Abstract
Background
Nodal staging of non–small-cell lung cancer (NSCLC) is crucial in evaluation of prognosis and determination of therapeutic strategy. This study aimed to determine the negative predictive value (NPV) of combined positron emission tomography and computed tomography (PET-CT) in patients with stage I (T1-2N0) NSCLC and to investigate the possible risk factors for occult nodal disease.
Methods
Studies investigating the performance of PET in conjunction with CT in the nodal staging of stage I NSCLC were identified in the MEDLINE database. The initiative of standards for reporting of diagnostic accuracy (STARD) was used to ensure study quality. Pathologic assessments through mediastinoscopy or thoracotomy were required as the reference standard for evaluation of PET-CT accuracy. Stata-based meta-analysis was applied to calculate the individual and pooled NPVs.
Results
Ten studies with a total of 1122 patients with stage I (T1-2N0) NSCLC were eligible for analysis. The NPVs of combined PET and CT for mediastinal metastases were 0.94 in T1 disease and 0.89 in T2 disease. Including both T1 disease and T2 disease, the NPVs were 0.93 for mediastinal metastases and 0.87 for overall nodal metastases. Adenocarcinoma histology type (risk ratio [RR], 2.72) and high fluorine-18 (18F) fluorodeoxyglucose (FDG) uptake in the primary lesion were associated with greater risk of occult nodal metastases.
Conclusions
Although overall occult nodal metastases in clinical stage T1-2N0 NSCLC is not infrequent, combined PET and CT provide a favorable NPV for mediastinal metastases in T1N0 NSCLC, suggesting a low yield from routine invasive staging procedures for this subgroup of patients.
Keywords: 18FDG-PET, Computed tomography, Lymph node metastasis, Meta-analysis, Negative predictive value, Non, small-cell lung cancer
Introduction
Lung cancer is the leading cause of cancer deaths worldwide. In 2008 there were an estimated 1.61 million new cases and 1.38 million deaths worldwide,1 among which 85% were non–small-cell lung cancer (NSCLC). Accurate staging, especially nodal staging, is a crucial factor for evaluation of prognosis and determination of treatment strategy in NSCLC.
Intravenous contrast-enhanced computed tomography (CT) is the most commonly used imaging modality for clinical staging. The predictive ability of CT for mediastinal lymph node metastasis has been well documented, with sensitivity and specificity of 57%–68% and 76–82%, respectively.2–6 Using the fluorine-18 (18F) fluorodeoxyglucose (FDG) tracer, positron emission tomography (PET) has much better performance in identification of nodal disease because abnormal metabolic uptake generally precedes anatomic change, providing a sensitivity of 79%–85% and a specificity of 87%–92%.2–6 Combined PET and CT (PET-CT), in particular integrated PET-CT, could further improve the accuracy of malignant node detection by combining information on spatial resolution, anatomic localization, and metabolic activity of the suspicious lesion.7,8
Traditionally, mediastinoscopy and systematic lymph node dissection have been regarded as the gold standard for the identification of mediastinal lymph node metastasis by offering pathologic proof of malignancy. The emerging transesophageal ultrasound-guided fine-needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) may serve as alternatives to mediastinoscopy but have not yet been validated.9–11 Nevertheless, all these modalities are invasive and highly dependent on operator expertise.
In theory, tumors in the early stage behave less aggressively and may have a lower risk of lymph node involvement. The reported presence of mediastinal lymph node metastasis in patients with stage I NSCLC determined by CT ranged from 6%–21%; this was verified by mediastinoscopy-based or thoracotomy-based lymph node sampling or dissection.12–14 The relatively low rate of nodal involvement calls into question how much benefit can be gained from routine invasive staging for patients with PET-CT–identified small primary lesions and negative nodal findings. We performed a meta-analysis to evaluate the negative predictive value (NPV) of PET-CT in patients with stage I NSCLC (AJCC 6th edition stage T1-2N0M0) and further investigate the potential risk factors for nodal involvement.
Materials and Methods
Study Eligibility and Identification
We attempted to identify all studies that investigated the diagnostic performance of combined FDG-PET and CT, either integrated or visually correlated, for nodal staging in patients with stage I (T1-2N0) NSCLC. Computerized search of the MEDLINE database was performed using the following keywords: positron emission tomography, non–small-cell lung cancer, stage I, lymph node. Mediastinoscopy, thoracotomy-based lymph node dissection, or lymph node sampling was required to verify mediastinal involvement. Abstracts were ruled out because of insufficient information for the evaluation of methodologic quality and the calculation of pertinent diagnostic parameters. In addition, we also reviewed references listed in the identified articles and included eligible studies for integrality of the literature search. Authors with more than 1 publication involving the same study population were included only once, and the one that was most relevant and complete was selected. Literature retrieval was terminated by February 2011.
Study Quality Control
To ensure that only high-quality studies were included in this analysis, we used methodology-related quality criteria from the Standards for Reporting of Diagnostic Accuracy (STARD) checklist15 covering 8 dimensions: (1) Description of study population, (2) Description of participant recruitment, (3) Cohort assembly, (4) Reference standard and its rationale, (5) Description of technical specifications of imaging, including how and when measurements were taken, (6) a clear definition of cutoffs, (7) Description of number and expertise of professionals reading the pet-ct scans or executing the invasive procedures, (8) Description of calculation methods for performance parameters. A score between 0 and 2 was assigned to each item: 2 represented the complete description or prospective design, 1 represented the partial depiction or retrospective design, and 0 indicated no matched description, making the maximum score 16.
