioning is critical in generating optimal neuroprotective effects.
A few groups have studied the protective effects of rapid postconditioning on transient global cerebral ischemia. Rehni and Singh have shown that rapid postconditioning attenuates behavioral deficits after global ischemia in mice [94]. However, they did not report how rapid postconditioning affects neuronal loss. Nevertheless, Wang et al. showed that rapid postconditioning applied immediately after reperfusion attenuates neuronal death in both the hippocampus and the parietal cortex after 10 minutes of transient global ischemia [95].
The protective effects of postconditioning were further studied using in vitro models. Scartabelli et al. showed that rapid postconditioning with brief oxygen glucose deprivation (OGD) blocks ischemic injury in rat organotypic hippocampal slice cultures [96]. In this study postconditioning with 3 minutes of OGD started at 5 minutes after reperfusion reduced cell injury by about 40%. In addition, Pignataro et al. found that postconditioning with oxygen glucose deprivation (OGD) reduced neuronal death in cortical culture [90]. Postconditioning with 30 minutes of OGD conducted at 10, 30 or 60 minutes after reperfusion did not reduce the cell death caused by 120 minutes OGD; however, with 10 minutes OGD initiated after 10 minutes of reperfusion, postconditioning robustly blocked cell death [90]. This study suggests that the onset time and duration of postconditioning are critical for generating neuroprotection for in vitro models, as seen in animal models.
DELAYED ISCHEMIC POSTCONDITIONING ATTENUATES BRAIN INJURY AFTER STROKE
Rapid ischemic postconditioning, which interrupts early reperfusion after stroke, reduces infarction. However, the extremely short therapeutic time windows of rapid postconditioning, which spans a few seconds to minutes after reperfusion, may hinder its clinical translation. In the case of heart injury, ischemic postconditioning must be induced immediately after reperfusion to generate protection; however, with brain injury the possibility still exists that delayed postconditioning performed at later time points could be protective. First, delayed neuronal death is usually observed after transient global ischemia. It may take 2 to 3 days for ischemic brain tissues to die, especially for those neurons in the hippocampus. Neurons in the ischemic penumbra after focal ischemia may also die in a delayed pattern. Second, like ischemic preconditioning, which has at least two therapeutic time windows (rapid preconditioning performed 1 to 3 hours before stroke onset and delayed preconditioning induced 1 to 7 days before stroke onset) [9, 17, 68], postconditioning may protect against cerebral ischemia at multiple time windows. Third, ischemic postconditioning with different paradigms has different therapeutic time windows. The fact that ischemic postconditioning with certain parameters in the delayed time window is not protective does not exclude the possibility that postconditioning with other parameters at later time points could be protective.
Delayed neuronal death occurs 2 to 3 days after transient global ischemia, therefore, a transient global ischemia model might be the most ideal to test whether delayed ischemic postconditioning is protective. In a global ischemia model with induction by 4-vessel occlusion, neuronal death was not detected by 2 days post-ischemia. Using this model, Burda and colleagues found that delayed postconditioning with a single 5 minute ischemia induced 2 days after reperfusion reduced neuronal death in the hippocampus by about 94% when measured 7 days after global ischemia, a time point at which neuronal death is considered to be matured [97]. Other groups have confirmed this protective phenomenon in similar global ischemia models[98, 99].
We tested the hypothesis that delayed postconditioning with different parameters reduces infarct size in focal ischemia. Our results showed that delayed postconditioning performed at 3 and 6 hours after stroke robustly reduces infarct size, with the strongest protection achieved by delayed postconditioning with 6 cycles of 15 minute occlusion/15 minute release of the ipsilateral CCA executed from 6 hours. We found that this delayed postconditioning attenuates reduction in 2-[(18)F]-fluoro-2-deoxy-D-glucose (FDG)-uptake, resulting in improved metabolism and reduced edema and blood brain barrier leakage. A prerequisite for performing postconditioning is that reperfusion must be first achieved; reperfusion in ischemic stroke patients is usually achieved by tissue plasminogen activator (tPA) application. Nevertheless, t-PA may have side effects that worsen ischemic injury. Thus, we were very interested in testing whether delayed postconditioning counteracts the exacerbating effects of t-PA. Our results showed that delayed postconditioning can reduce the infarct size increased by t-PA application [100].
Most recently, Feng and colleagues demonstrated that delayed postconditioning with 3 cycles of 5 min ischemia performed 4 h after thrombotic cerebral ischemia[101], which supports our results of delayed postconditioning in focal ischemia. Most recently, Leconte and colleagues tested an audacious hypothesis that delayed hypoxic postconditioning performed 5 days after stroke in mice, and 14 hours after OGD in culture, would protect against neuronal injury [102]. Focal ischemia was induced by 1 hour of MCA suture occlusion in mice. Delayed hypoxic postconditioning was performed by chronic intermittent hypoxia starting either 1 day or 5 days post-ischemia and lasting to day 43 when the animals were euthanized. Although both the 1 and 5 day time points offered no protection on infarction in the ischemic region, delayed postconditioning starting at 5 days attenuated the delayed thalamic atrophy measured at 43 days. In addition, the in vitro study showed that hypoxic postconditioning performed 14 hours after OGD reduces neuronal death measured 48 hours after OGD [102]. Taken together, this study further confirmed the protective effects of delayed postconditioning.
PHARMACOLOGICAL POSTCONDITIONING
Similarly to ischemic preconditioning, a major challenge for the clinical translation of ischemic postconditioning is the high risk of applying an ischemic event to an organ that has already been subjected to a severe ischemia. Other challenges include the availability of the artery leading to the ischemic area, and whether reperfusion can be achieved in a timely manner. To avoid these problems, it would be ideal to find drugs that match the actions of ischemic postconditioning, such as anesthetics or drugs that mimic a brief ischemia, or drugs that share common mechanisms of ischemic postconditioning. Thus, in recent years the protective effects of pharmacological postconditioning have been explored.
As a means to induce pharmacological postconditioning, the anesthetic isoflurane, which had already been used to induce preconditioning to protect against brain ischemia, was a good candidate to test. In a suture MCA occlusion model, Lee et al. conducted postconditioning by maintaining 2% isoflurane for 60 minutes starting at the time the MCA occluding suture was removed. In this study, no isoflurane was used during the MCA occlusion. For rats receiving control ischemia, only 1 minute of isoflurane was used for the MCA suture removal [103]. They found that isoflurane postconditioning robustly reduces brain infarction and attenuates neurological deficits. Lee et al. also found that rapid isoflurane postconditioning protects against ischemic injury in slice organ cultures [103] in which OGD was maintained for 15 minutes and postconditioning was instituted by application of isoflurane after OGD. They found that the protective effects of isoflurane postconditioning are dependent on the duration and concentration of isoflurane exposure. Lastly, isoflurane postconditioning started at 0 or 10 minutes, but not greater than 30 minutes post reperfusion, reduced cell damage, suggesting a similar therapeutic time window to ischemic postconditioning [103]. In addition to isoflurane, sevoflurane can also be used as postconditioning in both in vivo stroke and in vitro OGD model[104, 105]. Interestingly, postconditioning with isoflurane, sevoflurane or desflurane inhibited cell death in the SH-SY5Y cells, which are human-like neurons[106].
Rapid postconditioning with brief OGD also blocks ischemic injury in rat organotypic hippocampal slice cultures [96]. Results showed that postconditioning with 3 minutes of OGD started at 5 minutes after reperfusion reduced cell injury by about 40%. In the same study, the protective effects of postconditioning were also induced by adding a low dose of the pharmacological agent, 3,5-dihydroxyphenylglycine (DHPG, a group 1 mGlu receptor agonist), 5 minutes after reperfusion and incubating for 30 minutes. It has been reported that high doses of DHPG exacerbate, while low doses inhibit, neuronal injury [96].
Pharmacological treatments have also been used to mimic delayed postconditioning conducted at 2 days after reperfusion in a rat global ischemia model [97]. In these studies, global ischemia was induced by 4-vessel occlusion, and pharmacological postconditioning was conducted by injection of 3-nitropropionic acid (3-NP), norepinephrine or bradykinin [107, 108]. Delayed postconditioning at 2 days with an intraperitoneal injection of norepinephrine, bradykinin or 3NP resulted in increased neuronal survival after global ischemia [97] The protective effects in these cases are comparable to those induced by delayed postconditioning with 5–6 min of ischemia [97]. In support of these studies, a most recent study also reports that kanate application, one most recent study reports that kanate application 2 days post-ischemia inhibited hippocampal neuronal injury after global ischemia[99].
