Abstract
Gonadotropin releasing hormone (GnRH) has now been suggested as an important intraovarian regulatory factor. Gonadotropin inhibitory hormone (GnIH) a hypothalamic dodecapeptide, acts opposite to GnRH. GnRH, GnIH and their receptors have been demonstrated in the gonads. In order to find out the physiological significance of these neuropeptides in the ovary, we aim to investigate changes in the abundance of GnRH I and GnIH in the ovary of mice during estrous cycle. The present study investigated the changes in GnRH I, GnRH I-receptor and RFRP-3 protein expression in the ovary of mice during estrous cycle by immunohistochemistry and immunoblot analysis. The immunoreactivity of GnRH I and its receptor and RFRP-3 were mainly localized in the granulosa cells of the healthy and antral follicles during proestrus and estrus and in the luteal cells during diestrus 1 and 2 phases. The relative abundance of immunoreactivity of GnRH I, GnRH I-receptor and RFRP-3 undergo significant variation during proestrus and thus may be responsible for selection of follicle for growth and atresia. A significant increase in the concentration of RFRP-3 during late diestrus 2 coincided with the decline in corpus luteum activity and initiation of follicular growth and selection. In general, immunolocalization of GnRH I, GnRH I-receptor and RFRP-3 were found in close vicinity suggesting functional interaction between these peptides. It is thus, hypothesized that interaction between GnRH I-RFRP-3 neuropeptides may be involved in the regulation of follicular development and atresia.
Keywords: GnRH I, GnRH I-receptor, RFRP-3, Ovary, Mice, Estrous cycle
Introduction
Gonadotropin releasing hormone (GnRH) is a key regulator of reproductive functions in mammals, which acts mainly at the level of hypothalamo-hypophysis axis. In the past last several years, GnRH and its receptors have been demonstrated in the ovary and there are increasing evidences suggesting GnRH as an intraovarian regulatory factor (Leung et al. 2003; Metallinou et al. 2007). This is supported by the observation on direct effects of this neuropeptide on steroidogenesis (Andreu et al. 1998; Jones and Hsueh 1981), follicular development (Srivastava et al. 1995), apoptosis (Parborell et al. 2001; Parborell et al. 2002, Sridaran et al. 1998, 1999) and meiotic maturation of the oocyte (Hillensjo and LeMaire 1980). It is well documented that GnRH I exerts anti-gonadotropic effect in the rat ovary by down regulating the expression of follicular-stimulating hormone (FSH) and luteinizing hormone (LH) receptors (Piquette et al. 1991; Tilly et al. 1992), by inhibiting gonadotropin-stimulated cAMP production (Knecht et al. 1985; Richards 1994) and suppressing steroidogenic enzymes (Hsueh and Schaeffer 1985; Sridaran et al. 1999). In addition to its antigonadotropic effect, GnRH-I may function as a permissive factor for the process of luteolysis. GnRH I, its receptor and the transcriptional products from their genes have been demonstrated in the ovarian tissue of several vertebrate species including mammals (Aten et al. 1986; Birnbaumer et al. 1985; Chakrabarti et al. 2008; Jones et al. 1980; Kogo et al. 1995; Li et al. 1993; Peng et al. 1994; Sengupta et al. 2008). However, little is known about the changes in concentration of GnRH I and its receptor, regulation of their synthesis and interaction with other local endocrine/paracrine factors in the ovary during reproductive/estrous cycle of mammals. The cyclic variation in the GnRH I and GnRH I-receptor mRNA expression in the ovary throughout the estrous cycle has so far been studied only in the rat (Schirman-Hildesheim et al. 2005, 2006). The changes in the concentration of GnRH I and its receptor proteins have not yet been investigated in the ovaries of mice during estrous cycle (Table 1).
Table 1.
Cellular localization and relative score intensity of GnRH I, GnRH I-R and RFRP-3 in ovaries of adult cyclic mice
| Types of follicles | GnRH I | GnRH I-receptor | RFRP-3 |
|---|---|---|---|
| Oocytes | |||
| Primordial | − | − | − |
| Primary | − | − | − |
| Secondary | − | − | − |
| Tertiary | − | − | + |
| Dominant | − | − | + |
| Atretic | ++ | + | + |
| Granulosa cells | |||
| Primordial | − | − | − |
| Primary | + | − | − |
| Secondary | ++ | + | ++ |
| Tertiary | +++ | +++ | +++/+ |
| Dominant | +++ | +++ | +++/+ |
| Atretic | + | ++ | ++ |
| Theca cells | |||
| Primordial | * | * | * |
| Primary | * | * | * |
| Secondary | + | + | + |
| Tertiary | ++ | + | +++/+ |
| Dominant | ++ | + | +++/+ |
| Atretic | +/− | +/− | ++ |
| CL-healthy | +++ | +++ | +++ |
| CL-luteolytic | ++ | +++/+ | ++ |
| Secondary interstitial cells | ++ | + | + |
| Surface epithelium | ++ | + | − |
The intensities of signals indicated above represent a subjective consensus of sections examined from the ovaries collected from all of the animals over the estrous cycle (N = 6). The signals were estimated as score intensity of immunoreactivity on a scale of − to 3+ as: −, absence of immunoreactivity; +, mild; ++, moderate; +++, intense; +/−, there was heterogeneity in the signal, e.g., some of the histological units contained signal, and others did not;
, absence of the cells in the section
A dodeca neuropeptide, having RFamide (Arg-Phe-NH2) at its C-terminal end was isolated from quail brain in the year 2000, which inhibits gonadotropin release and thus was named as gonadotropin-inhibitory hormone (GnIH) (Tsutsui et al. 2000). GnIH orthologous peptides that possessed LPXRFamide (X = L or Q) motif at their C-termini have been documented in a variety of vertebrates, such as mammals including primates and humans [RFamide-related peptides (RFRPs)], reptiles, amphibians [frog growth hormone-releasing peptide (fGRP) and fGRP-RPs, Rana RFamide peptide (R-RFa)] and teleosts [gold-fish LPXRFamide peptide (gfLPXRFa)] (Tsutsui and Bentley 2008). In 2006, Kriegsfeld and coworkers proposed that structurally related RF-amide related peptides (RFRPs) are the mammalian counterpart of avian GnIH that has the potential to modulate GnRH secretion in brain (Kriegsfeld et al. 2006). RFRP-3-ir has been investigated in the brain of several rodent species, photoperiodic Syrian hamsters (Mesocricetus auratus), and less photoperiodic rats (Rattus novegicus) and mice (Mus musculus) (Kriegsfeld et al. 2006; Ukena and Tsutsui 2001; Sethi et al. 2010a). In vitro and in vivo administration of GnIH decreases synthesis and release of LH from pituitary in a dose dependent manner in both birds and mammals (Bentley et al. 2006). GnIH and its receptor and their mRNA have recently been demonstrated in gonads and accessory reproductive organs in sexually mature European starlings, white-crowned sparrows, Japanese quail and in Calotes versicolor (Bentley et al. 2008; Singh et al. 2008). Mammalian gonads also express GnIH ortholog peptide, RFRP. RFRP and its receptor have been demonstrated in the ovaries and testes of rhesus macaque (Macaca mulatta) (Tsutsui and Bentley 2008) and its mRNA and peptides in the testes Syrian hamster (Zhao et al. 2010). Motivated by the presence of RFRP-3, in the hypothalamus of several rodent species, it is worthwhile to investigate the distribution of RFRP-3 in the ovaries of mice. Quantitative and qualitative changes in the RFRP-3 together with the changes in GnRH I and its receptor may provide interactive relationship between the two neuropeptides in the ovary during the estrous cycle of the mice.
