Abstract
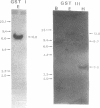
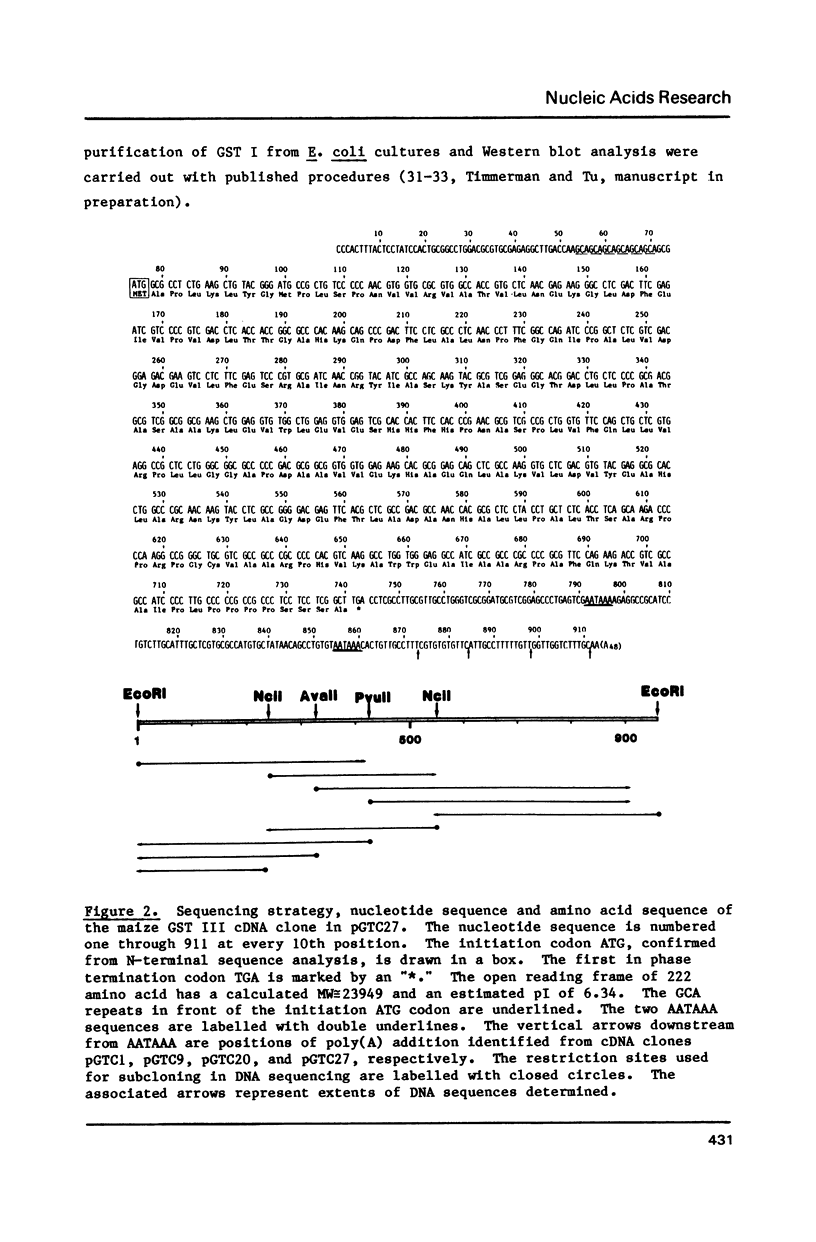
We have isolated from a constructed lambda gt11 expression library two classes of cDNA clones encoding the entire sequence of the maize GSH S-transferases GST I and GST III. Expression of a full-length GST I cDNA in E. coli resulted in the synthesis of enzymatically active maize GST I that is immunologically indistinguishable from the native GST I. Another GST I cDNA with a truncated N-terminal sequence is also active in heterospecific expression. Our GST III cDNA sequence differs from the version reported by Moore et al. [Moore, R. E., Davies, M. S., O'Connell, K. M., Harding, E. I., Wiegand, R. C., and Tiemeier, D. C. (1986) Nucleic Acids Res. 14:7227-7235] in eight reading frame shifts which result in partial amino acid sequence conservation with the rat GSH S-transferase sequences. The GST I and GST III sequences share approximately 45% amino acid sequence homology. Both the GST I and the GST III mRNAs contain different repeating motifs in front of the initiation codon ATG. Multiple poly(A) addition sites have been identified for these two classes of maize GSH S-transferase messages. Genomic Southern blotting results suggest that both GST I and GST III are present in single or low copies in the maize (GT112 RfRf) genome.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovitz M., Listowsky I. Selective expression of a unique glutathione S-transferase Yb3 gene in rat brain. J Biol Chem. 1987 Jun 5;262(16):7770–7773. [PubMed] [Google Scholar]
- Asaoka K. Affinity purification and characterization of glutathione S-transferases from bovine liver. J Biochem. 1984 Mar;95(3):685–696. doi: 10.1093/oxfordjournals.jbchem.a134658. [DOI] [PubMed] [Google Scholar]
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Berget S. M. Are U4 small nuclear ribonucleoproteins involved in polyadenylation? Nature. 1984 May 10;309(5964):179–182. doi: 10.1038/309179a0. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G. J., Ding V. D., Rodkey J. A., Bennett C. D., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. DNA sequence analysis of a Yb2 cDNA clone and regulation of the Yb1 and Yb2 mRNAs by phenobarbital. J Biol Chem. 1986 Jun 15;261(17):7952–7957. [PubMed] [Google Scholar]
- Ding G. J., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. Nucleotide sequence analysis of a Yb1 cDNA clone and prediction of the complete amino acid sequence of the Yb1 subunit. J Biol Chem. 1985 Oct 25;260(24):13268–13271. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Glutathione S-transferases (rat and human). Methods Enzymol. 1981;77:218–231. doi: 10.1016/s0076-6879(81)77029-0. [DOI] [PubMed] [Google Scholar]
- Hayes J. D. Purification and characterization of glutathione S-transferases P, S and N. Isolation from rat liver of Yb1 Yn protein, the existence of which was predicted by subunit hybridization in vitro. Biochem J. 1984 Dec 15;224(3):839–852. doi: 10.1042/bj2240839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H. C., Grove G., Tu C. P. Cloning and sequence analysis of a cDNA for a rat liver glutathione S-transferase Yb subunit. Nucleic Acids Res. 1986 Aug 11;14(15):6101–6114. doi: 10.1093/nar/14.15.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H. C., Li N., Weiss M. J., Reddy C. C., Tu C. P. The nucleotide sequence of a rat liver glutathione S-transferase subunit cDNA clone. J Biol Chem. 1984 May 10;259(9):5536–5542. [PubMed] [Google Scholar]