CONVENTIONAL ISCHEMIC POSTCONDITIONING IS EXTENDED TO REMOTE POSTCONDITIONING
As discussed above, classical pre- or postconditioning is conducted in the same organ that receives prolonged ischemia [3, 17, 109, 110]; its clinical application is limited by the risk of applying an additional ischemia to a vital organ, such as the brain or the heart. The concept of conventional ischemic pre- and postconditioning has been extended not only to pharmacological pre- and postconditioning, but also to remote ischemic pre- and postconditioning [111]. Previous studies have shown that remote preconditioning performed in limbs [112, 113], in a kidney [111], or in mesentery [114] protects against a subsequent ischemia in the heart. Similarly, many studies have shown that remote postconditioning reduces ischemic injury in the heart[115–117].
Although the protective effects of remote pre- and postconditioning have been studied extensively in the research field of myocardial ischemia [118], they have received much less attention in the field of stroke. In the area of remote preconditioning against brain ischemia, there are only a few studies demonstrating that limb ischemia reduces delayed neuronal death in the hippocampal CA 1 region [119–124]. More recently, we provided solid evidence that limb remote preconditioning reduces infarct size in a focal ischemia model in rats. Our results showed that when limb ischemia is conducted immediately, 12 hours, or 48 hours before cerebral ischemia, limb preconditioning protects against focal cerebral ischemia in rats. We found that the protective effects of remote preconditioning could be induced in the single hind limb ipsilateral to the ischemic hemisphere.
Based on this study in our own laboratory and other studies showing that remote postconditioning reduces heart injury after ischemia [125], we further tested our hypothesis that remote postconditioning conducted in the ipsilateral hind limb protects against focal ischemia. From this study we were able to demonstrate that remote postconditioning conducted immediately after reperfusion markedly reduces infarct size[126]. Stroke was generated in male rats by a permanent occlusion of the left distal middle cerebral artery combined with a 30 minute occlusion of the bilateral common carotid arteries (CCA). After CCA release, remote postconditioning was generated by 3 cycles of a 15 minute occlusion/15 minute release of the left hind femoral artery. We found that rapid remote postconditioning performed immediately after CCA release reduces infarction by 67% measured at 2 days after stroke. In addition, we found that, delayed remote postconditioning initiated as late as 3 hours, though not 6 hours, still robustly reduces infarction by 43% 2 days after stroke. Remote postconditioning’s protective effect was abolished by injecting the protein synthesis inhibitor, cycloheximide, and the afferent nerve blocker, capsaicin, suggesting that remote postconditioning blocks ischemic injury by modulating protein synthesis and nerve activity [126]. These results suggest that remote postconditioning provides a wide therapeutic time window for clinical translation. Our studies are supported by other recent reports showing that limb ischemic postconditioning is neuroprotective in neonatal and adult rats[127, 128].
POSTCONDITIONING OFFERS LONG-TERM PROTECTIVE EFFECTS AND IMPROVES BRAIN FUNCTION AFTER STROKE
Whether postconditioning provides lasting protection and preserves brain function has been studied. This is another critical issue demanding confirmation because some neuroprotectants such as post-ischemic hypothermia [129] and rapid ischemic preconditioning [68] have been shown to provide protection for only a few days after ischemia. In addition, reducing injured brain tissue may not translate into the preservation of neurological function [130].
In a long-term study we found that rapid postconditioning performed immediately after reperfusion reduces lesion size ~40% in rats subjected to ischemia when measured 30 days after ischemia [131]. In addition, using a vibrissae test detecting asymmetrical forelimb usage, we found that rapid postconditioning improves neurological function [131]. In global ischemia rapid postconditioning also improves subject performance on spatial learning and memory in a water maze test 3 weeks after reperfusion [95], suggesting that rapid postconditioning provides long-term protection in global ischemia, which is consistent with its protective effects on neuronal survival.
We found that both rapid postconditioning and delayed postconditioning offer long-term protection and improve functional recovery. Our results showed that delayed ischemic postconditioning conducted at 6 hours after stroke attenuates brain injury and improves the outcomes of behavioral tests up to 2 months using 4 standard methods, including the vibrissae test, postural reflex test, tail hang test, and home cage test [100].
In contrast, our recent study showed that remote postconditioning did not reduce infarct size measured at 2 months after stroke, although the infarction measured at 2 days post-stroke was reduced, suggesting that remote preconditioning in our study only executes transient protection on infarct size. Nevertheless, the same study that remote postconditioning improved the outcome of the behavioral test up to 2 months post-ischemia. The underlying mechanisms of the improved neurological function without infarction remain to be clarified.
COMBINATION OF RAPID POSTCONDITIONING WITH PRECONDITIONING OFFERS NO SYNERGISTIC PROTECTION
The protective mechanisms of preconditioning and postconditioning have been extensively studied in the heart, and results suggest that they have some mechanisms in common. For example, both pre- and postconditioning protect the ischemic organ by enhancing adenosine activity, reducing the products of reactive oxygen species and lipid peroxidation [2, 132], inhibiting JNK/P38 activities [133], and promoting ERK1/2 activity [134]. The above discussed mechanisms regarding postconditioning against cerebral ischemia also share similarities to those of preconditioning in cerebral ischemia. Therefore, the combination study may provide further clues to understanding how both pre- and postconditioning exert their protective effects. In addition, because they may both be feasible in certain clinical settings in which stroke occurrence is predictable, a combination study may help clinicians determine whether a combination of both pre-and postconditioning is an option for clinical translation.
Our laboratory recently compared the protective effects of rapid and delayed preconditioning to those of postconditioning, and studied the protective effects of postconditioning combined with either rapid or delayed preconditioning [91], which refers to two therapeutic time windows well-defined for preconditioning. Rapid preconditioning in rats was induced by transiently occluding the left dMCA for 15 minutes at 60 minutes before stroke [91], while delayed preconditioning was induced by occluding the left dMCA for 5 or 15 minutes at 3 days before the stroke. Postconditioning of 10 cycles of 10 second occlusion/10 second reperfusion was conducted immediately after reperfusion. We found that the protective effects of postconditioning are comparable to those of rapid preconditioning, but are less effective than delayed preconditioning. We further addressed whether postconditioning plus preconditioning can generate a synergistic effect [91]. We found that postconditioning combined with either rapid or delayed preconditioning provides no additional reduction in infarction. Our results are consistent with a previous study where Pignataro and colleagues reported that the protective effects of postconditioning are comparable to those of delayed preconditioning in a suture MCA occlusion model in rats, while a combination of postconditioning with preconditioning provided no greater protection [90].
In the above combination studies, both pre- and postconditioning were induced by a similar modality: a brief ischemia, or a series of brief ischemia. These scenarios offered no synergistic protection. However, combining two different modalities, for example, ischemic postconditioning with hypoxic preconditioning or with 3-NP preconditioning, or vice versa, may provide greater protection, and this needs further study.
PROTECTIVE MECHANISMS OF POSTCONDITIONING AGAINST STROKE
Current studies about the underlying protective mechanisms of postconditioning focus on rapid ischemic postconditioning (Table-1). Since rapid ischemic postconditioning interrupts early reperfusion, its protective effects must be closely associated with changes in cerebral blood flow (CBF) after reperfusion, and with subsequent events, such as free radical production, endothelial function, and changes in BBB integrity and inflammation that occur due to interrupted CBF. In our studies, we first confirmed whether rapid postconditioning attenuates the hyperemic response induced by reperfusion, and whether it mitigates hypotension thereafter. CBF was measured by a laser Doppler probe in the penumbra in rats subjected to 15 or 30 minutes of bilateral CCA occlusion combined with permanent MCA occlusion [1, 91]. We detected a clear hyperemic response after reperfusion in rats subjected to 15 minutes occlusion, and CBF was recovered to pre-ischemic levels in rats with 30 minutes occlusion [1, 91]. We showed that rapid postconditioning with mechanical interruption results in CBF changes, and CBF at 30 minutes after reperfusion was improved [91]. Wang and colleagues confirmed this effect in a global ischemia model [95].
Next, we examined whether rapid postconditioning attenuates ROS production and apoptosis, as early reperfusion is considered to cause significant ROS products leading to apoptosis. We found that rapid postconditioning profoundly attenuated the amount of superoxide at 30 minutes after reperfusion in a model of 30 minutes CCA occlusion plus permanent MCA occlusion [1]. Consistent with our findings, Xing and colleagues reported that rapid postconditioning attenuates lipid peroxidase levels in a focal ischemia model [92]. Moreover, delayed postconditioning performed 2 days after global ischemia increased the activity of antioxidant enzymes, including superoxide dismutase and catalase [108] Furthermore, we showed that rapid postconditioning blocked terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling (TUNEL) positive staining, a marker of apoptosis, in the penumbra 2 days after stroke [1]. Wang and colleagues further showed that rapid postconditioning reduces cytochrome c release from the mitochondria to the cytosol, a critical cascade for apoptosis induction [95]. Most recently, delayed postconditioning performed 2 days after global ischemia, and remote limb postconditioning were also reported to inhibit ROS activity and promote SOD expression[127, 135]. Taken together, these data suggest postconditioning may reduce ischemic injury by blocking ROS activity and apoptosis.