The cyclic variation in the local expression of GnRH I and its receptor mRNA has so far been studied in the ovary of rat and compared with their levels in the pituitary throughout the estrous cycle (Schirman-Hildesheim et al. 2005, 2006). However, physiological significance of GnRH I peptide in the mammalian ovary in relation to follicular development, ovulation and luteinization have not yet been investigated. Ovary also contains RFRP neuropeptide (Bentley et al. 2008). In brain, RFRP was found to be in close association GnRH I neurons (Kriegsfeld et al. 2006). Thus, in the present study the quantitative changes in GnRH I and its receptor together with the changes in RFRP-3, a mammalian ortholog of GnIH were estimated, in order to determine the significance of these neuropeptides in the ovaries of mice during estrous cycle. We therefore suggest, that locally produced GnRH I may have some interactions with other closely related neuropeptide, RFRP-3 in the ovary to regulate ovarian activities like follicular development, ovulation and luteinization during the estrous cycle of mice.
Materials and methods
Animal
All experiments were conducted in accordance with the principles and procedures approved by the Departmental Research Committee at Banaras Hindu University, Varanasi, India. Parkes strain mice were housed under controlled temperature (24 ± 2°C), humidity and lights on between 07.00 and 20.00 h. Food and water were available ad libitum. Adult female mice (10–12 weeks old) were used in this study. Estrous cycles were monitored through daily examination of vaginal cytology. Mice that exhibited at least two consecutive regular estrous cycles were selected for the experiment. Animals were sacrificed by decapitation under mild anesthesia (anesthetic ether) at 10.00, 12.00, 14.00 and 19.00 h on proestrus, 10.00 h and 14.00 h on estrus, diestrus 1 and diestrus 2 of the estrous cycle. Ovaries were immediately dissected out and one side of the ovaries were snap frozen and kept at −40°C until protein extraction for immunoblots, whereas contralateral ovaries were fixed in 4% paraformaldehyde in 0.1 M sodium phosphate buffer overnight for immunohistochemistry. The fixed tissues were dehydrated in ethanol, embedded in paraffin wax and sectioned at 5 μm.
Antibodies and chemicals
GnIH was purified and GnIH antibody was raised in the laboratory of Prof. K. Tsutsui. GnRH I antibody (Cat No. IHC-7201) was purchased from Peninsula laboratory, San Carlos, CA, USA and GnRH I-R antibody (Cat No. sc 8028) was purchased from Santacruz Biotechnology Inc. Santa Cruz, CA, USA.
In vitro study
To determine the effects of GnIH on the expression of ovarian GnRH I-receptor protein, the in vitro study was performed. Three different doses were adopted for the study. Proestrus ovaries were used for culture, because they contain many maturing follicles, as well as to maintain uniformity in the stage of the ovaries used in all of the groups. The ovaries were quickly dissected out and cleaned from any adhered fat tissue and oviduct in medium Dulbecco Modified Eagle’s Medium (DMEM; Himedia, Mumbai, India) containing penicillin (250 U/ml) and streptomycin sulfate (250 μg/ml). Ovaries were cultured by the method as described previously (Singh et al. 2011). Culture media was a mixture of DMEM (with sodium pyruvate and L-glutamine) and Ham’s F-12 (1:1; v:v) (Himedia, Mumbai, India) containing penicillin (100 U/ ml), streptomycin sulfate (100 μg/ml) and BSA (0.1% w/v, Sigma Chemicals Co., St Louis, USA). Intact ovaries (one per tube) were cultured in 1 ml medium in a humidified atmosphere with 95% air and 5% CO2 to maintain pH 7.4 for 24 h at 37°C. Treatment of different doses of GnIH (1, 10, and 100 ng) was given in a total volume of 10 μl/ml medium per tube. Doses of GnIH treatment and the duration of the in vitro study were selected based on our preliminary study. Each treatment and control groups were run in triplicate and the experiment was repeated two times. Ovaries collected at the end of culture were found to be healthy and kept frozen at −40°C for immunoblot study.
Immunohistochemistry
The ovarian sections were processed through standard protocols of immunohistochemistry (Singh et al. 2007). The sections were deparaffinized, rehydrated in graded ethanol and endogenous peroxidase was quenched with 0.3% H2O2 in methanol. Background blocking was performed with 10% normal horse serum in PBS for 1 h. The tissue sections were then incubated for 1 h at room temperature either with GnRH I antibody (dilution 1:1,500) or with GnRH I-R antibody (dilution 1:25) or with quail GnIH antibody (specific for RFRP-3, dilution 1:1,000) diluted in phosphate buffered saline (PBS). The specificity of the GnIH antibody has been checked by a competitive ELISA (Ukena and Tsutsui 2001; Ukena et al. 2002; Kriegsfeld et al. 2006; Gibson et al. 2008; Ubuka et al. 2009; Sethi et al. 2010a, b; Zhao et al. 2010). Following three rinse in PBS for 15 min, the slides were then incubated with secondary antibody. Detection system used was ABC universal staining kit (sc-PK-6200; Vector Laboratories, Inc, Burlingame, CA, USA). Peroxidase activity was revealed with 0.03% 3,3′-diaminobenzidine tetra hydrochloride (DAB; Sigma Chemicals Co., St Louis, USA) in 0.05 M Tris pH 7.6 and 0.1% H2O2 for 5 min. Nucleus was counterstained with Elrich’s hematoxylin. Slides were then dehydrated and mounted with DPX. To test the specificity of the immunoreaction in the control section the primary antiserum was replaced by: (1) 1% normal horse serum; (2) after preadsorption of GnRH I antiserum with GnRH I antigen (200 ng/ml), and RFRP-3 antiserum with GnIH antigen (10 μg/ml). For preadsorption, the antigens were added to diluted antisera (in the same dilution as used for localization), incubated overnight at 4°C, centrifuged and then the supernatant was used.