Data Extraction
As the patients in this pooled setting carried clinical N-negative diseases (T1-2N0M0), the primary operating characteristic in this study was negative predictive value [NPV = true negatives/(true negatives + false negatives)]. The presence of occult nodal metastasis was defined as the likelihood that a patient with a normal PET-CT finding at nodal regions actually had pathologically proven nodal involvement (1-NPV). Patients with hilar nodal metastases alone were classified as having false-negative N1 disease, whereas those with synchronous mediastinal involvement were classified as having false-negative N2 disease. Patients with mediastinal metastases (with or without N1 disease) were defined as having false-negative N2 disease. As far as overall nodal analysis, patients with histologically proven nodal metastases (at any nodal station) were categorized as having false-negative disease.
Statistical Analysis
Meta-analysis was performed with the METAN routine in the Stata/SE 11 (StataCorp LP, College Station, TX). The heterogeneity across different studies was assessed with the Cochran Q test and also described with I square.16–19 A P value of .05 was elected as the threshold, < .05 indicating significant heterogeneity across studies. The pooled summary estimate of NPVs and corresponding 95% confidence intervals (CIs) were obtained using the fixed effects method of inverse-variance (I-V pooled effect size) if there was homogeneity among studies.20 In case of heterogeneity across studies, the random effects model of DerSimonian & Laird (D + L pooled effect size) was applied.21 The risk ratios (RRs) of occult nodal metastasis for different subgroups were estimated based on a 2 × 2 table using the fixed effects method of Mantel-Haenszel for homogeneous studies or the random effects model by DerSimonian and Laird for heterogeneous studies.21–23
Results
Study Identification and Quality
Sixty-seven English-language articles were retrieved in our initial literature search. After reviewing these articles and corresponding references, 10 studies were identified as eligible for this analysis.24–33 Results of the methodology quality assessment for all studies are shown in Table 1. Quality scores in the series ranged from 10 to 16, with both mean and median values of 13. The worst described item was technical specifications of imaging, with a total score of only 11 in the 10 studies. Eight of 10 studies provided particular depiction of CT and PET scanning procedures, 24–27,30–33 whereas only 3 studies specified the timing of PET and CT imaging acquisition, showing a mean interval of 10 days between PET-CT scanning and pathologic staging in 1 study27 and less than 1 month in another 2 studies.31,32 No correlation was observed between the publication year and quality score (P = .62).
Table 1.
Characteristics of 10 Individual Studies
| Author | Year | Quality Score | Study Type | Modality | Positive Nodal Definition | Reference | Patients (No.) | Stage | N1a NPV | N2a NPV | Overalla,b NPV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Farrell et al24 | 2000 | 12 | Retro | PET & CTc | Visuald or sizee | M, Tf | 65 | I | 0.97 | 1.0 | 0.97 |
| Konishi et al25 | 2003 | 14 | Retro | PET & CT | Visual or size | T | 41 | I | 0.95 | 0.98 | 0.93 |
| Cerfolio et al26 | 2005 | 15 | Pro | PET/CT,g CTh | SUV (≥ 2.5) or size | M, T, EUS-FNA | 140 | I | NA | 0.91 | NA |
| Kim et al27 | 2006 | 16 | Pro | PET/CT, CT | Visual or SUV (≥ 3.5) | M, T | 134 | IA | NA | 0.87 | NA |
| Lee et al28 | 2007 | 10 | Retro | PET & CT | SUV or size | M, T | 224 | I | NA | 0.93 | NA |
| 155 | IA | NA | 0.94 | NA | |||||||
| 69 | IB | NA | 0.91 | NA | |||||||
| Veeramachaneni et al29 | 2008 | 11 | Pro | PET & CT | PET-NAi or size | M, T | 108 | IA | 0.94 | 0.95 | 0.90 |
| Herth et al30 | 2008 | 15 | Pro | PET & CT | SUV (≥ 2.5) or size | M, T, EBUS-TBNA | 97 | I | 0.97 | 0.94b | 0.91 |
| Maeda et al31 | 2009 | 10 | Retro | PET/CT, CT | SUV or size | T | 58 | IA | 0.85 | 0.95 | 0.79 |
| Gómez-Caro et al32 | 2010 | 16 | Pro | PET/CT, CT | Visual or size | T | 108 | I | 0.82 | 0.86 | 0.68 |
| 47 | IA | 0.89 | 0.87 | 0.76 | |||||||
| 61 | IB | 0.75 | 0.85 | 0.60 | |||||||
| Park et al33 | 2010 | 11 | Retro | PET/CT, CT | Visual or size | M, T | 147 | IA | 0.91 | 0.95 | 0.86 |
Abbreviations: EBUS-TBNA = endobronchial ultrasound-guided transbronchial needle aspiration; EUS-FNA = endoscopic ultrasound-guided fine-needle aspiration; M = mediastinoscopy; NA = not available; NPV = negative predictive value; Pro = prospective; Retro = retrospective; SUV = maximum standard uptake value.
N1, hilar nodal metastasis alone; N2, mediastinal nodal metastasis with or without N1 disease; overall, positive nodal disease at N1 or N2.
Includes both N2 and N3 metastases.
Visually reviewed PET and CT side by side.
Visually higher uptake than surrounding normal tissue.
Short axis of lymph nodes.
Thoracotomy with nodal dissection/sampling.
Integrated PET and CT.
Dedicated CT.