The protective effects of rapid postconditioning on inflammation after stroke have also been explored. Rapid postconditioning inhibits myeloperoxidase (MPO) activity, an indicator of leukocyte accumulation, IL-1β and TNF-α mRNA expression, and ICAM-1 protein expression in the ischemic cortex at 24 hours after ischemia [92]. In addition, protein levels of the key innate immune response mediator, TLR-4 receptor, were inhibited by delayed postconditioning performed 4 hours post-ischemia[101]. These results suggest that both rapid and delayed postconditioning may produce an anti-inflammatory effect.
Consistent with the improvement in CBF after rapid postconditioning, we have reported that delayed postconditioning enhances glucose uptake or metabolism as detected by micro PET imaging [100]. In addition, delayed postconditioning attenuates edema formation and blood brain barrier (BBB) leakage.
Multiple pathways are involved in neuronal death after stroke, including PKC pathways, MAPK pathways, and the PI3K/Akt Pathway (Fig.2). These pathways contain both pro- and anti-apoptotic signals; it is their balance that decides the fate of ischemic neurons after stroke. In the PKC pathways, at least 11 isozymes of the PKC family exist, including δPKC and εPKC [136]. While δPKC activity usually leads to cell death [87], εPKC activity promotes neuronal survival [86]. PKC isozymes differ as to their intracellular location and function, and their activities are regulated by their cleavage form, phosphorylation, and subcellular location. Our results showed that rapid postconditioning has no affect on the protein levels of total δPKC, but blocks the increase in levels of its cleaved form at 1 hour after stroke in the penumbra, which is indicative of δPKC activity [131]. Although rapid postconditioning had no effect on phosphorylated δPKC (thr 505) levels, which were decreased by 24 hours after stroke onset, it strongly inhibited decreases in phosphorylated εPKC after stroke. Thus, rapid postconditioning may reduce ischemic damage by inhibiting the worsening effect of δPKC while promoting a beneficial effect of εPKC activity [131]. A recent study also showed that remote limb ischemic postconditioning inhibited δPKC activity [127].
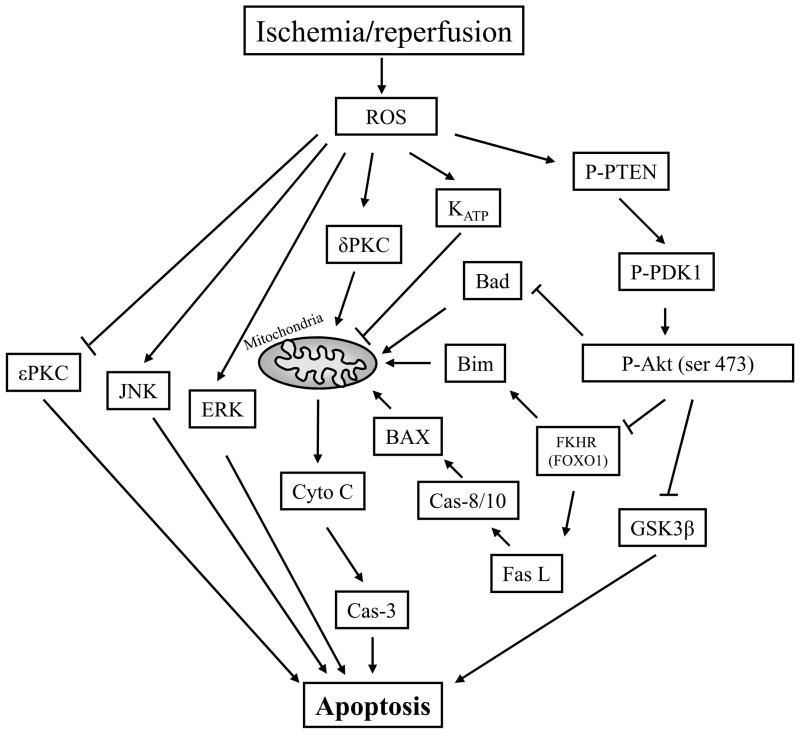
Fig. 2.
The cell signaling pathways involved in ischemic postconditioning. Reactive oxygen species (ROS) eruption after reperfusion causes dysfunction of the Akt cell signaling pathway, increases sσPKC activity while decreases εPKC activity. ROS also activates JNK and ERK activity. Furthermore, the PI3K/Akt inhibition directly results in dephosphorylation of GSK3β and Bad, and indirectly causes cytochrome c release from the mitochondria and caspases activity. Akt inhibition also results in activation of the transcription factor, FKHR (FOXO1), which increases Fas ligand and Bim expression. ROS, reactive oxygen species; Cyto C, cytochrome c; Cas-3, caspase-3; GSK 3 b, glycogen synthase kinase 3b; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; P-Akt, phosphorylated Akt; P-PTEN, phosphorylated phosphatase and tensin homolog deleted on chromosome 10; P-PDK1, phosphorylated phosphoinositide-dependent protein kinase-1; JNK, c-Jun N-terminal kinases; ERK, extracellular signal-regulated kinases; KATP channels, ATP-sensitive potassium channels.
Ischemic injury and neuronal survival are modulated by the MAPK pathways, including the extracellular signal-regulated kinase 1/2 (ERK1/2), P38, and c-Jun N-terminal kinase (JNK) pathways [137]. As we have reviewed, JNK and p38 appear to be clearly detrimental after stroke, and their inhibition blocks apoptosis in many neuronal death paradigms [137]. However, ERK1/2’s activity is involved in both neuroprotection as well as injury exacerbation [137]. In general, most studies agree that ERK1/2 phosphorylation is transiently increased after stroke, suggesting increases in ERK1/2 activity are induced by ischemia/reperfusion. Such an increase, however, appears to be a double-edged sword that involves both the beneficial effects of growth factors, estrogen, preconditioning, and hypothermia on the ischemic brain, but also the promotion of inflammation and oxidative stress, and, when inhibited, a reduction in ischemic damage [137]. Given such incongruity, we were very interested in studying which changes in ERK1/2 activity are involved in the protective effects of postconditioning.
In our pilot study, ERK1/2 phosphorylation (P-ERK1/2) was increased from 1 to 24 hours after stroke, and rapid postconditioning reduced its level in the penumbra [131]. Our results imply a detrimental role for P-ERK1/2 after ischemia, but we did not determine whether or not its inhibition contributed to the protection of rapid postconditioning in our report. Our observation conflicted with Pignataro and colleagues’ study showing that rapid postconditioning enhances ERK1/2 phosphorylation [90]. Furthermore, they showed that increases in P-ERK1/2 may be unrelated to the protective effect of rapid postconditioning because U0126, the antagonist of ERK1/2, did not block the protective effects of rapid postconditioning. More detailed experiments will be required to resolve the discrepancy between our results and those of Pignataro et al.
The Akt pathway plays a critical role in neuronal survival after stroke (Fig.2). Akt dysfunction results in apoptosis induction, while Akt activity blocks apoptosis by phosphorylating its substrates, including GSK3β, FKHR and Bad. Akt activity is considered to be regulated by phosphorylation, which is modulated by upstream molecular signals, such as PTEN (phosphatase and tensin homologue deleted on chromosome 10) and PDK1 (phosphoinositide-dependent protein kinase-1). Akt activity is increased when phosphorylation of PTEN and PDK1 is improved, and GSK3β (glycogen synthase kinase 3β) phosphorylation supports cell survival [138]. Dephosphorylation of GSK3β leads to its activation and to phosphorylation of β-catenin, which results in β-catenin degradation and apoptosis [138]. We and others found that rapid postconditioning increases both Akt phosphorylation (measured by Western blot) [90, 98, 105, 131, 139] and Akt activity (assayed by in vitro kinase assay) [131]. Furthermore, Akt inhibition by injection of the PI3K inhibitor, LY294002, partially blocks the protective effects of rapid postconditioning [90, 131]. However, rapid postconditioning does not affect phosphorylation of PTEN or PDK1 but it does inhibit an increase in GSK3β phosphorylation. We found that rapid postconditioning blocks β-catenin phosphorylation, but has no effect on total or non-phosphorylated β-catenin protein levels [131]. Taken together, the Akt pathway plays a critical protective role in postconditioning. Our results are further supported by a recent in vitro experiment showing that Akt inhibition abolishes the postconditioning protective effect of OGD and DHPG in hippocampal slice cultures [96].