Slot blot
The ovaries were collected during each phase of the estrous cycle and homogenized for protein extraction (Singh et al. 2007). The samples with equal amount of protein, as determined by Lowry’s method (Lowry et al. 1951), were adjusted to equal volume with PBS and 10 μl of these samples were loaded on PVDF membranes using Millipore slot blot apparatus (Millipore, Billerica, MA, USA). Non-specific sites were blocked with 5% non-fat dried milk in PBS, 0.02% Tween 20. Membranes were then incubated for 1 h at room temperature either with GnRH I or RFRP-3 (dilution 1:2,000) or β-actin antibodies in PBS. Immunodetection was performed with anti-rabbit IgG conjugated horseradish peroxidase (1:500 v/v). Finally, signal was detected with enhanced chemiluminescence (ECL, Bio-Rad, Hercules, CA, USA). Experiments were repeated three times with the same results. To test the specificity of the immunoreaction primary antibodies for GnRH-I and RFRP-3 were replaced by the antibodies preadsorbed with GnRH-I or GnIH antigen, respectively. The blots developed with preadsorbed antibodies showed no visible bands up to 3 min of exposure on X-ray film. Accordingly, the exposure time of 30 s was chosen to get specific immunoreactive bands for the experiments.
Western blot
Equal amount of protein as determined by Lowry’s method (Lowry et al. 1951) was loaded on 10% SDS PAGE for electrophoresis. Separated proteins were then transferred on PVDF membrane (Millipore, Billerica, MA, USA). Non-specific sites were blocked with 5% non-fat dried milk in PBS, 0.02% Tween 20. Membrane was then incubated for 1 h at room temperature with GnRH I-receptor antibody (dilution 1:250). Immunodetection was performed with secondary antibody conjugated with horseradish peroxidase (1:500 v/v). Finally, signal was detected with ECL (Bio-Rad, Hercules, CA, USA). Experiments were repeated thrice with the same result.
Densitometry
X-ray films were later scanned and then quantified by densitometry (Image J vs. 1.36, NIH, USA). Quality of loading and transfer was assessed with Ponceau S staining and/or β-actin. All immunoblots were normalized to β-actin. The data are presented as the ± S.E.M. of three blots of each peptide.
Statistics
Cyclic changes were analyzed by one-way ANOVA followed by Duncan’s multiple range post hoc tests. The difference was considered to be significant if P < 0.05 or P < 0.01. Correlation studies were performed by using SPSS 12.0 software (SPSS Inc., Chicogo, IL) to compare the data from different phases of estrous cycle.
Results
Relative concentration of GnRH I and GnRH I-receptor in the ovaries of mice during estrous cycle
Presence of GnRH I and GnRH I-receptor were demonstrated immunohistochemically in the ovaries of mice during different phases of estrous cycle (Fig. 1i, ii).
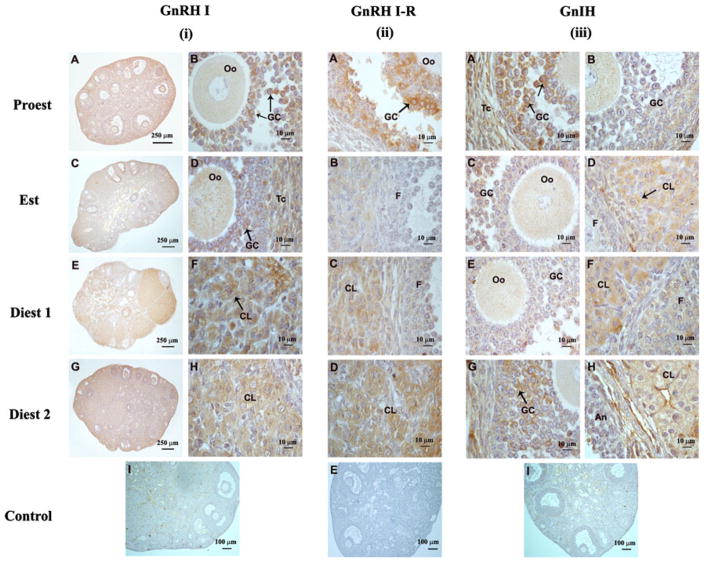
Fig. 1.
Immunohistochemical localization of GnRH I, GnRH I-receptor and RFRP-3 proteins in the ovaries of mice: (i) GnRH I A, B Ovary showing intense immunoreactivity for GnRH I mainly in the granulosa cells (GCs) of the antral follicles during proestrus phase. C, D Strong immunoreactivity for GnRH I in the granulosa cells (GCs) and theca cells (Tcs) and mild in the oocyte (Oo) during estrus phase. E, F During diestrus 1 phase, GnRH I strong immunoreactivity was mainly observed in the corpus luteum. G, H Strong immunoreactivity for GnRH I mainly in the corpus luteum (CL) and moderate in the follicles. I Absence of immunoreactivity in preadsorbed control for GnRH I. (ii) GnRH I-Receptor A Strong immunoreactivity for GnRH I-receptor in the granulosa cells (GCs) and mild immunoreactivity in the theca cells (Tcs) during proestrus phase. B Mild immunoreactivity for GnRH I-receptor in the corpus luteum (CL) and absence of immunoreactivity in the follicles F during estrus phase. C Strong immunoreactivity for GnRH I-receptor in the corpus luteum and mild in the follicle (F) during diestrus 1 during diestrus 1. D GnRH I-receptor is mainly localized in the corpus luteum during diestrus 2. E Negative control showing no immunoreactivity. (iii) RFRP-3A Intense immunoreactivity for RFRP-3 in the granulosa cells (GCs) and theca cells (Tcs) during early proestrus, while b mild immunoreactivity for RFRP-3 into the granulosa and theca cells of proliferating follicles during late proestrus. C Mild RFRP-3 immunoreactivity in the follicles and D moderate in newly formed corpus luteum and mild in the follicles during estrus phase. E Mild immunoreactivity in the antral follicles and F moderate immunoreactivity into the corpus luteum (CL) during diestrus 1. G Moredate RFRP-3 immunoreactivity in the GCs and Tcs of antral follicles and H strong immunoreactivity into the corpus luteum during diestrus 2. I Absence of immunoreactivity in preadsorbed control for RFRP-3
The immunoreactivity of both GnRH I and GnRH I-receptor proteins were mainly localized in the granulosa cells (GC) of the healthy and atretic antral follicles during proestrus and estrus phases, and moderate to intense staining in the luteal cells during diestrus 1 and diestrus 2 phases. Interstitial cells showed mild to moderate immunoreactivity during estrous cycle. Theca cells (Tc) of antral follicles also showed mild to moderate immunoreactivity. Small preantral follicles generally showed no to mild immunoreactivity. During proestrus phase, immunoreactivity of GnRH I was mainly observed in the antral follicles (Fig. 1iA, B). Granulosa cells located around oocytes or lining antral cavity generally showed intense immunoreactivity. Theca cells showed mild immunoreactivity. Oocytes of the large antral follicles showed no immunoreactivity. During estrus phase, antral follicles showed moderate immunoreactivity in granulosa cells and theca cells, whereas preantral follicles showed mild immunoreactivity (Fig. 1iC, D). During diestrus 1 phase, newly formed corpus luteum showed intense GnRH I immunoreactivity in luteal cells, whereas antral follicles showed mild to moderate immunoreactivity (Fig. 1iE, F). During diestrus 2 phase, corpus luteum showed moderate immunoreactivity, whereas follicles showed mild immunoreactivity (Fig. 1iG, H). Preadsorbed control section for GnRH I showed no immunoreactivity (Fig. 1iI).