Positive PET finding without description of cutoff.
Study Description
The characteristics of eligible studies are shown in Table 1. The number of patients with clinical stage I (T1-2N0) NSCLC in each study ranged from 41 to 224, with a median number of 108. Five studies were prospectively designed26,27,29–32 and 5 applied retrospective cohort assembly.24,25,28,33 Clinical staging was determined by side-by-side visual review of PET and CT (PET & CT) in 5 studies24,25,28–30 and by integrated PET-CT in the other 5 studies.26,27,31–33 Patients with overall stage I disease (T1-2N0) were evaluated in 6 studies,24–26,28,30,32 and patients with exclusively stage IA (T1N0) disease were enrolled in 4 studies.27,29,31,33 N2-related information was available in all studies and N1 disease was analyzable in only 7 studies.24,25,29–33 Eight studies declared no pathologic N3 disease24,25,27–29,31–33 and 1 study found 1 patient with N3 metastasis.30 N3 data was not shown in 1 study.26 Regarding a PET-based definition of positive nodal disease, 5 studies used higher FDG uptake on visualization than seen in surrounding normal tissue,24,25,27,32,33 and 5 studies used certain thresholds of maximum standard uptake value (SUV) to distinguish between benign and malignant manifestations.26–28,30,31 With 1 exception,27 all studies also took CT-based nodal size into account to identify positive nodal disease.
Negative Predictive Value for Mediastinal Metastases
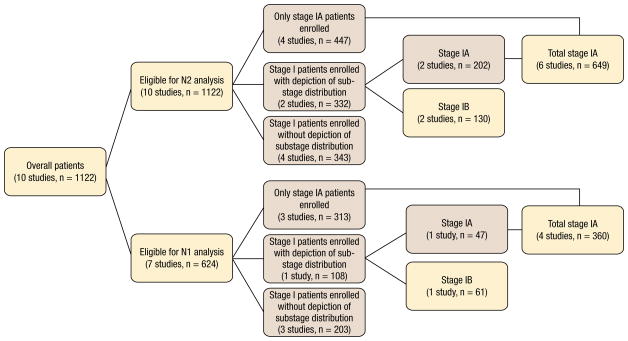
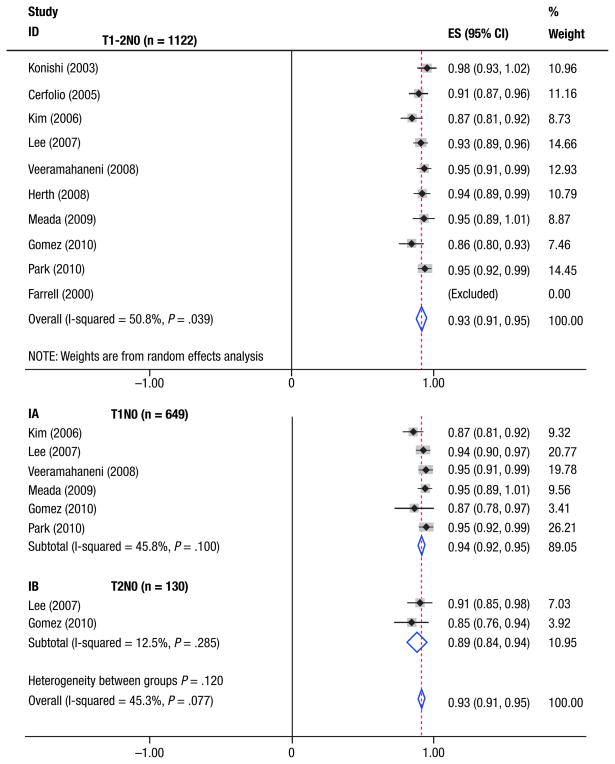
A total of 1122 patients from 10 studies with PET-CT– determined stage I (T1-2N0) NSCLC were eligible for the analysis. Figure 1 displays the stratification diagram, and the analyzed sub-population is emphasized in the pink boxes. Table 1 shows the NPVs of combined PET and CT for individual studies, ranging from 0.82–0.97 for hilar metastasis (N1 alone), 0.86–1.0 for N2 mediastinal metastasis, and 0.68–0.97 for overall nodal involvement. All studies were eligible for the calculation of the NPV for occult N2 mediastinal metastasis, and the summary estimates for NPVs are shown in Figure 2. Across all 10 studies, there was marked heterogeneity in NPVs (P = .04). The weighted estimate of NPV for N2 metastases in the whole population was 0.93 (95% CI, 0.91, 0.95), corresponding to 7% of occult diseases. Figure 2 also displays the NPVs for subgroup patients. For 649 patients from 6 studies with specific stage IA (T1N0) NSCLC,27–29,31–33 the NPVs ranged from 0.87–0.95, resulting in the summary estimated NPV of 0.94 (95% CI, 0.92, 0.96). Only 130 patients with stage IB (T2N0) NSCLC from 2 studies were eligible for N2 analysis,28,32 yielding a summary NPV of 0.89 (95% CI, 0.84, 0.95).
Figure 1.
Patient Stratification Diagram
Figure 2. Individual and Summary Estimated NPVs of Combined PET and CT for Patients With T1-2N0M0 NSCLC (Top) and Subgroup Patients With Specific T1N0M0 and T2N0M0 NSCLC (Bottom). Of Note, 1 Study was Excluded (Weighted 0.0) for the Pooled NPV Computation Regarding N2 Disease Because of Lack of False-Negative Event.