In addition, KATP channels may also play a critical role in brain injury after stroke. After ischemia, ATP depletion results in the opening of KATP channels, which is critical for the induction of the protective effect of ischemic preconditioning as well as postconditioning in the heart. There are two types of KATP channels that vary by location: sarcolemmal and mitochondrial. Mitochondrial KATP channels have been studied the most as their opening generates an outward current that stabilizes the mitochondrial membrane and blocks cell death. In the same vein, Lee and colleagues reported that both a general channel blocker, glibenclamide, and a mitochondrial channel blocker, 5-HD, abolish the protective effect of isoflurane postconditioning, suggesting that KATP channels may be involved in the protective mechanisms of postconditioning[103].
Compared to traditional rapid postconditioning, little is known about the underlying protective mechanisms of remote postconditioning. Nevertheless, in the heart, accumulating evidence suggests that neural pathways serve as a connection between the remote preconditioned organ and the heart. Wolfrum et al. reported that remote preconditioning with brief mesenteric artery occlusion/reperfusion reduces heart infarction by activating εPKC in rats [140]. This protection was blocked by pretreatment with the ganglion blocker, hexamethonium [140]. Another study showed that sensory nerve stimulation resulting from bradykinin release after remote preconditioning confers a protective effect on the heart; this effect was abolished by hexamethonium [141]. Moreover, inhibition of afferent nerves with capsaicin also abolished the protective effects of remote preconditioning against gastric ischemia when remote preconditioning is conducted in the heart or liver by two-5 minute ischemic occlusions of the coronal or hepatic arteries [142]. Consistent with these findings, we recently demonstrated that capsaicin treatment reverses the protective effects of remote postconditioning, suggesting that afferent nerve pathways may sever a connection between the remote organ or limb, and the ischemic brain [126]. Moreover, we have demonstrated that the protein synthesis inhibitor, cycloheximide, also robustly attenuates the protective effects of remote postconditioning; however, the underlying mechanisms of action are not clear. Cycloheximide is usually used to test the hypothesis that preconditioning protects against ischemic injury via protein synthesis [143]. It is not surprising that a protein synthesis inhibitor would block the protective effects of preconditioning because preconditioning is carried out a few hours to days before ischemia onset [143–145], and preconditioning may have time to stimulate the organ to adapt to a future ischemic event, including protein synthesis. In the case of remote postconditioning, the brain may have no time to synthesize new proteins for neuroprotection because postconditioning is performed immediately after reperfusion. Therefore, why protein synthesis inhibition abolishes the protective effects of remote postconditioning remains elusive. Additionally, recent studies have examined the effects of remote postconditioning on some cell signaling pathways, and reported that remote postconditioning promoted Akt phosphorylation and inhibited Bax protein levels, δPKC activity and ROS production[127, 128], as observed in the protective effects of conventional postconditioning.
SUMMARY, CONCLUSION, AND FUTURE DIRECTION
Extensive studies on the protective effects and underlying mechanisms of ischemic preconditioning have evolved into research focused on rapid, delayed and remote ischemic postconditioning. The concept of rapid ischemic postconditioning against cerebral ischemia is now well-established. It seems to reduce ischemic injury by blocking the overproduction of ROS and lipid peroxidation, and by inhibiting apoptosis. Akt and KATP channel activity also contributes to its protective effects. In addition, there are associated changes in the MAPK pathway, and δPKC and εPKC activities. However, the underlying protective mechanisms of delayed and remote postconditioning remain unclear and require further study.
Because rapid postconditioning is applied immediately following reperfusion, it is able to attenuate the detrimental responses induced by reperfusion, such as free radical products and the associated interruption of various cell signaling pathways. However, delayed postconditioning is applied a few hours, or even a few days after reperfusion; therefore, it may modulate the secondary responses that occur much later after reperfusion injury. For instance, it may attenuate CBF inhibition that occurs at later time points after initial reperfusion, or regulate the inflammatory response, a relatively delayed detrimental event following stroke. Additionally, it may promote angiogenesis and neurogenesis. Future studies will be required to determine exactly how delayed postconditioning affects these late cascades after stroke.
Acknowledgments
The author wishes to thank Ms. Cindy Samos for editorial assistance. This work was partially supported by AHA grant-in-aid (HZ), NIH grant 1R21NS057750-01A2 (HZ), 1R01NS 064136-01A1 (HZ), and RGC-GRF grant 774408 (SJG).
ABBREVIATIONS
- BBB
Blood brain barrier
- bCCA
Bilateral ommon carotid artery
- CBF
Cerebral blood flow
- CREB
cAMP response element-binding protein
- dMCA
Distal middle cerebral artery
- DHPG
3,5-Dihydroxyphenylglycine
- ERK1/2
Extracellular signal-regulated kinase ½
- eNOS
Endothelial nitric oxide synthase
- GSK3β
Glycogen synthase kinase 3β
- HIF
hypoxia-inducible factors
- JNK
c-Jun N-terminal kinase
- KATP channels
ATP-sensitive potassium channel channels
- LAD
Left anterior descending artery
- MPO
Myeloperoxidase
- NFkB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- 3-NP
3-Nitropropionic acid
- NMDA
N-methyl-D-aspartate
- NO
Nitric oxide
- OGD
Oxygen glucose deprivation
- PTEN
Phosphatase and tensin homologue deleted on chromosome 10
- PDK1
Phosphoinositide-dependent protein kinase-1
- PKC
Protein kinase C
- ROS
Reactive oxygen species
- tPA
Tissue plasminogen activator
- TUNEL
Terminal deoxynucleotidyl transferasemediated uridine 5′-triphosphate-biotin nick end labeling
- VEGF
Vascular endothelial growth factor
Footnotes
From preconditioning to postconditioning Heng Zhao, PhD, Department of Neurosurgery, Stanford University School of Medicine, MSLS Bldg., Room P306, 1201 Welch Rd., Room P306, Stanford, CA 94305-5327, Phone: 650-725-7723, Fax: 650-498-4134, hzhao@stanford.edu
References
- 1.Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1114–21. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- 2.Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–88. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 3.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 4.Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–54. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 5.Schaller B, Graf R. Cerebral ischemic preconditioning. An experimental phenomenon or a clinical important entity of stroke prevention? J Neurol. 2002;249:1503–11. doi: 10.1007/s00415-002-0933-8. [DOI] [PubMed] [Google Scholar]
- 6.Schaller B, Graf R, Jacobs AH. Ischaemic tolerance: a window to endogenous neuroprotection? Lancet. 2003;362:1007–8. doi: 10.1016/S0140-6736(03)14446-7. [DOI] [PubMed] [Google Scholar]
- 7.Schaller BJ. Influence of age on stroke and preconditioning-induced ischemic tolerance in the brain. Exp Neurol. 2007;205:9–19. doi: 10.1016/j.expneurol.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Zubakov D, Hoheisel JD, Kluxen FW, et al. Late ischemic preconditioning of the myocardium alters the expression of genes involved in inflammatory response. FEBS Lett. 2003;547:51–5. doi: 10.1016/s0014-5793(03)00667-7. [DOI] [PubMed] [Google Scholar]