The pattern of GnRH I-receptor immunoreactivity was nearly the same as described for GnRH I. Maximum GnRH I-receptor immunoreactivity was observed in the granulosa and cumulus oophorus of antral follicles during proestrus, theca cells showed very mild immunoreactivity while oocytes showed no immunoreactivity (Fig. 1iiA). The antral follicles during estrus phase showed mild immunoreactivity (Fig. 1iiB). Corpus luteum showed mild staining during estrus whereas strong during diestrus 1 (Fig. 1iiC) and diestrus 2 (Fig. 1iiD) phases.
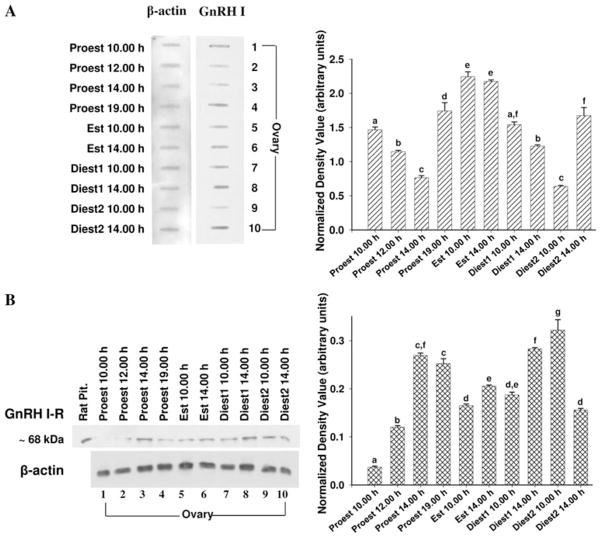
GnRH I and GnRH I-receptor protein concentration in the ovaries of mice were detected by immunoblots (slot/ western blot). The densitometric analysis of GnRH I slot blot showed significant variation (P < 0.05) in the concentration in the ovaries during different phases of estrous cycle (Fig. 2a). The ovaries showed most significant changes during proestrus. During proestrus phase, GnRH I expression decreased gradually from 10.00 to 14.00 h. The intensity of GnRH I expression increased sharply in the evening at 19.00 h during proestrus and remain high during the estrus phase. Subsequently, it gradually declined during diestrus 1 to attain a lowest level in the morning of diestrus 2. A sharp increase in the concentration of GnRH I was found in the afternoon of diestrus 2.
Fig. 2.
Slot blot for GnRH I (a) and western blot for GnRH I-receptor (b) in the ovaries of mice during estrous cycle. Densitometric analyses of the blots are given in bar graphs. Results were normalized with β-actin and are expressed in arbitrary units of signal intensity as ± SEM (N = 6). Bar bearing the different superscript (a–g) indicate significant difference between the mean values (P <0.05)
The western blot analysis of GnRH I-receptor in mice showed a single immunoreactive band at ~68 kDa (Fig. 2b). Densitometric analysis of western blot for GnRH I-receptor during different phases of estrous cycle showed a marked variation. The intensity of GnRH I-receptor expression increased sharply at 14.00 h on proestrus and remained high until late evening in proestrus. Subsequently, intensity of GnRH I-receptor expression declined during estrus and remained unchanged until early diestrus 1. The intensity of GnRH I-receptor expression increased again during late diestrus 1 and remained high during diestrus 2.
Relative concentration of RFRP-3 in the ovaries of mice during estrous cycle
Presence of RFRP-3 was demonstrated immunohistochemically in the ovaries of mice during different phases of estrous cycle (Fig. 1iii).
RFRP-3 immunoreactivity was mainly observed in the granulosa and theca cells of large antral follicles during proestrus (Fig. 1iiiA, B). Strong RFRP-3 immunoreactivity was observed in granulosa and theca cells of the antral follicles during 14.00 h (Fig. 1iiiA) while low immunoreactivity was observed at 19.00 h of proestrus (Fig. 1iiiB). Mild RFRP-3 immunoreactivity was observed in the follicles during estrus phases (Fig. 1iiiC) while moderate in the corpus luteum (Fig. 1iiiD). Follicles showed mild immunoreactivity during diestrus 1 phase (Fig. 1iiiE) while strong during diestrus 2 (Fig. 1iiiG). Moderate RFRP-3 immunoreactivity was observed in the corpus luteum during diestrus 1 (Fig. 1iiiF) and diestrus 2 (Fig. 1iiiH) phases. The newly formed corpus luteum showed stronger immunoreactivity than the older corpus luteum. Preadsorbed control section for RFRP-3 showed no immunoreactivity (Fig. 1iiiI).
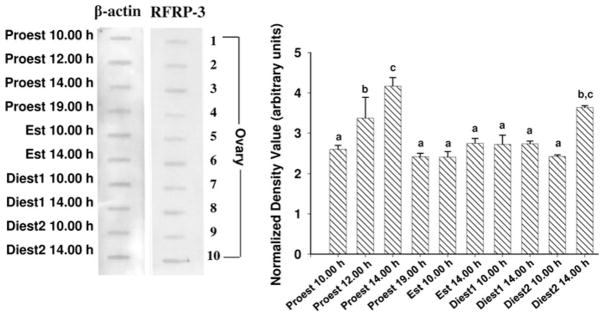
RFRP-3 protein concentration in the ovaries of mice was detected by slot blot. The densitometric analysis of RFRP-3 slot blot in the ovaries of mice during different phases of estrous cycle showed significant variation (P < 0.05; Fig. 3). The relative intensity of RFRP-3 slot blot revealed the most significant variation during proestrus. The intensity of RFRP-3 gradually increased from morning to attain a peak at 14.00 h and subsequently it declined sharply to a low level at 19.00 h on proestrus. The RFRP-3 concentration showed no significant change from late proestrus until early diestrus 2 phase. The intensity of RFRP-3 increased again to attain a second peak during diestrus 2 afternoon.
Fig. 3.