Abbreviation: ES = effect size.
Negative Predictive Value Per Nodal Station
Table 2 shows the pooled NPVs of combined PET and CT per nodal station within the same population. Based on 7 studies involving 624 patients with T1-2N0M0 disease,24,25,29–33 the estimated NPVs for N1 alone, N2, and overall nodal involvement were 0.92, 0.95, and 0.87, respectively. As far as specific T1 disease, 360 patients from 4 studies were eligible for estimation of NPV per nodal station,29,31–33 resulting in pooled NPVs of 0.92, 0.95, and 0.86 for N1, N2, and overall nodal involvement, respectively. Based on only 1 study, 32 the corresponding NPVs for each station in T2 diseases were 0.75, 0.85, and 0.61, respectively.
Table 2.
Summary Estimated NPV Per Nodal Station
| Stage | No. of Studies | No. of Patients | N1a | N2b | Overallc | |||
|---|---|---|---|---|---|---|---|---|
| NPV | 95% CI | NPV | 95% CI | NPV | 95% CI | |||
| I | 7 | 624 | 0.92 | 0.89, 0.96 | 0.95 | 0.93, 0.97 | 0.87 | 0.80, 0.93 |
| IA | 4 | 360 | 0.92 | 0.89, 0.94 | 0.95 | 0.93, 0.97 | 0.86 | 0.82, 0.89 |
| IB | 1 | 61 | 0.75 | 0.62, 0.85 | 0.85 | 0.73, 0.92 | 0.61 | 0.47, 0.73 |
Abbreviations: CI = confidence interval; NPV = negative predictive value.
Hilar nodal metastasis alone.
Mediastinal nodal metastasis with or without N1 disease.
Positive nodal disease at N1 or N2.
Potential Risk Factors for Occult Nodal Metastases
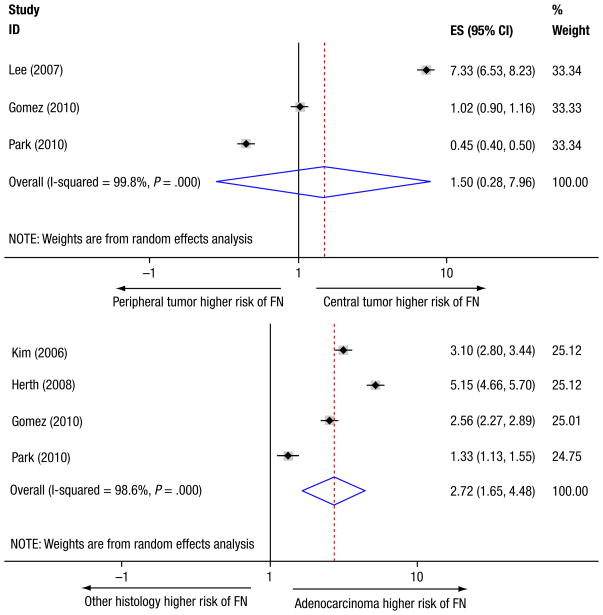
As mentioned before, occult nodal metastasis referred to the false-negative rate of combined PET and CT in detecting nodal diseases and could reflect NPV from another point of view. Several factors may be associated with the risk of occult nodal disease, such as tumor location, size, histologic type, and metabolic activity. On the basis of 3 studies,28,32,33 NPVs for nodal metastasis per primary tumor location (central vs. peripheral) are shown in Table 3. Figure 3 (top) displays the summary RR of central tumors having false-negative disease compared with peripheral tumors, suggesting no significant difference between locations (RR = 1.496; P = .64). Four studies examined whether primary tumor size had an impact on the rate of occult nodal metastasis.28,29,32,33 Two of these studies demonstrated that larger tumor size significantly predicted a higher presence of unforeseen nodal involvement.28,29 However based on 2 studies available for respective false-negative calculation in both T1 and T2 disease,28,32 no significant difference was found in pooled occult N2 metastases between T1 and T2 tumors (P = .53). Five studies investigated the effect of tumor histologic type on the presence of occult nodal metastasis.27,28,30,32,33 Table 4 lists the NPVs according to histologic type for each study as well as the summary estimation based on the pooled population. A marginally higher proportion of false-negative disease in adenocarcinoma was observed in 3 individual studies28,30,32; such a trend turned out to be statistically significant in the pooled patients, corresponding to an RR of 2.72 (Figure 3, bottom). In addition, 5 studies evaluated the correlation between maximum SUV and the presence of unsuspected nodal metastasis.26,28,31–33 Four of these studies reported that higher SUV in primary tumor was a predictor of nodal involvement, although there was great heterogeneity in the definition of SUV threshold among studies (range, 2.0–7.3).26,28,31,33
Table 3.
Correlation between Tumor Location and NPV
| Author | Year | Nodal Location | No. of Patients | NPV | P Valuea | |
|---|---|---|---|---|---|---|
| Central | Peripheral | |||||
| Lee et al28 | 2007 | N2b | 224 | 0.78 | 0.97 | <.001 |
| Gómez-Caro et al32c | 2010 | N2 | 125 | 0.85 | 0.86 | NS |
| Park et al33 | 2010 | N1d or N2 | 147 | 0.93 | 0.84 | NS |
| Total | 496 | 0.86 | 0.89 | .64 | ||
Abbreviations: NPV = negative predictive value; NS = no significant difference.
Crosstab χ2 test was used for proportion comparison in individual study; DerSimonian & Laird method was applied for pooling results.