- 9.Kuzuya T, Hoshida S, Yamashita N, et al. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993;72:1293–9. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- 10.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–72. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 11.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–51. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 12.Jin LM, Liu YX, Zhou L, et al. Ischemic preconditioning attenuates morphological and biochemical changes in hepatic ischemia/reperfusion in rats. Pathobiology. 77:136–46. doi: 10.1159/000292647. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Liu X, Wan X, et al. Ischemic preconditioning attenuates renal ischemia-reperfusion injury by inhibiting activation of IKKbeta and inflammatory response. Am J Nephrol. 2009;30:287–94. doi: 10.1159/000225928. [DOI] [PubMed] [Google Scholar]
- 14.Dahl NA, Balfour WM. Prolonged Anoxic Survival Due to Anoxia Pre-Exposure: Brain Atp, Lactate, and Pyruvate. Am J Physiol. 1964;207:452–6. doi: 10.1152/ajplegacy.1964.207.2.452. [DOI] [PubMed] [Google Scholar]
- 15.Schurr A, Reid KH, Tseng MT, West C, Rigor BM. Adaptation of adult brain tissue to anoxia and hypoxia in vitro. Brain Res. 1986;374:244–8. doi: 10.1016/0006-8993(86)90418-x. [DOI] [PubMed] [Google Scholar]
- 16.Kitagawa K, Matsumoto M, Kuwabara K, et al. ‘Ischemic tolerance’ phenomenon detected in various brain regions. Brain Res. 1991;561:203–11. doi: 10.1016/0006-8993(91)91596-s. [DOI] [PubMed] [Google Scholar]
- 17.Kitagawa K, Matsumoto M, Tagaya M, et al. ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res. 1990;528:21–4. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- 18.Hashiguchi A, Yano S, Morioka M, et al. Up-regulation of endothelial nitric oxide synthase via phosphatidylinositol 3-kinase pathway contributes to ischemic tolerance in the CA1 subfield of gerbil hippocampus. J Cereb Blood Flow Metab. 2004;24:271–9. doi: 10.1097/01.WCB.0000110539.96047.FC. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Bartels M, Lu A, Sharp FR. Microglia/macrophages proliferate in striatum and neocortex but not in hippocampus after brief global ischemia that produces ischemic tolerance in gerbil brain. J Cereb Blood Flow Metab. 2001;21:361–73. doi: 10.1097/00004647-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Yano S, Morioka M, Fukunaga K, et al. Activation of Akt/protein kinase B contributes to induction of ischemic tolerance in the CA1 subfield of gerbil hippocampus. J Cereb Blood Flow Metab. 2001;21:351–60. doi: 10.1097/00004647-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Dave KR, Lange-Asschenfeldt C, Raval AP, et al. Ischemic preconditioning ameliorates excitotoxicity by shifting glutamate/gamma-aminobutyric acid release and biosynthesis. J Neurosci Res. 2005;82:665–73. doi: 10.1002/jnr.20674. [DOI] [PubMed] [Google Scholar]
- 22.Kawahara N, Wang Y, Mukasa A, et al. Genome-wide gene expression analysis for induced ischemic tolerance and delayed neuronal death following transient global ischemia in rats. J Cereb Blood Flow Metab. 2004;24:212–23. doi: 10.1097/01.WCB.0000106012.33322.A2. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Pinzon MA, Xu GP, Dietrich WD, Rosenthal M, Sick TJ. Rapid preconditioning protects rats against ischemic neuronal damage after 3 but not 7 days of reperfusion following global cerebral ischemia. J Cereb Blood Flow Metab. 1997;17:175–82. doi: 10.1097/00004647-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Atochin DN, Clark J, Demchenko IT, Moskowitz MA, Huang PL. Rapid cerebral ischemic preconditioning in mice deficient in endothelial and neuronal nitric oxide synthases. Stroke. 2003;34:1299–303. doi: 10.1161/01.STR.0000066870.70976.57. [DOI] [PubMed] [Google Scholar]
- 25.Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Perez-Pinzon MA. Epsilon PKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J Neurosci. 2003;23:384–91. doi: 10.1523/JNEUROSCI.23-02-00384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grabb MC, Choi DW. Ischemic tolerance in murine cortical cell culture: critical role for NMDA receptors. J Neurosci. 1999;19:1657–62. doi: 10.1523/JNEUROSCI.19-05-01657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X, Qian Z, Ke Y, Du F, Zhu L. Ginkgolide B preconditioning protects neurons against ischaemia-induced apoptosis. J Cell Mol Med. 2009;13:4474–83. doi: 10.1111/j.1582-4934.2008.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Della-Morte D, Dave KR, DeFazio RA, et al. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson AL, Stone TW. Adenosine preconditions against ouabain but not against glutamate on CA1-evoked potentials in rat hippocampal slices. Eur J Neurosci. 2008;28:2084–98. doi: 10.1111/j.1460-9568.2008.06490.x. [DOI] [PubMed] [Google Scholar]
- 30.Sasaoka N, Kawaguchi M, Kawaraguchi Y, et al. Isoflurane exerts a short-term but not a long-term preconditioning effect in neonatal rats exposed to a hypoxic-ischaemic neuronal injury. Acta Anaesthesiol Scand. 2009;53:46–54. doi: 10.1111/j.1399-6576.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 31.Raval AP, Bramlett H, Perez-Pinzon MA. Estrogen preconditioning protects the hippocampal CA1 against ischemia. Neuroscience. 2006;141:1721–30. doi: 10.1016/j.neuroscience.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Meloni BP, Tilbrook PA, Boulos S, Arthur PG, Knuckey NW. Erythropoietin preconditioning in neuronal cultures: signaling, protection from in vitro ischemia, and proteomic analysis. J Neurosci Res. 2006;83:584–93. doi: 10.1002/jnr.20755. [DOI] [PubMed] [Google Scholar]
- 33.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tejero-Taldo MI, Gursoy E, Zhao TC, Kukreja RC. Alpha-adrenergic receptor stimulation produces late preconditioning through inducible nitric oxide synthase in mouse heart. J Mol Cell Cardiol. 2002;34:185–95. doi: 10.1006/jmcc.2001.1500. [DOI] [PubMed] [Google Scholar]
- 35.Hampton CR, Shimamoto A, Rothnie CL, et al. HSP70.1 and −70. 3 are required for late-phase protection induced by ischemic preconditioning of mouse hearts. Am J Physiol Heart Circ Physiol. 2003;285:H866–74. doi: 10.1152/ajpheart.00596.2002. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko T, Yokoyama K, Makita K. Late preconditioning with isoflurane in cultured rat cortical neurones. Br J Anaesth. 2005;95:662–8. doi: 10.1093/bja/aei228. [DOI] [PubMed] [Google Scholar]
- 37.Cadet JL, Krasnova IN. Cellular and molecular neurobiology of brain preconditioning. Mol Neurobiol. 2009;39:50–61. doi: 10.1007/s12035-009-8051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyagi P, Tayal G. Ischemic preconditioning of myocardium. Acta Pharmacol Sin. 2002;23:865–70. [PubMed] [Google Scholar]
- 39.Bond A, Lodge D, Hicks CA, Ward MA, O’Neill MJ. NMDA receptor antagonism, but not AMPA receptor antagonism attenuates induced ischaemic tolerance in the gerbil hippocampus. Eur J Pharmacol. 1999;380:91–9. doi: 10.1016/s0014-2999(99)00523-3. [DOI] [PubMed] [Google Scholar]
- 40.Zheng S, Zuo Z. Isoflurane preconditioning reduces purkinje cell death in an in vitro model of rat cerebellar ischemia. Neuroscience. 2003;118:99–106. doi: 10.1016/s0306-4522(02)00767-4. [DOI] [PubMed] [Google Scholar]
- 41.Zheng S, Zuo Z. Isoflurane preconditioning induces neuroprotection against ischemia via activation of P38 mitogen-activated protein kinases. Mol Pharmacol. 2004;65:1172–80. doi: 10.1124/mol.65.5.1172. [DOI] [PubMed] [Google Scholar]
- 42.Nakajima T, Iwabuchi S, Miyazaki H, et al. Preconditioning prevents ischemia-induced neuronal death through persistent Akt activation in the penumbra region of the rat brain. J Vet Med Sci. 2004;66:521–7. doi: 10.1292/jvms.66.521. [DOI] [PubMed] [Google Scholar]
- 43.Wick A, Wick W, Waltenberger J, et al. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J Neurosci. 2002;22:6401–7. doi: 10.1523/JNEUROSCI.22-15-06401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao X, Zhang H, Steinberg G, Zhao H. The Akt pathway is involved in rapid ischemic tolerance in focal ischemia in Rats. Transl Stroke Res. 2010;1:202–209. doi: 10.1007/s12975-010-0017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di-Capua N, Sperling O, Zoref-Shani E. Protein kinase C-epsilon is involved in the adenosine-activated signal transduction pathway conferring protection against ischemia-reperfusion injury in primary rat neuronal cultures. J Neurochem. 2003;84:409–12. doi: 10.1046/j.1471-4159.2003.01563.x. [DOI] [PubMed] [Google Scholar]