Slot blot for RFRP-3 in the ovaries of mice during estrous cycle. Densitometric analyses of the blots are given in bar graphs. Results were normalized with β-actin and are expressed in arbitrary units of signal intensity as ± SEM (N = 6). Bar bearing the different superscript (a–g) indicate significant difference between the mean values (P < 0.05)
Correlation between ovarian GnRH I, GnRH I-receptor and GnIH
A correlation study between relative density value of the immunoblots with specific antibodies for GnRH I, GnRH I receptor and GnIH based on analysis of total ovarian extract (shown in Figs. 3 and 4) was performed. GnRH I showed negative correlation with GnRH I-receptor (r = 0.375; P < 0.05) during estrous cycle of mouse.
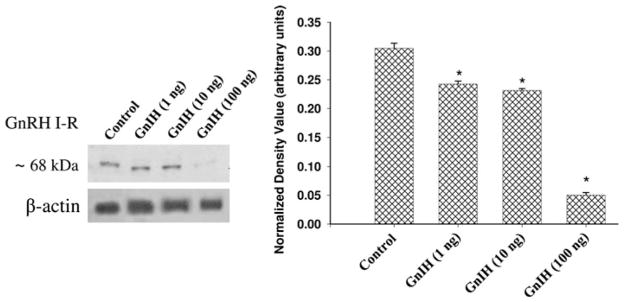
Fig. 4.
Effect of in vitro treatment of different doses of gonadotropin-inhibitory hormone (GnIH) on GnRH I-R protein expression in the ovaries of mice. Densitometric analysis of the blot is shown in bar graph. Values are represented as ± SEM (N = 3). *Values are significantly different (P < 0.01) versus control
Effect of GnIH on ovarian GnRH I-receptor expression in vitro
The in vitro effect of different doses of GnIH on GnRH I-receptor expression is shown in Fig. 4. The treatment of all the three doses of GnIH in vitro caused the significant (P < 0.01) decline in the expression of GnRH I-receptor protein as compared with the control. The declined in GnRH I-receptor protein expression was more pronounced in the ovary of mice treated in vitro with 100 ng dose of GnIH.
Discussion
The present study provides evidence for the presence of GnRH I and GnRH I-receptor together with RFRP-3 in the ovaries of mice during estrous cycle. Thus, it provides further support for the notion that there is a close relationship between GnRH I and RFRP-3 system in the gonad as suggested recently in the brain of the other vertebrate classes (Bentley et al. 2006; Ubuka et al. 2008). This is the first detailed study demonstrating the distribution of GnRH I, GnRH I-receptor together with RFRP-3 peptides using immunohistochemical localization and immunoblot analysis in the ovaries of mice in order to understand their role during estrous cycle. The study also showed a significant variation in the relative concentration of GnRH I, GnRH I-receptor and RFRP-3 during different phases of estrous cycle, which suggests the importance of GnRH-RFRP-3 system in the cyclic activity of mouse ovary.
Relative abundance of both GnRH I and GnRH I-receptor immunoreactivity in the ovary increases significantly in the evening of proestrus. This may suggests the possible involvement of gonadotropin surge in increased production of GnRH system during late proestrus. GnRH continued to remain high during estrus, although GnRH I-receptor concentration decreased significantly during this period. GnRH I-receptor levels seem to be somewhat of an inverse to GnRH I levels. It is well known that GnRH down-regulates its own receptor. When GnRH signals, the membrane GnRH-R is internalized and then degraded. So, high GnRH signaling may induce lower GnRH I-R levels. Similar correlation was also obtained in our present study (Fig. 2). In an earlier study on rat (Schirman-Hildesheim et al. 2005), relatively high GnRH—GnRH-receptor mRNA expression was observed in the early afternoon of proestrus. The difference in the time of peak GnRH–GnRH I-receptor concentration may be due to different rodent species.
The immunolocalization of GnRH I–GnRH I-receptor showed distinct cell specific distribution in the ovaries of mice during estrous cycle. Relatively most abundant immunoreactivity of GnRH I–GnRH I-receptor was mainly found in the antral follicles during proestrus and estrus, whereas immunoreactivity of these peptides were more abundant in corpus luteum during diestrus 1 and diestrus 2 phases. This suggests a tissue specific regulation of GnRH I–GnRH I-receptor proteins expression, which is likely a part of local auto-regulatory system. During proestrus and estrus phases, the immunoreactivity was mainly in the granulosa cells and theca cells of both healthy and atretic antral follicles. This is in accordance with the earlier study showing localization of both GnRH I and its receptor in the granulosa cells of the rat ovary (Schirman-Hildesheim et al. 2005; Whitelaw et al. 1995). The abundance of immunoreactivity of GnRH I and its receptor undergo significant variation during proestrus, which may be responsible for selection of follicles for ovulation. In human, a temporary increase in GnRH I-receptor concentration was found in the ovary at the time of ovulation (Brus et al. 1997). The presence of GnRH I and its receptor in theca cells suggests possible involvement of these peptides in regulation of gonadal steroid synthesis and release. It has earlier been suggested that GnRH may affect ovarian steroidogenesis possibly as a local autocrine and paracrine regulator by modulation of gonadotropin stimulated steroidogenesis in the ovary (Peng et al. 1994; Vaananen et al. 1997). The high concentration of GnRH I during estrus phase suggests that this peptide might be needed for growth and differentiation of new wave of follicular recruitment in mammals with repeated reproductive cycle (Schirman-Hildesheim et al. 2005). Changes in the immunolocalization of these peptides during diestrus 2 coincide with the beginning of luteolytic activity in the corpus luteum and a decline in progesterone concentration. It has earlier been suggested that GnRH may function as a permissive factor for the process of luteolysis (Leung and Cheng 2004). GnRH I-receptor mRNA is also demonstrated in human luteinized granulosa cells (Peng et al. 1994) and in human ovarian homogenate across different functional stages of the menstrual cycle (Minaretzis et al. 1995). The present finding together with earlier studies thus implies that multiple ovarian compartments of the mice may express GnRH I–GnRH I-receptor system.
GnRH has been involved in a variety of both inhibitory and stimulatory responses in the mammalian ovary (Leung et al. 2003) including a role in follicular atresia or selection (Whitelaw et al. 1995). Localization of GnRH I and GnRH I-receptor immunoresctivity in the granulosa cells of atretic follicles in the present study provide further support for a role of GnRH in the induction of follicular atresia in the mice ovary, as previously have been suggested for the rat (Andreu et al. 1998; Parborell et al. 2001). Also, the finding that ovarian GnRH activation blocks the trophic action of gonadotropins, led to suggests that it may be regulating follicular selection by promoting artesia in the ovary (Birnbaumer et al. 1985). More direct evidence was demonstrated later, in that GnRH agonist treatment in vivo has been shown to significantly reduced the meiotic activity of granulosa cells, but induce apoptotic cell death in granulosa cells (Billig et al. 1994) and stimulate the production of insulin like growth factor-binding protein 4 (IGFBP4), a marker for follicular atresia (Erickson et al. 1994).