Mediastinal nodal metastasis.
Additional 17 patients with cN0 NSCLC, including 10 with stage IIB and 7 with stage IIIB were also included for risk factor analysis.
Hilar nodal metastasis.
Figure 3. Tumor Location (Top) and Histologic Type (Bottom) for the Risk of Occult Nodal Metastases. Of Note, 1 Study was Excluded from the Pooled Risk Ratio Computation Regarding Histologic Subtypes for the Risk of Occult Nodal Metastasis due to Having No False-Negative Event in Nonadenocarcinoma Subgroup.
Abbreviations: ES = effect size; FN = false negative.
Table 4.
Correlation between Tumor Histologic Type and NPV
| Author | Year | Nodal Location | Patient No. | NPV | P Valuea | |
|---|---|---|---|---|---|---|
| Adenocarcinoma | Other | |||||
| Kim et al27 | 2006 | N2 | 134 | 0.84 | 0.95 | NS |
| Lee et al28 | 2007 | N2 | 224 | 0.91 | 1.0 | .047 |
| Herth et al30 | 2008 | N1b or N2c | 97 | 0.86 | 0.97 | .070 |
| Gómez-Caro et al32d | 2010 | N2 | 125 | 0.79 | 0.92 | .045 |
| Park et al33 | 2010 | N1 or N2 | 147 | 0.85 | 0.89 | NS |
| Total | 727 | 0.87 | 0.95 | <.001 | ||
Abbreviations: NPV = negative predictive value; NS = no significant difference.
Crosstab χ2 test was used for proportion comparison in individual study; DerSimonian & Laird method was applied for pooling results.
Hilar nodal metastasis.
Mediastinal nodal metastasis.
Additional 17 patients with cN0 NSCLC, including 10 with stage IIB and 7 with stage IIIB were also included for risk factor analysis.
Discussion
In this meta-analysis of 10 studies including 1122 patients with PET-CT–determined stage I (T1-2N0) NSCLC, the summary estimated NPV was 0.93 for mediastinal metastasis. The NPVs for mediastinal metastases in T1 and T2 subgroups were 0.94 and 0.89, respectively. In terms of the overall nodal metastases, the summary estimated NPV of PET-CT was 0.87 for stage I NSCLC. To our knowledge, this is the first combined study systemically evaluating the diagnostic performance of combined FDG-PET and CT for nodal staging in specific stage I NSCLC. All studies included in this meta-analysis were reasonably designed and presented with acceptable quality scores.
In the present analysis, the presence of N2 metastasis in stage I (T1-2N0) and exclusive stage IA (T1N0) disease was almost identical, which resulted from the predominance of the T1 component among the whole study population. Except for 2 studies with 237 patients not specifying substage distribution (T1 vs. T2),26,30 only 153 of 885 patients from the other 8 studies had stage IB (T2N0) disease. Therefore the general results of the present analysis were more likely to represent features of stage IA disease. The estimated 6% presence of occult N2 metastasis is pretty similar to a recent retrospective report, 34 which demonstrated a 7% N2 involvement in patients with pathologic T1 and clinical N0 NSCLC. Notably, a substantial number of patients with clinical T1 disease do not truly have pathologic T1 disease. Stiles et al reported that only 72.2% of patients with stage IA disease retained a pathologic T1 classification after surgical resection and 27.8% had postoperative upstaging of their T classification; the majority changed to stage stage IB/T2 primarily because of the visceral pleural invasion.35 Therefore the patients with truly pathologic T1 disease in our pooled study may have an even lower likelihood of occult mediastinal metastasis.
The low presence of mediastinal metastasis in patients with T1N0 disease that was clinically staged with PET-CT arouses much controversy on the routine practice of invasive staging for this group of patients. The average sensitivity of cervical mediastinoscopy for mediastinal staging is around 80% in an unselected population9,36 and it is likely to be even lower in selected patients with previous normal findings on PET-CT. Given the 6% presence of false-negative findings among patients with T1N0 disease in this study, mediastinal disease would be discovered in < 5% of patients undergoing additional mediastinoscopy. This estimation was consistent with a prospective report, which revealed a 6% detection rate and a 60% sensitivity rate for unsuspected N2 disease by performing both mediastinoscopy and EUS-FNA in patients with NSCLC who are clinically staged as N2 negative after integrated PET-CT and CT scanning.37 Therefore at least 16 patients with T1 disease and normal mediastinal findings on PET and CT would undergo futile invasive staging procedures to prevent 1 unnecessary thoracotomy, suggesting a low yield from routine invasive staging for this group of patients. In terms of T2N0 NSCLC in this pooled study, mediastinal involvement seemed more frequent, showing an NPV of 0.89 and a >10% incidence of unforeseen N2 metastases based on the limited number of patients. This finding is partially consistent with the standpoint that nodal metastasis increases with the tumor size,38,39 suggesting the necessity of further invasive staging procedures for patients with T2 disease despite negative nodal findings on PET & CT.
Conventionally, mediastinal status matters significantly for the determination of treatment strategy, such as the use of neoadjuvant therapy or immediate thoracotomy. N1 disease generally has limited influence on this part of decision making. Nevertheless, occult lymph node involvement regardless of nodal station is one of the main considerations for the implementation of stereotactic body radiation therapy. In this pooled study, the summary estimated NPV for overall nodal metastases in stage I NSCLC by PET-CT was 0.87, indicating up to a 13% false-negative rate. Thereby the invasive procedures should still be strongly recommended to achieve more accurate staging for those who are medically unfit for surgery.