- Lai H. C., Tu C. P. Rat glutathione S-transferases supergene family. Characterization of an anionic Yb subunit cDNA clone. J Biol Chem. 1986 Oct 15;261(29):13793–13799. [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Mason P. J., Elkington J. A., Lloyd M. M., Jones M. B., Williams J. G. Mutations downstream of the polyadenylation site of a Xenopus beta-globin mRNA affect the position but not the efficiency of 3' processing. Cell. 1986 Jul 18;46(2):263–270. doi: 10.1016/0092-8674(86)90743-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Moore R. E., Davies M. S., O'Connell K. M., Harding E. I., Wiegand R. C., Tiemeier D. C. Cloning and expression of a cDNA encoding a maize glutathione-S-transferase in E. coli. Nucleic Acids Res. 1986 Sep 25;14(18):7227–7235. doi: 10.1093/nar/14.18.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozer T. J., Tiemeier D. C., Jaworski E. G. Purification and characterization of corn glutathione S-transferase. Biochemistry. 1983 Mar 1;22(5):1068–1072. doi: 10.1021/bi00274a011. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett C. B., Telakowski-Hopkins C. A., Ding G. J., Argenbright L., Lu A. Y. Rat liver glutathione S-transferases. Complete nucleotide sequence of a glutathione S-transferase mRNA and the regulation of the Ya, Yb, and Yc mRNAs by 3-methylcholanthrene and phenobarbital. J Biol Chem. 1984 Apr 25;259(8):5182–5188. [PubMed] [Google Scholar]
- Rhoads D. M., Zarlengo R. P., Tu C. P. The basic glutathione S-transferases from human livers are products of separate genes. Biochem Biophys Res Commun. 1987 May 29;145(1):474–481. doi: 10.1016/0006-291x(87)91345-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K., Kitahara A., Soma Y., Inaba Y., Hatayama I., Sato K. Purification, induction, and distribution of placental glutathione transferase: a new marker enzyme for preneoplastic cells in the rat chemical hepatocarcinogenesis. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3964–3968. doi: 10.1073/pnas.82.12.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro R. H., Frear D. S., Swanson H. R., Walsh W. C. Glutathione conjugation. An enzymatic basis for atrazine resistance in corn. Plant Physiol. 1971 Jan;47(1):10–14. doi: 10.1104/pp.47.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suguoka Y., Kano T., Okuda A., Sakai M., Kitagawa T., Muramatsu M. Cloning and the nucleotide sequence of rat glutathione S-transferase P cDNA. Nucleic Acids Res. 1985 Sep 11;13(17):6049–6057. doi: 10.1093/nar/13.17.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telakowski-Hopkins C. A., Rodkey J. A., Bennett C. D., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. Construction of a cDNA clone complementary to a Yc mRNA and prediction of the complete amino acid sequence of a Yc subunit. J Biol Chem. 1985 May 10;260(9):5820–5825. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Lai H. C., Li N. Q., Weiss M. J., Reddy C. C. The Yc and Ya subunits of rat liver glutathione S-transferases are the products of separate genes. J Biol Chem. 1984 Aug 10;259(15):9434–9439. [PubMed] [Google Scholar]
- Tu C. P., Reddy C. C. On the multiplicity of rat liver glutathione S-transferases. J Biol Chem. 1985 Aug 25;260(18):9961–9964. [PubMed] [Google Scholar]
- Tu C. P., Weiss M. J., Karakawa W. W., Reddy C. C. Cloning and sequence analysis of a cDNA plasmid for one of the rat liver glutathione S-transferase subunits. Nucleic Acids Res. 1982 Sep 25;10(18):5407–5419. doi: 10.1093/nar/10.18.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]