The presence of RFRP-3 peptide, a mammalian ortholog of GnIH was also detected in the ovaries of mice by immunohistochemistry and further confirmed by immunoblotting. In the mice ovary, RFRP-3 immunoreactivity, was mainly localized in the granulosa cells and theca cells of antral follicles and interstitial cells. Bentley et al. 2008 has also reported the presence of GnIH and GnIH receptor in avian reproductive system including gonads and accessory reproductive organs. They showed the presence of GnIH peptide in ovarian theca and granulosa cells, testicular interstitial and germ cells and pseudostratified columnar epithelial cells in the epididymis in European starlings and white-crowned sparrows. RFRP-3-ir has been investigated in the brain of several rodent species like Syrian hamster, rat and mice (Kriegsfeld et al. 2006; Sethi et al. 2010a, b; Ukena and Tsutsui 2001) and mammalian testes during spermatogenic cycle (Zhao et al. 2010).
Densitometric analysis of the immonoblots suggests a gradual increase in relative concentration of RFRP-3 in the ovaries during proestrus with the highest concentration achieved during afternoon of proestrus. RFRP-3 concentration decreased sharply at 19.00 h of proestrus when GnRH I and GnRH I-receptor both were high. The relative concentration of RFRP-3 immunorectivity in the ovaries showed no significant variation from late proestrus to early diestrus 2 phases. A significant increase in the concentration of RFRP-3 immunoreactivity in the ovaries of mice during late diestrus 2 concided with the declined in corpus luteum activity and initiation of follicular growth and selection. This is also confirmed by immunohistochemistry which showed strong RFRP-3 immunoreactivity in the granulosa cells antral follicles as well as in corpus luteum. This suggests the potential for direct effects of RFRP-3 on GnRH I release also in the ovary as suggested in the brain (Bentley et al. 2006). In vitro experiment in the present study showed suppressive effect of GnIH on GnRH I-receptor in the ovary of mice. We have also demonstrated previously the suppressive effect of GnIH on GnRH I-receptor expression in the ovaries of Calotes versicolor (Singh et al. 2008). However, it is possible that GnIH regulates multiple ovarian functions in mice which may be the result of change in the concentration of GnRH I-receptor. It has earlier been described that GnIH/RFRP neurons project to GnRH neurons as well as to the median eminence in birds and mammals (Tsutsui 2009; Tsutsui et al. 2009, 2010a, b; Kriegsfeld et al. 2010). Additionally, in birds and hamster, confocal microscopy indicates that GnRH and RFRP-3 peptides were on the same optical plane suggesting functional interaction between these peptides (Bentley et al. 2006; Kriegsfeld et al. 2006). As in the brain, there are possible that RFRP-3 may inhibit GnRH and/or GnRH receptor levels in the ovary. Previously, it was demonstrated that GnIH inhibit GnRH elicited LH release in sparrow (Osugi et al. 2004). Our data demonstrate the presence of GnRH I, GnRH I-receptor and RFRP-3 are localized in close vicinity in the mouse ovary. Further study is required to find out the significance of interaction between GnRH - RFRP-3 in the ovaries of mice.
In summary, the present study showed significant variation in the localization and abundance of immunoreactivity of the neuropeptides, GnRH I, GnRH I-receptor and RFRP-3 in the ovary of mice during estrous cycle. GnRH I, and its receptors and RFRP-3 were mainly localized in the granulosa cells of the antral follicles. The immunoreactivity subsequently shifted from follicular cells to the luteal cells during diestrus. A significant increase in the concentration of RFRP-3 during diestrus 2 coincides with the decline in the activity of corpus luteum and initiation of follicular growth. It is thus hypothesized that the mouse ovary possess GnRH - RFRP-3 system and these neuropeptides may be involved in the regulation of follicular development and atresia.
Acknowledgments
This work was supported in part by Grant-in aid from UGC, New Delhi to (F. 34-420/2008 SR) AK; Scientific research from the Ministry of Education, Science and Culture, Japan (15207007, 16086206 and 18107002) to KT and grants from NIH, USA (HD41749, HD52155 and RR03034) to RS. PS is thankful to CSIR, India for providing research fellowships.
Footnotes
Conflict of Interest The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Contributor Information
Padmasana Singh, Department of Zoology, Banaras Hindu University, Varanasi 221005, India.
Amitabh Krishna, Email: akrishna_ak@yahoo.co.in, Department of Zoology, Banaras Hindu University, Varanasi 221005, India.
Rajagopala Sridaran, Department of Physiology, Morehouse School of Medicine, 720 Westview Dr SW, Atlanta, GA 30310-1495, USA.
Kazuyoshi Tsutsui, Laboratory of Integrative Bain Sciences, Department of Biology, Waseda University, Shinjuku-ku, Tokyo 162-8480, Japan.
References
- Andreu C, Parborell F, Vanzulli S, Chemes H, Tesone M. Regulation of follicular luteinization by a gonadotropin-releasing hormone agonist: relationship between steroidogenesis and apoptosis. Mol Reprod Dev. 1998;51:287–294. doi: 10.1002/(SICI)1098-2795(199811)51:3<287::AID-MRD8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Aten RF, Williams AT, Behrman HR. Ovarian gonadotropin-releasing hormone-like protein(s): demonstration and characterization. Endocrinology. 1986;118:961–967. doi: 10.1210/endo-118-3-961. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Kriegsfeld LJ, Osugi T, Ukena K, O’Brien S, Perfito N, Moore IT, Tsutsui K, Wingfield JC. Interactions of gonadotropin-releasing hormone (GnRH) and gonadotropin-inhibitory hormone (RFRP-3) in birds and mammals. J Exp Zool A Comp Exp Biol. 2006;305:807–814. doi: 10.1002/jez.a.306. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Ubuka T, McGuire NL, Chowdhury VS, Morita Y, Yano T, Hasunuma I, Binns M, Wingfield JC, Tsutsui K. Gonadotropin-inhibitory hormone and its receptor in the avian reproductive system. Gen Comp Endocrinol. 2008;156:34–43. doi: 10.1016/j.ygcen.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Billig H, Furuta I, Hsueh AJ. Gonadotropin-releasing hormone directly induces apoptotic cell death in the rat ovary: biochemical and in situ detection of deoxyribonucleic acid fragmentation in granulosa cells. Endocrinology. 1994;134:245–252. doi: 10.1210/endo.134.1.8275940. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, Shahabi N, Rivier J, Vale W. Evidence for a physiological role of gonadotropin-releasing hormone (GnRH) or GnRH-like material in the ovary. Endocrinology. 1985;116:1367–1370. doi: 10.1210/endo-116-4-1367. [DOI] [PubMed] [Google Scholar]