In the present analysis, studies using either integrated PET-CT or a visual combination of PET and CT were all included. Regarding the sensitivity, specificity, and accuracy, a large number of studies have shown that integrated PET-CT is superior to the manual combination of PET and CT in detecting nodal disease.7,8 However limited data are available for the comparison of NPV between these two modalities. One study retrospectively assessed the diagnostic accuracies of fused PET-CT, PET and CT viewed side by side, PET alone, and CT alone for nodal staging in 260 patients with various oncologic diseases, corresponding to the NPVs of 96%, 92%, 91%, and 73%, respectively.40 Another preliminary study including 27 NSCLC patients observed an NPV of 94% for both coregistered PET-CT and dedicated PET.41 One study reported a slight advantage of integrated PET-CT over PET alone when assessing the NPV of N2 disease, though the difference was not significant.42 In light of the similar NPVs of integrated PET-CT and visual correlation of PET and CT for nodal staging, as well as the limited number of eligible studies, it was reasonable to pool these two combination patterns together for this analysis.
Multiple factors such as scanning equipment, observer interpretation of tests, and inherent characteristics of tumors may contribute to false-negative findings on PET-CT. Limited spatial resolution may be the primary cause for PET-based false-negative findings. Spatial resolution of current-generation PET scanners is typically 5–7 mm and theoretically it is difficult to detect lesions <7 mm based on PET imaging. Even so, one report showed that PET correctly detected 88% of false-negative nodes <10 mm that were missed on CT.43 The author speculated that the high-contrast resolution may compensate for the limited spatial resolution and thus enhance the detection of micrometastases. Another study demonstrated that 38% of false-negative nodes on CT (<10 mm in the short axis) were successfully detected by integrated PET-CT and the smallest node on CT that showed a true-positive uptake on integrated PET-CT was 3.8 mm.27 This evidence suggested that positive FDG uptake for even very small nodes should not be ignored. Besides the FDG uptake, CT-de-fined nodal size can still be of great value for the identification of nodal malignancy. A meta-analysis revealed that the predicted posttest probability of malignancy was 5% for enlarged nodes measuring 10 to 15 mm with a normal finding on PET and 20% for those larger than 15 mm.44 This result suggested that nodes larger than 15 mm, although without abnormal FDG uptake, should not be neglected either. In our analysis, 9 of 10 studies took both tumor metabolic activity and size into consideration; either showing abnormality would be considered suspicious for malignancy.
Stage I NSCLC disease is seen in a heterogeneous group of patients with varying tumor size, location, histologic type, and metabolic activity. Besides the previously mentioned lymph node features such as size and metabolic activity, characteristics of primary tumor may also be predictive of nodal involvement. In addition to the aforementioned tumor size and location,45,46 histologic type 38,47 and FDG uptake of primary tumor 39,48,49 may be potential predictors of nodal metastases as well. Based on the specific patient pool, we quantitatively validated that adenocarcinoma carried a much greater risk of nodal involvement, whereas no significant effect was found for tumor location on nodal involvement. Abnormal FDG uptake was the most consistently reported risk factor across studies, although there was dramatic discrepancy in SUV threshold for risk hierarchy. Prospective studies enrolling larger numbers of patients to explore the optimal SUV threshold for prediction of nodal metastasis are warranted. A multiinstitutional clinical trial is ongoing to prospectively investigate the presence of occult N2/3 metastases and the sensitivity of routine cervical mediastinoscopy in potentially high-risk patients with stage I NSCLC (PET- and CT-staged T2N0 NSCLC as well as T1N0 with a maximum SUV > 10 of the primary tumor, NCT01146366).50
Conclusions
In summary, combined PET and CT provide a favorable NPV for mediastinal metastases in clinical T1N0 NSCLC, and the presence of occult mediastinal involvement is around 6%, inferring a low yield from routine invasive staging procedures for this group of patients. Patients with T2 disease, adenocarcinoma histology, or high FDG uptake in primary lesions have a higher risk of nodal metastases, and the invasive staging procedures are recommended before the initiation of any active treatment.
Acknowledgments
We would like to thank Dr. Morand Piert and Dr. Kirk Frey for their valuable comments. We also thank Matthew Schipper for his comments on statistical analysis. We are appreciative of the efforts of Paul Stanton for editing the manuscript.
This work was funded in part by R21CA127057 and R01 CA142840.
Footnotes
This study was presented as a Mini oral presentation at the 14th World Conference on Lung Cancer, Amsterdam, Netherland, (July 3–7, 2011).
Disclosure
All authors report that they have no relevant relationships to disclose.