- 46.Kurkinen K, Busto R, Goldsteins G, Koistinaho J, Perez-Pinzon MA. Isoform-specific membrane translocation of protein kinase C after ischemic preconditioning. Neurochem Res. 2001;26:1139–44. doi: 10.1023/a:1012322906824. [DOI] [PubMed] [Google Scholar]
- 47.Raval AP, Dave KR, DeFazio RA, Perez-Pinzon MA. epsilonPKC phosphorylates the mitochondrial K(+) (ATP) channel during induction of ischemic preconditioning in the rat hippocampus. Brain Res. 2007;1184:345–53. doi: 10.1016/j.brainres.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen J, Ma S, Chan P, et al. Nitric oxide down-regulates caveolin-1 expression in rat brains during focal cerebral ischemia and reperfusion injury. J Neurochem. 2006;96:1078–89. doi: 10.1111/j.1471-4159.2005.03589.x. [DOI] [PubMed] [Google Scholar]
- 49.Jasmin JF, Malhotra S, Singh Dhallu M, et al. Caveolin-1 deficiency increases cerebral ischemic injury. Circ Res. 2007;100:721–9. doi: 10.1161/01.RES.0000260180.42709.29. [DOI] [PubMed] [Google Scholar]
- 50.Shen J, Lee W, Li Y, et al. Interaction of caveolin-1, nitric oxide, and nitric oxide synthases in hypoxic human SK-N-MC neuroblastoma cells. J Neurochem. 2008;107:478–87. doi: 10.1111/j.1471-4159.2008.05630.x. [DOI] [PubMed] [Google Scholar]
- 51.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–33. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 52.Couet J, Sargiacomo M, Lisanti MP. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem. 1997;272:30429–38. doi: 10.1074/jbc.272.48.30429. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Cardena G, Martasek P, Masters BS, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–40. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 54.Lisanti MP, Scherer PE, Tang Z, Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 1994;4:231–5. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 55.Oka N, Yamamoto M, Schwencke C, et al. Caveolin interaction with protein kinase C. Isoenzyme-dependent regulation of kinase activity by the caveolin scaffolding domain peptide. J Biol Chem. 1997;272:33416–21. doi: 10.1074/jbc.272.52.33416. [DOI] [PubMed] [Google Scholar]
- 56.Das M, Das S, Das DK. Caveolin and MAP kinase interaction in angiotensin II preconditioning of the myocardium. J Cell Mol Med. 2007;11:788–97. doi: 10.1111/j.1582-4934.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Kapinya K, Penzel R, Sommer C, Kiessling M. Temporary changes of the AP-1 transcription factor binding activity in the gerbil hippocampus after transient global ischemia, and ischemic tolerance induction. Brain Res. 2000;872:282–93. doi: 10.1016/s0006-8993(00)02503-8. [DOI] [PubMed] [Google Scholar]
- 58.Hara T, Hamada J, Yano S, et al. CREB is required for acquisition of ischemic tolerance in gerbil hippocampal CA1 region. J Neurochem. 2003;86:805–14. doi: 10.1046/j.1471-4159.2003.01847.x. [DOI] [PubMed] [Google Scholar]
- 59.Meller R, Minami M, Cameron JA, et al. CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:234–46. doi: 10.1038/sj.jcbfm.9600024. [DOI] [PubMed] [Google Scholar]
- 60.Blondeau N, Widmann C, Lazdunski M, Heurteaux C. Activation of the nuclear factor-kappaB is a key event in brain tolerance. J Neurosci. 2001;21:4668–77. doi: 10.1523/JNEUROSCI.21-13-04668.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bergeron M, Gidday JM, Yu AY, et al. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann Neurol. 2000;48:285–96. [PubMed] [Google Scholar]
- 62.Bernaudin M, Nedelec AS, Divoux D, et al. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:393–403. doi: 10.1097/00004647-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 63.Ryu H, Lee J, Zaman K, et al. Sp1 and Sp3 are oxidative stress-inducible, antideath transcription factors in cortical neurons. J Neurosci. 2003;23:3597–606. doi: 10.1523/JNEUROSCI.23-09-03597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ratan RR, Siddiq A, Aminova L, et al. Translation of ischemic preconditioning to the patient: prolyl hydroxylase inhibition and hypoxia inducible factor-1 as novel targets for stroke therapy. Stroke. 2004;35:2687–9. doi: 10.1161/01.STR.0000143216.85349.9e. [DOI] [PubMed] [Google Scholar]
- 65.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ratan RR, Siddiq A, Smirnova N, et al. Harnessing hypoxic adaptation to prevent, treat, and repair stroke. J Mol Med. 2007;85:1331–8. doi: 10.1007/s00109-007-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baranova O, Miranda LF, Pichiule P, et al. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27:6320–32. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perez-Pinzon MA. Neuroprotective effects of ischemic preconditioning in brain mitochondria following cerebral ischemia. J Bioenerg Biomembr. 2004;36:323–7. doi: 10.1023/B:JOBB.0000041762.47544.ff. [DOI] [PubMed] [Google Scholar]
- 69.Zhao H. The protective effect of ischemic postconditioning against ischemic injury: from the heart to the brain. Journal of Neuroimmune Pharmacology. 2007;2:313–8. doi: 10.1007/s11481-007-9089-8. [DOI] [PubMed] [Google Scholar]
- 70.Tsang A, Hausenloy DJ, Yellon DM. Myocardial postconditioning: reperfusion injury revisited. Am J Physiol Heart Circ Physiol. 2005;289:H2–7. doi: 10.1152/ajpheart.00091.2005. [DOI] [PubMed] [Google Scholar]
- 71.Sewell WH, Koth DR, Huggins CE. Ventricular fibrillation in dogs after sudden return of flow to the coronary artery. Surgery. 1955;38:1050–3. [PubMed] [Google Scholar]
- 72.Grech ED, Ramsdale DR. Termination of reperfusion arrhythmia by coronary artery occlusion. Br Heart J. 1994;72:94–5. doi: 10.1136/hrt.72.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Na HS, Kim YI, Yoon YW, et al. Ventricular premature beat-driven intermittent restoration of coronary blood flow reduces the incidence of reperfusion-induced ventricular fibrillation in a cat model of regional ischemia. Am Heart J. 1996;132:78–83. doi: 10.1016/s0002-8703(96)90393-2. [DOI] [PubMed] [Google Scholar]
- 74.Zhao ZQ, Vinten-Johansen J. Postconditioning: reduction of reperfusion-induced injury. Cardiovasc Res. 2006;70:200–11. doi: 10.1016/j.cardiores.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 75.Obal D, Dettwiler S, Favoccia C, et al. The influence of mitochondrial KATP-channels in the cardioprotection of preconditioning and postconditioning by sevoflurane in the rat in vivo. Anesth Analg. 2005;101:1252–60. doi: 10.1213/01.ANE.0000181336.96511.32. [DOI] [PubMed] [Google Scholar]
- 76.Kin H, Zatta AJ, Lofye MT, et al. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005;67:124–33. doi: 10.1016/j.cardiores.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 77.Krolikowski JG, Weihrauch D, Bienengraeber M, et al. Role of Erk1/2, p70s6K, and eNOS in isoflurane-induced cardioprotection during early reperfusion in vivo. Can J Anaesth. 2006;53:174–82. doi: 10.1007/BF03021824. [DOI] [PubMed] [Google Scholar]
- 78.Iliodromitis EK, Georgiadis M, Cohen MV, et al. Protection from postconditioning depends on the number of short ischemic insults in anesthetized pigs. Basic Res Cardiol. 2006;101:502–7. doi: 10.1007/s00395-006-0606-3. [DOI] [PubMed] [Google Scholar]
- 79.Dosenko VE, Nagibin VS, Tumanovskaya LV, et al. Proteasome inhibitors eliminate protective effect of postconditioning in cultured neonatal cardiomyocytes. Fiziol Zh. 2006;52:15–24. [PubMed] [Google Scholar]
- 80.Staat P, Rioufol G, Piot C, et al. Postconditioning the human heart. Circulation. 2005;112:2143–8. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 81.Zhao H, Wang JQ, Sun G, et al. Conditions of protection by hypothermia and effects on apoptotic pathways in a model of permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2005;25:S474–S474. [Google Scholar]
- 82.Zhao H, Yenari M, Cheng D, Sapolsky R, Steinberg G. Biphasic cytochrome c release after transient global ischemia and its inhibition by hypothermia. J Cereb Blood Flow Metab. 2005;25:1119–1129. doi: 10.1038/sj.jcbfm.9600111. [DOI] [PubMed] [Google Scholar]
- 83.Merkle S, Frantz S, Schon MP, et al. A role for caspase-1 in heart failure. Circ Res. 2007;100:645–53. doi: 10.1161/01.RES.0000260203.55077.61. [DOI] [PubMed] [Google Scholar]