- Brus L, Lambalk CB, de Koning J, Helder MN, Janssens RM, Schoemaker J. Specific gonadotrophin-releasing hormone analogue binding predominantly in human luteinized follicular aspirates and not in human pre-ovulatory follicles. Hum Reprod. 1997;12:769–773. doi: 10.1093/humrep/12.4.769. [DOI] [PubMed] [Google Scholar]
- Chakrabarti N, Subbarao T, Sengupta A, Xu F, Stouffer RL, Sridaran R. Expression of mRNA and proteins for GnRH I and II and their receptors in primate corpus luteum during menstrual cycle. Mol Reprod Dev. 2008;75:1567–1577. doi: 10.1002/mrd.20898. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Li D, Sadrkhanloo R, Liu XJ, Shimasaki S, Ling N. Extrapituitary actions of gonadotropin-releasing hormone: stimulation of insulin-like growth factor-binding protein-4 and atresia. Endocrinology. 1994;134:1365–1372. doi: 10.1210/endo.134.3.7509739. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Humber SA, Jain S, Williams WP, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149:4958–4969. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillensjo T, LeMaire WJ. Gonadotropin releasing hormone agonists stimulate meiotic maturation of follicle-enclosed rat oocytes in vitro. Nature. 1980;287:145–146. doi: 10.1038/287145a0. [DOI] [PubMed] [Google Scholar]
- Hsueh AJ, Schaeffer JM. Gonadotropin-releasing hormone as a paracrine hormone and neurotransmitter in extra-pituitary sites. J Steroid Biochem Mol Biol. 1985;23:757–764. doi: 10.1016/s0022-4731(85)80011-x. [DOI] [PubMed] [Google Scholar]
- Jones PB, Hsueh AJ. Regulation of ovarian 20 alpha-hydroxysteroid dehydrogenase by gonadotropin releasing hormone and its antagonist in vitro and in vivo. J Steroid Biochem Mol Biol. 1981;14:1169–1175. doi: 10.1016/0022-4731(81)90047-9. [DOI] [PubMed] [Google Scholar]
- Jones PB, Conn PM, Marian J, Hsueh AJ. Binding of gonadotropin releasing hormone agonist to rat ovarian granulosa cells. Life Sci. 1980;27:2125–2132. doi: 10.1016/0024-3205(80)90494-4. [DOI] [PubMed] [Google Scholar]
- Knecht M, Ranta T, Feng P, Shinohara O, Catt KJ. Gonadotropin-releasing hormone as a modulator of ovarian function. J Steroid Biochem Mol Biol. 1985;23:771–778. doi: 10.1016/s0022-4731(85)80013-3. [DOI] [PubMed] [Google Scholar]
- Kogo H, Kudo A, Park MK, Mori T, Kawashima S. In situ detection of gonadotropin-releasing hormone (GnRH) receptor mRNA expression in the rat ovarian follicles. J Exp Zool. 1995;272:62–68. doi: 10.1002/jez.1402720108. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Nat Acad Sci USA. 2006;103:2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Gibson EM, Williams WP, III, Zhao S, Mason AO, Bentley GE, Tsutsui K. The roles of RFamide-related peptide-3 in mammalian reproductive function and behaviour. J Neuroendocrinol. 2010;22:692–700. doi: 10.1111/j.1365-2826.2010.02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung PKC, Cheng CK. GnRH as an autocrine regulator in the human ovary. In: Leung PKC, Adashi EY, editors. The ovary. Elsevier Academic press; Amsterdam: 2004. pp. 289–304. [Google Scholar]
- Leung PC, Cheng CK, Zhu XM. Multi-factorial role of GnRH-I and GnRH-II in the human ovary. Mol Cell Endocrinol. 2003;202:145–153. doi: 10.1016/s0303-7207(03)00076-5. [DOI] [PubMed] [Google Scholar]
- Li WI, Jiao S, Chin PP. Immunoreactive gonadotropin-releasing hormone in porcine reproductive tissues. Peptides. 1993;14:543–549. doi: 10.1016/0196-9781(93)90143-5. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Metallinou C, Asimakopoulos B, Schroer A, Nikolettos N. Gonadotropin-releasing hormone in the ovary. Reprod Sci. 2007;14:737. doi: 10.1177/1933719107310707. [DOI] [PubMed] [Google Scholar]
- Minaretzis D, Jakubowski M, Mortola JF, Pavlou SN. Gonadotropin-releasing hormone receptor gene expression in human ovary and granulosa-lutein cells. J Clin Endocrinol Metab. 1995;80:430–434. doi: 10.1210/jcem.80.2.7852501. [DOI] [PubMed] [Google Scholar]
- Osugi T, Ukena K, Bentley GE, O’Brien S, Moore IT, Wingfield JC, Tsutsui K. Gonadotropin-inhibitory hormone in Gambel’s white-crowned sparrow (Zonotrichia leucophrys gambelii): cDNA identification, transcript localization and functional effects in laboratory and field experiments. J Endocrinol. 2004;182:33–42. doi: 10.1677/joe.0.1820033. [DOI] [PubMed] [Google Scholar]
- Parborell F, Dain L, Tesone M. Gonadotropin-releasing hormone agonist affects rat ovarian follicle development by interfering with FSH and growth factors on the prevention of apoptosis. Mol Reprod Dev. 2001;60:241–247. doi: 10.1002/mrd.1084. [DOI] [PubMed] [Google Scholar]
- Parborell F, Pecci A, Gonzalez O, Vitale A, Tesone M. Effects of a gonadotropin-releasing hormone agonist on rat ovarian follicle apoptosis: regulation by epidermal growth factor and the expression of Bcl-2-related genes. Biol Reprod. 2002;67:481–486. doi: 10.1095/biolreprod67.2.481. [DOI] [PubMed] [Google Scholar]
- Peng C, Fan NC, Ligier M, Vaananen J, Leung PC. Expression and regulation of gonadotropin-releasing hormone (GnRH) and GnRH receptor messenger ribonucleic acids in human granulosa-luteal cells. Endocrinology. 1994;135:1740–1746. doi: 10.1210/endo.135.5.7956897. [DOI] [PubMed] [Google Scholar]
- Piquette GN, LaPolt PS, Oikawa M, Hsueh AJ. Regulation of luteinizing hormone receptor messenger ribonucleic acid levels by gonadotropins, growth factors, and gonadotropin-releasing hormone in cultured rat granulosa cells. Endocrinology. 1991;128:2449–2456. doi: 10.1210/endo-128-5-2449. [DOI] [PubMed] [Google Scholar]
- Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev. 1994;15:725–751. doi: 10.1210/edrv-15-6-725. [DOI] [PubMed] [Google Scholar]
- Schirman-Hildesheim TD, Bar T, Ben-Aroya N, Koch Y. Differential gonadotropin-releasing hormone (GnRH) and GnRH receptor messenger ribonucleic acid expression patterns in different tissues of the female rat across the estrous cycle. Endocrinology. 2005;146:3401–3408. doi: 10.1210/en.2005-0240. [DOI] [PubMed] [Google Scholar]