References
- 1.Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No 10 [Internet] Lyon, France: International Agency for Research on Cancer; 2010. [Accessed: August 30, 2011]. Available at: http://globocan.iarc.fr. [Google Scholar]
- 2.Alongi F, Ragusa P, Montemaggi P, et al. Combining independent studies of diagnostic fluorodeoxyglucose positron-emission tomography and computed tomography in mediastinal lymph node staging for non-small cell lung cancer. Tumori. 2006;92:327–33. doi: 10.1177/030089160609200412. [DOI] [PubMed] [Google Scholar]
- 3.Birim O, Kappetein A, Stijnen T, et al. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg. 2005;79:375–82. doi: 10.1016/j.athoracsur.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 4.Gould M, Kuschner W, Rydzak C, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med. 2003;139:879–92. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- 5.Toloza E, Harpole L, McCrory D. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest. 2003;123:137S–46S. doi: 10.1378/chest.123.1_suppl.137s. [DOI] [PubMed] [Google Scholar]
- 6.Dwamena B, Sonnad S, Angobaldo J, et al. Metastases from non-small cell lung cancer: mediastinal staging in the 1990s—meta-analytic comparison of PET and CT. Radiology. 1999;213:530–6. doi: 10.1148/radiology.213.2.r99nv46530. [DOI] [PubMed] [Google Scholar]
- 7.Lardinois D, Weder W, Hany T, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–7. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 8.Cerfolio R, Ojha B, Bryant A, et al. The accuracy of integrated PET-CT compared with dedicated PET alone for the staging of patients with nonsmall cell lung cancer. Ann Thorac Surg. 2004;78:1017–23. doi: 10.1016/j.athoracsur.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 9.Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA. 2010;304:2245–52. doi: 10.1001/jama.2010.1705. [DOI] [PubMed] [Google Scholar]
- 10.Defranchi SA, Edell ES, Daniels CE, et al. Mediastinoscopy in patients with lung cancer and negative endobronchial ultrasound guided needle aspiration. Ann Thorac Surg. 2010;90:1753–7. doi: 10.1016/j.athoracsur.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 11.Cerfolio RJ, Bryant AS, Eloubeidi MA, et al. The true false negative rates of esophageal and endobronchial ultrasound in the staging of mediastinal lymph nodes in patients with non-small cell lung cancer. Ann Thorac Surg. 2010;90:427–34. doi: 10.1016/j.athoracsur.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 12.Choi YS, Shim YM, Kim J, et al. Mediastinoscopy in patients with clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2003;75:364–6. doi: 10.1016/s0003-4975(02)04411-9. [DOI] [PubMed] [Google Scholar]
- 13.Seely J, Mayo J, Miller R, et al. T1 lung cancer: prevalence of mediastinal nodal metastases and diagnostic accuracy of CT. Radiology. 1993;186:129–32. doi: 10.1148/radiology.186.1.8416552. [DOI] [PubMed] [Google Scholar]
- 14.Defranchi SA, Cassivi SD, Nichols FC, et al. N2 disease in T1 non-small cell lung cancer. Ann Thorac Surg. 2009;88:924–8. doi: 10.1016/j.athoracsur.2009.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Fam Pract. 2004;21:4–10. doi: 10.1093/fampra/cmh103. [DOI] [PubMed] [Google Scholar]
- 16.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 17.Gavaghan DJ, Moore RA, McQuay HJ. An evaluation of homogeneity tests in meta-analyses in pain using simulations of individual patient data. Pain. 2000;85:415–24. doi: 10.1016/S0304-3959(99)00302-4. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutton A, Abrams K, Jones D, et al. Methods for Meta-Analysis in Medical Research. Chichester, UK: John Wiley & Sons Ltd; 2000. [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 23.Robins J, Breslow N, Greenland S. Estimators of the Mantel-Haenszel variance consistent in both sparse data and large-strata limiting models. Biometrics. 1986;42:311–23. [PubMed] [Google Scholar]
- 24.Farrell M, McAdams H, Herndon J, et al. Non-small cell lung cancer: FDG PET for nodal staging in patients with stage I disease. Radiology. 2000;215:886–90. doi: 10.1148/radiology.215.3.r00jn29886. [DOI] [PubMed] [Google Scholar]
- 25.Konishi J, Yamazaki K, Tsukamoto E, et al. Mediastinal lymph node staging by FDG-PET in patients with non-small cell lung cancer: analysis of false-positive FDG-PET findings. Respiration. 2003;70:500–6. doi: 10.1159/000074207. [DOI] [PubMed] [Google Scholar]
- 26.Cerfolio RJ, Bryant AS, Ojha B, et al. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg. 2005;80:1207–13. doi: 10.1016/j.athoracsur.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Kim B, Lee K, Shim S, et al. Stage T1 non-small cell lung cancer: preoperative mediastinal nodal staging with integrated FDG PET/CT—a prospective study. Radiology. 2006;241:501–9. doi: 10.1148/radiol.2412051173. [DOI] [PubMed] [Google Scholar]
- 28.Lee P, Port J, Korst R, et al. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:177–81. doi: 10.1016/j.athoracsur.2007.03.081. [DOI] [PubMed] [Google Scholar]
- 29.Veeramachaneni N, Battafarano R, Meyers B, et al. Risk factors for occult nodal metastasis in clinical T1N0 lung cancer: a negative impact on survival. Eur J Cardiothorac Surg. 2008;33:466–9. doi: 10.1016/j.ejcts.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Herth FJ, Eberhardt R, Krasnik M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest. 2008;133:887–91. doi: 10.1378/chest.07-2535. [DOI] [PubMed] [Google Scholar]