- 84.Zhao H, Shimohata T, Wang JQ, et al. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Noshita N, Sugawara T, Hayashi T, et al. Copper/zinc superoxide dismutase attenuates neuronal cell death by preventing extracellular signal-regulated kinase activation after transient focal cerebral ischemia in mice. J Neurosci. 2002;22:7923–30. doi: 10.1523/JNEUROSCI.22-18-07923.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shimohata T, Zhao H, Steinberg GK. Epsilon PKC may contribute to the protective effect of hypothermia in a rat focal cerebral ischemia model. Stroke. 2007;38:375–80. doi: 10.1161/01.STR.0000254616.78387.ee. [DOI] [PubMed] [Google Scholar]
- 87.Shimohata T, Zhao H, Sung JH, et al. Suppression of deltaPKC activation after focal cerebral ischemia contributes to the protective effect of hypothermia. J Cereb Blood Flow Metab. 2007;27:1463–75. doi: 10.1038/sj.jcbfm.9600450. [DOI] [PubMed] [Google Scholar]
- 88.Chen J, Nagayama T, Jin K, et al. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci. 1998;18:4914–28. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamashima T. Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Prog Neurobiol. 2000;62:273–95. doi: 10.1016/s0301-0082(00)00006-x. [DOI] [PubMed] [Google Scholar]
- 90.Pignataro G, Meller R, Inoue K, et al. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cereb Blood Flow Metab. 2008;28:232–41. doi: 10.1038/sj.jcbfm.9600559. [DOI] [PubMed] [Google Scholar]
- 91.Gao X, Ren C, Zhao H. Protective effects of ischemic postconditioning compared with gradual reperfusion or preconditioning. J Neurosci Res. 2008;86:2505–11. doi: 10.1002/jnr.21703. [DOI] [PubMed] [Google Scholar]
- 92.Xing B, Chen H, Zhang M, et al. Ischemic post-conditioning protects brain and reduces inflammation in a rat model of focal cerebral ischemia/reperfusion. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05276.x. [DOI] [PubMed] [Google Scholar]
- 93.Pignataro G, Xiong Z, Simon RP. Program No. 760.3. 2006 Neuroscience Meeting Planner. Vol. 2006. Atlanta, GA: Society for Neuroscience; 2006. Ischemic post-conditioning: a new neuroprotective strategy. Online. [Google Scholar]
- 94.Rehni AK, Singh N. Role of phosphoinositide 3-kinase in ischemic postconditioning-induced attenuation of cerebral ischemia-evoked behavioral deficits in mice. Pharmacol Rep. 2007;59:192–8. [PubMed] [Google Scholar]
- 95.Wang JY, Shen J, Gao Q, et al. Ischemic postconditioning protects against global cerebral ischemia/reperfusion-induced injury in rats. Stroke. 2008;39:983–90. doi: 10.1161/STROKEAHA.107.499079. [DOI] [PubMed] [Google Scholar]
- 96.Scartabelli T, Gerace E, Landucci E, Moroni F, Pellegrini-Giampietro DE. Neuroprotection by group I mGlu receptors in a rat hippocampal slice model of cerebral ischemia is associated with the PI3K-Akt signaling pathway: a novel postconditioning strategy? Neuropharmacology. 2008;55:509–16. doi: 10.1016/j.neuropharm.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 97.Burda J, Danielisova V, Nemethova M, et al. Delayed postconditionig initiates additive mechanism necessary for survival of selectively vulnerable neurons after transient ischemia in rat brain. Cell Mol Neurobiol. 2006;26:1141–51. doi: 10.1007/s10571-006-9036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou C, Tu J, Zhang Q, et al. Delayed ischemic postconditioning protects hippocampal CA1 neurons by preserving mitochondrial integrity via Akt/GSK3beta signaling. Neurochem Int. 2011 doi: 10.1016/j.neuint.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 99.Nagy D, Kocsis K, Fuzik J, et al. Kainate postconditioning restores LTP in ischemic hippocampal CA1: onset-dependent second pathophysiological stress. Neuropharmacology. 2011;61:1026–32. doi: 10.1016/j.neuropharm.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 100.Ren C, Gao X, Niu G, et al. Delayed postconditioning protects against focal ischemic brain injury in rats. PLoS One. 2008;3:e3851. doi: 10.1371/journal.pone.0003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Feng R, Li S, Li F. Toll-like receptor 4 is involved in ischemic tolerance of postconditioning in hippocampus of tree shrews to thrombotic cerebral ischemia. Brain Res. 2011;1384:118–27. doi: 10.1016/j.brainres.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 102.Leconte C, Tixier E, Freret T, et al. Delayed hypoxic postconditioning protects against cerebral ischemia in the mouse. Stroke. 2009;40:3349–55. doi: 10.1161/STROKEAHA.109.557314. [DOI] [PubMed] [Google Scholar]
- 103.Lee JJ, Li L, Jung HH, Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–62. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Adamczyk S, Robin E, Simerabet M, et al. Sevoflurane pre- and post-conditioning protect the brain via the mitochondrial K ATP channel. Br J Anaesth. 2010;104:191–200. doi: 10.1093/bja/aep365. [DOI] [PubMed] [Google Scholar]
- 105.Wang JK, Yu LN, Zhang FJ, et al. Postconditioning with sevoflurane protects against focal cerebral ischemia and reperfusion injury via PI3K/Akt pathway. Brain Res. 2010;1357:142–51. doi: 10.1016/j.brainres.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 106.Lin D, Li G, Zuo Z. Volatile anesthetic post-treatment induces protection via inhibition of glycogen synthase kinase 3beta in human neuron-like cells. Neuroscience. 2011;179:73–9. doi: 10.1016/j.neuroscience.2011.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Burda J, Danielisova V, Nemethova M, et al. Delayed postconditionig initiates additive mechanism necessary for survival of selectively vulnerable neurons after transient ischemia in rat brain. Cell Mol Neurobiol. 2006;26:1139–49. doi: 10.1007/s10571-006-9036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Danielisova V, Nemethova M, Gottlieb M, Burda J. The changes in endogenous antioxidant enzyme activity after postconditioning. Cell Mol Neurobiol. 2006;26:1181–91. doi: 10.1007/s10571-006-9034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Perez-Pinzon MA. Mechanisms of neuroprotection during ischemic preconditioning: lessons from anoxic tolerance. Comp Biochem Physiol A Mol Integr Physiol. 2007;147:291–9. doi: 10.1016/j.cbpa.2006.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raval AP, Dave KR, Perez-Pinzon MA. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2006;26:1141–7. doi: 10.1038/sj.jcbfm.9600262. [DOI] [PubMed] [Google Scholar]
- 111.McClanahan T, Nao B, Wolke L, et al. Brief renal occlusion and reperfusion reduces myoardial infarct size in rabbits. FASEB J. 1993:7. [Google Scholar]
- 112.Oxman T, Arad M, Klein R, Avazov N, Rabinowitz B. Limb ischemia preconditions the heart against reperfusion tachyarrhythmia. Am J Physiol. 1997;273:H1707–12. doi: 10.1152/ajpheart.1997.273.4.H1707. [DOI] [PubMed] [Google Scholar]
- 113.Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96:1641–6. doi: 10.1161/01.cir.96.5.1641. [DOI] [PubMed] [Google Scholar]
- 114.Liem DA, Verdouw PD, Ploeg H, Kazim S, Duncker DJ. Sites of action of adenosine in interorgan preconditioning of the heart. Am J Physiol Heart Circ Physiol. 2002;283:H29–37. doi: 10.1152/ajpheart.01031.2001. [DOI] [PubMed] [Google Scholar]
- 115.Gritsopoulos G, Iliodromitis EK, Zoga A, et al. Remote postconditioning is more potent than classic postconditioning in reducing the infarct size in anesthetized rabbits. Cardiovasc Drugs Ther. 2009;23:193–8. doi: 10.1007/s10557-009-6168-5. [DOI] [PubMed] [Google Scholar]
- 116.Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis. 2009;204:334–41. doi: 10.1016/j.atherosclerosis.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 117.Kerendi F, Kin H, Halkos ME, et al. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100:404–12. doi: 10.1007/s00395-005-0539-2. [DOI] [PubMed] [Google Scholar]
- 118.Przyklenk K, Darling CE, Dickson EW, Whittaker P. Cardioprotection ‘outside the box’--the evolving paradigm of remote preconditioning. Basic Res Cardiol. 2003;98:149–57. doi: 10.1007/s00395-003-0406-y. [DOI] [PubMed] [Google Scholar]
- 119.Zhao HG, Sun XC, Xian XH, et al. The role of nitric oxide in the neuroprotection of limb ischemic preconditioning in rats. Neurochem Res. 2007;32:1919–26. doi: 10.1007/s11064-007-9381-2. [DOI] [PubMed] [Google Scholar]