- Schirman-Hildesheim TD, Ben-Aroya N, Koch Y. Daily GnRH and GnRH-receptor mRNA expression in the ovariectomized and intact rat. Mol Cell Endocrinol. 2006;252:120–125. doi: 10.1016/j.mce.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Chakrabarti N, Sridaran R. Presence of immunoreactive gonadotropin releasing hormone (GnRH) and its receptor (GnRHR) in rat ovary during pregnancy. Mol Reprod Dev. 2008;75:1031–1044. doi: 10.1002/mrd.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Tsutsui K, Chaturvedi CM. Temporal phase relation of circadian neural oscillations alters RFamide-related peptide-3 and testicular function in the mouse. Neuroendocrinology. 2010a;91:189–199. doi: 10.1159/000265760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Tsutsui K, Chaturvedi CM. Age-dependent variation in the RFRP-3 neurons is inversely correlated with gonadal activity of mice. Gen Comp Endocrinol. 2010b;168:326–332. doi: 10.1016/j.ygcen.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Singh P, Krishna A, Sridaran R. Localization of gonadotrophin-releasing hormone I, bradykinin and their receptors in the ovaries of non-mammalian vertebrates. Reproduction. 2007;133:969–981. doi: 10.1530/REP-06-0106. [DOI] [PubMed] [Google Scholar]
- Singh P, Krishna A, Sridaran R, Tsutsui K. Changes in GnRH I, bradykinin and their receptors and GnIH in the ovary of Calotes versicolor during reproductive cycle. Gen Comp Endocrinol. 2008;159:158–169. doi: 10.1016/j.ygcen.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Krishna A, Tsutsui K. Effects of Gonadotropin-inhibitory hormone on folliculogenesis and steroidogenesis of cyclic mice. Fert Stert. 2011;95:1397–1404. doi: 10.1016/j.fertnstert.2010.03.052. [DOI] [PubMed] [Google Scholar]
- Sridaran R, Hisheh S, Dharmarajan AM. Induction of apoptosis by a gonadotropin-releasing hormone agonist during early pregnancy in the rat. Apoptosis. 1998;3:51–57. doi: 10.1023/a:1009611203705. [DOI] [PubMed] [Google Scholar]
- Sridaran R, Philip GH, Li H, Culty M, Liu Z, Stocco DM, Papadopoulos V. GnRH agonist treatment decreases progesterone synthesis, luteal peripheral benzodiazepine receptor mRNA, ligand binding and steroidogenic acute regulatory protein expression during pregnancy. J Mol Endocrinol. 1999;22:45–54. doi: 10.1677/jme.0.0220045. [DOI] [PubMed] [Google Scholar]
- Srivastava RK, Krishna A, Sridaran R. Follicular development, steroidogenesis and gonadotrophin secretion in response to long-term treatment with a gonadotrophin-releasing hormone agonist in the rat. J Endocrinol. 1995;146:349–357. doi: 10.1677/joe.0.1460349. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Billig H, Kowalski KI, Hsueh AJ. Epidermal growth factor and basic fibroblast growth factor suppress the spontaneous onset of apoptosis in cultured rat ovarian granulosa cells and follicles by a tyrosine kinase-dependent mechanism. Mol Endocrinol. 1992;6:1942–1950. doi: 10.1210/mend.6.11.1480180. [DOI] [PubMed] [Google Scholar]
- Tsutsui K. A new key neurohormone controlling reproduction, gonadotropin-inhibitory hormone (GnIH): biosynthesis, mode of action and functional significance. Progr Neurobiol. 2009;88:76–88. doi: 10.1016/j.pneurobio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Bentley GE. Gonadotropin-inhibitory hormone (GnIH): biosynthesis, mode of action and functional significance in birds. Avian Biol Res. 2008;1:177–188. [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Saigoh E, Yin H, Ubuka T, Chowdhury VS, Osugi T, Ukena K, Sharp PJ, Wingfield JC, Bentley GE. A new key neurohormone controlling reproduction, gonadotrophin-inhibitory hormone in birds: discovery, progress and prospects. J Neuroendocrinol. 2009;21:271–275. doi: 10.1111/j.1365-2826.2009.01829.x. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Bentley GE, Bedecarrats G, Osugi T, Ubuka T, Kriegsfeld LJ. Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Frontiers Neuroendocrinol. 2010a;31:284–295. doi: 10.1016/j.yfrne.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Bentley GE, Kriegsfeld LJ, Osugi T, Seong JY, Vaudry H. Discovery and evolutionary history of gonadotrophin-inhibitory hormone and kisspeptin: new key neuropeptides controlling reproduction. J Neuroendocrinol. 2010b;22:716–727. doi: 10.1111/j.1365-2826.2010.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubuka T, Kim S, Huang Yc, Reid J, Jiang J, Osugi T, Chowdhury VS, Tsutsui K, Bentley GE. Gonadotropin-inhibitory hormone neurons interact directly with gonadotropin-releasing hormone-I and -II neurons in European starling brain. Endocrinology. 2008;149:268–278. doi: 10.1210/en.2007-0983. [DOI] [PubMed] [Google Scholar]
- Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, Tsutsui K, Millar RP, Bentley GE. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS ONE. 2009;4:1–7. doi: 10.1371/journal.pone.0008400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena K, Tsutsui K. Distribution of novel RFamide-related peptide-like immunoreactivity in the mouse central nervous system. Neurosci Lett. 2001;300:153–156. doi: 10.1016/s0304-3940(01)01583-x. [DOI] [PubMed] [Google Scholar]
- Ukena K, Iwakoshi E, Minakata H, Tsutsui K. A novel rat hypothalamic RFamide-related peptide identified by immunoaffinity chromatography and mass spectrometry. FEBS Lett. 2002;512:255–258. doi: 10.1016/s0014-5793(02)02275-5. [DOI] [PubMed] [Google Scholar]
- Vaananen JE, Tong BL, Vaananen CM, Chan IH, Yuen BH, Leung PC. Interaction of prostaglandin F2alpha and gonadotro-pin-releasing hormone on progesterone and estradiol production in human granulosa-luteal cells. Biol Reprod. 1997;57:1346–1353. doi: 10.1095/biolreprod57.6.1346. [DOI] [PubMed] [Google Scholar]
- Whitelaw PF, Eidne KA, Sellar R, Smyth CD, Hillier SG. Gonadotropin-releasing hormone receptor messenger ribonucleic acid expression in rat ovary. Endocrinology. 1995;136:172–179. doi: 10.1210/endo.136.1.7828528. [DOI] [PubMed] [Google Scholar]
- Zhao S, Zhu E, Yang C, Bentley GE, Tsutsui K, Kriegsfeld LJ. RFamide-related peptide and messenger ribonucleic acid expression in mammalian testis: association with the spermatogenic cycle. Endocrinology. 2010;151:617–627. doi: 10.1210/en.2009-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]