- 31.Maeda R, Isowa N, Onuma H, et al. The maximum standardized 18F-fluorodeoxyglucose uptake on positron emission tomography predicts lymph node metastasis and invasiveness in clinical stage IA non-small cell lung cancer. Interact Cardiovasc Thorac Surg. 2009;9:79–82. doi: 10.1510/icvts.2008.201251. [DOI] [PubMed] [Google Scholar]
- 32.Gómez-Caro A, Garcia S, Reguart N, et al. Incidence of occult mediastinal node involvement in cN0 non-small-cell lung cancer patients after negative uptake of positron emission tomography/computed tomography scan. Eur J Cardiothorac Surg. 2010;37:1168–74. doi: 10.1016/j.ejcts.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Park HK, Jeon K, Koh WJ, et al. Occult nodal metastasis in patients with non-small cell lung cancer at clinical stage IA by PET/CT. Respirology. 2010;15:1179–84. doi: 10.1111/j.1440-1843.2010.01793.x. [DOI] [PubMed] [Google Scholar]
- 34.Casiraghi M, Travaini LL, Maisonneuve P, et al. Lymph node involvement in T1 non-small-cell lung cancer: could glucose uptake and maximal diameter be predictive criteria? Eur J Cardiothorac Surg. 2011;39:e38–43. doi: 10.1016/j.ejcts.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 35.Stiles BM, Servais EL, Lee PC, et al. Point: clinical stage IA non-small cell lung cancer determined by computed tomography and positron emission tomography is frequently not pathologic IA non-small cell lung cancer: the problem of understaging. J Thorac Cardiovasc Surg. 2009;137:13–9. doi: 10.1016/j.jtcvs.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 36.Shrager JB. Mediastinoscopy: still the gold standard. Ann Thorac Surg. 2010;89:S2084–9. doi: 10.1016/j.athoracsur.2010.02.098. [DOI] [PubMed] [Google Scholar]
- 37.Cerfolio RJ, Bryant AS, Eloubeidi MA. Routine mediastinoscopy and esophageal ultrasound fine-needle aspiration in patients with non-small cell lung cancer who are clinically N2 negative: a prospective study. Chest. 2006;130:1791–5. doi: 10.1378/chest.130.6.1791. [DOI] [PubMed] [Google Scholar]
- 38.Asamura H, Nakayama H, Kondo H, et al. Lymph node involvement, recurrence, and prognosis in resected small, peripheral, non-small-cell lung carcinomas: are these carcinomas candidates for video-assisted lobectomy? J Thorac Cardiovasc Surg. 1996;111:1125–34. doi: 10.1016/s0022-5223(96)70213-1. [DOI] [PubMed] [Google Scholar]
- 39.Ishida T, Yano T, Maeda K, et al. Strategy for lymphadenectomy in lung cancer three centimeters or less in diameter. Ann Thorac Surg. 1990;50:708–13. doi: 10.1016/0003-4975(90)90666-t. [DOI] [PubMed] [Google Scholar]
- 40.Antoch G, Saoudi N, Kuehl H, et al. Accuracy of whole-body dual-modality fluorine-18-2-fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography (FDG-PET/CT) for tumor staging in solid tumors: comparison with CT and PET. J Clin Oncol. 2004;22:4357–68. doi: 10.1200/JCO.2004.08.120. [DOI] [PubMed] [Google Scholar]
- 41.Antoch G, Stattaus J, Nemat AT, et al. Non-small cell lung cancer: dual-modality PET/CT in preoperative staging. Radiology. 2003;229:526–33. doi: 10.1148/radiol.2292021598. [DOI] [PubMed] [Google Scholar]
- 42.Halpern BS, Schiepers C, Weber WA, et al. Presurgical staging of non-small cell lung cancer: positron emission tomography, integrated positron emission tomography/CT, and software image fusion. Chest. 2005;128:2289–97. doi: 10.1378/chest.128.4.2289. [DOI] [PubMed] [Google Scholar]
- 43.Gupta NC, Graeber GM, Bishop HA. Comparative efficacy of positron emission tomography with fluorodeoxyglucose in evaluation of small (<1 cm), intermediate (1 to 3 cm), and large (>3 cm) lymph node lesions. Chest. 2000;117:773–8. doi: 10.1378/chest.117.3.773. [DOI] [PubMed] [Google Scholar]
- 44.de Langen AJ, Raijmakers P, Riphagen I, et al. The size of mediastinal lymph nodes and its relation with metastatic involvement: a meta-analysis. Eur J Cardiothorac Surg. 2006;29:26–9. doi: 10.1016/j.ejcts.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Ketchedjian A, Daly BD, Fernando HC, et al. Location as an important predictor of lymph node involvement for pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 2006;132:544–8. doi: 10.1016/j.jtcvs.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 46.Kanzaki R, Higashiyama M, Fujiwara A, et al. Occult mediastinal lymph node metastasis in NSCLC patients diagnosed as clinical N0-1 by preoperative integrated FDG-PET/CT and CT: risk factors, pattern, and histopathological study. Lung Cancer. 2011;71:333–7. doi: 10.1016/j.lungcan.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Al-Sarraf N, Aziz R, Gately K, et al. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardiothorac Surg. 2008;33:104–9. doi: 10.1016/j.ejcts.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 48.Li M, Liu N, Hu M, et al. Relationship between primary tumor fluorodeoxyglucose uptake and nodal or distant metastases at presentation in T1 stage non-small cell lung cancer. Lung Cancer. 2009;63:383–6. doi: 10.1016/j.lungcan.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Higashi K, Ito K, Hiramatsu Y, et al. 18F-FDG uptake by primary tumor as a predictor of intratumoral lymphatic vessel invasion and lymph node involvement in non-small cell lung cancer: analysis of a multicenter study. J Nucl Med. 2005;46:267–73. [PubMed] [Google Scholar]
- 50.Clinicaltrials.gov [Web site] [Accessed: August 30, 2011];Utility of routine cervical medisatinoscopy in clinical stage I non-small cell lung cancer (NSCLC) Available at: http://clinicaltrials.gov/ct2/show/NCT01146366.