- 120.Steiger HJ, Hanggi D. Ischaemic preconditioning of the brain, mechanisms and applications. Acta Neurochir (Wien) 2007;149:1–10. doi: 10.1007/s00701-006-1057-1. [DOI] [PubMed] [Google Scholar]
- 121.Jin RL, Li WB, Li QJ, et al. The role of extracellular signal-regulated kinases in the neuroprotection of limb ischemic preconditioning. Neurosci Res. 2006;55:65–73. doi: 10.1016/j.neures.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 122.Zhao HG, Li WB, Li QJ, et al. Limb ischemic preconditioning attenuates apoptosis of pyramidal neurons in the CA1 hippocampus induced by cerebral ischemia-reperfusion in rats. Sheng Li Xue Bao. 2004;56:407–12. [PubMed] [Google Scholar]
- 123.Kakimoto M, Kawaguchi M, Sakamoto T, et al. Evaluation of rapid ischemic preconditioning in a rabbit model of spinal cord ischemia. Anesthesiology. 2003;99:1112–7. doi: 10.1097/00000542-200311000-00017. [DOI] [PubMed] [Google Scholar]
- 124.Dave KR, Saul I, Prado R, Busto R, Perez-Pinzon MA. Remote organ ischemic preconditioning protect brain from ischemic damage following asphyxial cardiac arrest. Neurosci Lett. 2006;404:170–5. doi: 10.1016/j.neulet.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 125.Andreka G, Vertesaljai M, Szantho G, et al. Remote ischaemic postconditioning protects the heart during acute myocardial infarction in pigs. Heart. 2007;93:749–52. doi: 10.1136/hrt.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ren C, Yan Z, Wei D, et al. Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Res. 2009;1288:88–94. doi: 10.1016/j.brainres.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Q, Zhang X, Ding Q, et al. Limb Remote Postconditioning Alleviates Cerebral Reperfusion Injury Through Reactive Oxygen Species-Mediated Inhibition of Delta Protein Kinase C in Rats. Anesth Analg. 2011 doi: 10.1213/ANE.0b013e31822b885f. [DOI] [PubMed] [Google Scholar]
- 128.Zhou Y, Fathali N, Lekic T, et al. Remote limb ischemic postconditioning protects against neonatal hypoxic-ischemic brain injury in rat pups by the opioid receptor/Akt pathway. Stroke. 2011;42:439–44. doi: 10.1161/STROKEAHA.110.592162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dietrich WD, Busto R, Alonso O, Globus MY, Ginsberg MD. Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1993;13:541–9. doi: 10.1038/jcbfm.1993.71. [DOI] [PubMed] [Google Scholar]
- 130.Dumas TC, Sapolsky RM. Gene therapy against neurological insults: sparing neurons versus sparing function. Trends Neurosci. 2001;24:695–700. doi: 10.1016/s0166-2236(00)01956-1. [DOI] [PubMed] [Google Scholar]
- 131.Gao X, Zhang H, Takahashi T, et al. The Akt signaling pathway contributes to postconditioning’s protection against stroke; the protection is associated with the MAPK and PKC pathways. J Neurochem. 2008;105:943–55. doi: 10.1111/j.1471-4159.2008.05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Halkos ME, Kerendi F, Corvera JS, et al. Myocardial protection with postconditioning is not enhanced by ischemic preconditioning. Ann Thorac Surg. 2004;78:961–9. doi: 10.1016/j.athoracsur.2004.03.033. discussion 969. [DOI] [PubMed] [Google Scholar]
- 133.Sun HY, Wang NP, Halkos M, et al. Postconditioning attenuates cardiomyocyte apoptosis via inhibition of JNK and p38 mitogen-activated protein kinase signaling pathways. Apoptosis. 2006;11:1583–93. doi: 10.1007/s10495-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 134.Yang XM, Proctor JB, Cui L, et al. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–10. doi: 10.1016/j.jacc.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 135.Nemethova M, Danielisova V, Gottlieb M, Kravcukova P, Burda J. Ischemic postconditioning in the rat hippocampus: mapping of proteins involved in reversal of delayed neuronal death. Arch Ital Biol. 2010;148:23–32. [PubMed] [Google Scholar]
- 136.Casabona G. Intracellular signal modulation: a pivotal role for protein kinase C. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:407–25. doi: 10.1016/s0278-5846(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 137.Sawe N, Steinberg G, Zhao H. Dual roles of the MAPK/ERK1/2 cell signaling pathway after stroke. J Neurosci Res. 2008;86:1659–69. doi: 10.1002/jnr.21604. [DOI] [PubMed] [Google Scholar]
- 138.Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34:249–70. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]
- 139.Prasad SS, Russell M, Nowakowska M. Neuroprotection induced in vitro by ischemic preconditioning and postconditioning: modulation of apoptosis and PI3K-Akt pathways. J Mol Neurosci. 2011;43:428–42. doi: 10.1007/s12031-010-9461-7. [DOI] [PubMed] [Google Scholar]
- 140.Wolfrum S, Schneider K, Heidbreder M, et al. Remote preconditioning protects the heart by activating myocardial PKCepsilon-isoform. Cardiovasc Res. 2002;55:583–9. doi: 10.1016/s0008-6363(02)00408-x. [DOI] [PubMed] [Google Scholar]
- 141.Schoemaker RG, van Heijningen CL. Bradykinin mediates cardiac preconditioning at a distance. Am J Physiol Heart Circ Physiol. 2000;278:H1571–6. doi: 10.1152/ajpheart.2000.278.5.H1571. [DOI] [PubMed] [Google Scholar]
- 142.Brzozowski T, Konturek PC, Pajdo R, et al. Importance of brain-gut axis in the gastroprotection induced by gastric and remote preconditioning. J Physiol Pharmacol. 2004;55:165–77. [PubMed] [Google Scholar]
- 143.Barone FC, White RF, Spera PA, et al. Ischemic preconditioning and brain tolerance: temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke. 1998;29:1937–50. doi: 10.1161/01.str.29.9.1937. discussion 1950–1. [DOI] [PubMed] [Google Scholar]
- 144.Meldrum DR, Cleveland JC, Jr, Rowland RT, et al. Early and delayed preconditioning: differential mechanisms and additive protection. Am J Physiol. 1997;273:H725–33. doi: 10.1152/ajpheart.1997.273.2.H725. [DOI] [PubMed] [Google Scholar]
- 145.Thornton J, Striplin S, Liu GS, et al. Inhibition of protein synthesis does not block myocardial protection afforded by preconditioning. Am J Physiol. 1990;259:H1822–5. doi: 10.1152/ajpheart.1990.259.6.H1822. [DOI] [PubMed] [Google Scholar]
- 146.Xing B, Chen H, Zhang M, et al. Ischemic postconditioning inhibits apoptosis after focal cerebral ischemia/reperfusion injury in the rat. Stroke. 2008;39:2362–9. doi: 10.1161/STROKEAHA.107.507939. [DOI] [PubMed] [Google Scholar]
- 147.Zhang W, Miao Y, Zhou S, et al. Involvement of Glutamate Transporter-1 in Neuroprotection against Global Brain Ischemia-Reperfusion Injury Induced by Postconditioning in Rats. Int J Mol Sci. 2010;11:4407–16. doi: 10.3390/ijms11114407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang W, Miao Y, Zhou S, et al. Neuroprotective effects of ischemic postconditioning on global brain ischemia in rats through upregulation of hippocampal glutamine synthetase. J Clin Neurosci. 2011;18:685–9. doi: 10.1016/j.jocn.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 149.Yuan Y, Guo Q, Ye Z, et al. Ischemic postconditioning protects brain from ischemia/reperfusion injury by attenuating endoplasmic reticulum stress-induced apoptosis through PI3K-Akt pathway. Brain Res. 2011;1367:85–93. doi: 10.1016/j.brainres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 150.Robin E, Simerabet M, Hassoun SM, et al. Postconditioning in focal cerebral ischemia: role of the mitochondrial ATP-dependent potassium channel. Brain Res. 2011;1375:137–46. doi: 10.1016/j.brainres.2010.12.054. [DOI] [PubMed] [Google Scholar]
- 151.Pignataro G, Esposito E, Cuomo O, et al. The NCX3 isoform of the Na+/Ca2+ exchanger contributes to neuroprotection elicited by ischemic postconditioning. J Cereb Blood Flow Metab. 2011;31:362–70. doi: 10.1038/jcbfm.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Danielisova V, Nemethova M, Gottlieb M, Burda J. The changes in endogenous antioxidant enzyme activity after postconditioning. Cell Mol Neurobiol. 2006;26:1179–89. doi: 10.1007/s10571-006-9034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Danielisova V, Gottlieb M, Nemethova M, Burda J. Effects of bradykinin postconditioning on endogenous antioxidant enzyme activity after transient forebrain ischemia in rat. Neurochem Res. 2008;33:1057–64. doi: 10.1007/s11064-007-9550-3. [DOI] [PubMed] [Google Scholar]