Abstract
Because stem cells are often found to improve repair tissue including heart without evidence of engraftment or differentiation, mechanisms underlying wound healing are still elusive. Several studies have reported that stem cells can fuse with cardiomyocytes either by permanent or partial cell fusion processes. However, the respective physiological impact of these 2 processes remains unknown in part because of the lack of knowledge of the resulting hybrid cells. To further characterize cell fusion, we co-cultured mouse fully differentiated cardiomyocytes with human multipotent adipose-derived stem cells (hMADS cells) as a model of adult stem cells. We found that heterologous cell fusion promoted cardiomyocyte reprogramming back to a progenitor-like state. The resulting hybrid cells expressed early cardiac commitment and proliferation markers such as GATA-4, myocyte enhancer factor 2C, Nkx2.5 and Ki67 and exhibited a mouse genotype. Interestingly, human bone-marrow derived stem cells shared similar reprogramming properties than hMADS cells but not human fibroblasts, which suggests that these features might be common to multipotent cells. Furthermore, cardiac hybrid cells were preferentially generated by partial rather than permanent cell fusion and that intercellular structures composed of f-actin and microtubule filaments were involved in the process. Finally, we showed that stem cell mitochondria were transferred into cardiomyocytes, persisted in hybrids and were required for somatic cell reprogramming. In conclusion, by providing new insights into previously reported cell fusion processes, our data might contribute to a better understanding of stem cell-mediated regenerative mechanisms and thus, the development of more efficient stem cell-based heart therapies.
Keywords: Animals; Cell Differentiation; genetics; physiology; Cell Fusion; Cell Line; Cells, Cultured; Fibroblasts; cytology; metabolism; Humans; Immunohistochemistry; In Situ Hybridization; Male; Mesenchymal Stem Cells; cytology; metabolism; Mice; Mitochondria; metabolism; Myocytes, Cardiac; cytology; metabolism; Nuclear Reprogramming; genetics; physiology; Reverse Transcriptase Polymerase Chain Reaction
Keywords: mesenchymal stem cells, cardiomyocytes, cell fusion, nuclear reprogramming.
INTRODUCTION
Heart degenerative diseases such as myocardial infarction and heart failure are among the main causes of death in western countries. Such mortality has its roots in the poor ability of the myocardium to restore cardiomyocyte loss upon injury, which explains why rebuilding the damaged heart remains a critical challenge. One of the most promising approaches to repair this organ is cell therapy, particularly that based on multipotent adult stem cells [1–3]because such cells might benefit from immune priviledge [3,4]and can be expanded on a large scale in vitro [1–3]. However, although many studies suggested the benefit of stem cell grafting, the mechanisms by which such cells contribute to cardiac wound healing remain a matter of debate.
The functional benefit of stem cells may result from several processes, including transdifferentiation into cardiac and endothelial cells [5–7]or secretion of paracrine factors with various effects such as protection against apoptosis and induction of angiogenesis [8]. Another intriguing possibility is that permanent cell fusion between stem and resident cardiac cells may promote replacement of the dead myocardium by generating new cardiomyocytes in response to injury. Indeed, several in vivo and in vitro studies involving the Cre/loxP system or transgenic rodents expressing GFP or β-galactosidase reporter genes showed that both adult and embryonic stem cells could fuse with somatic cells such as neurons, hepatocytes, and cardiomyocytes [9–11]. Permanent cell fusion results in the formation of binucleated heterokaryons, which in some cases undergo nuclear fusion to give rise to mononucleated hyperploid synkaryons. In heart tissue, permanent cell fusion has been detected after stem cell delivery in various settings, including the healthy neonatal heart [12], myocardial infarction [13–15], mdx dystrophin-deficient cardiomyopathy [16], and monocrotaline-induced hypertension [17]. However, despite evidence of hybrid cells with a cardiac phenotype [13–17], such a phenomenon was described as being extremely rare and led critics to cast doubts on its biological significance [6,18,19].
Recently, 2 in vitro studies mimicking the cardiac environment by co-culturing stem cells and cardiomyocytes suggested that hybrid cells may be generated by a mechanism other than permanent cell fusion, one that consists of partial cell fusion through transient direct cell-to-cell communication and intercellular exchange of various compounds [20, 21]. This new transient cell fusion pathway, discovered in 2004, is based on the formation of membrane thin channels, referred to as tunneling nanotubes, that mediate membrane continuity between connected cells, sometimes over long distances [22]. These nanotubular structures have been found to connect a broad range of cultured mammalian cells [23]and to permit transfer of several components such as multi-protein complexes [24], organelles [25–27]and pathogens [28–30]. Specially, stem cells and cardiomyocytes have been reported to exchange in vitro cytoplasm macromolecules and organelles through intercellular structures resembling nanotubes [20, 21]. However, whether these cellular transfers play a role in cell fate change and tissue repair remains to be formally demonstrated.
Thus, the phenomenon of cell fusion in heart tissue appears to be poorly understood. In particular, the respective physiological importance of partial versus permanent cell fusion processes is unclear, and little is known about the cellular and molecular mechanisms underlying spontaneous generation of hybrid cells, as well as the characterization of their phenotypes.
Here, we hypothesized that stem-cell–mediated fusion with terminally differentiated cells constitutes a regenerative mechanism by which the nuclei of somatic cells are reprogrammed, thus resulting in selective survival and proliferative advantages. To promote cell fusion events, we developed a species mismatch co-culture approach using mouse terminally differentiated cardiomyocytes and human multipotent adipose-derived stem cells (hMADS cells)[3]as a model of adult stem cells. Using a Cre/loxP system, we found that hMADS cells could reprogram post-mitotic murine cardiomyocytes into proliferating cardiac progenitor-like cells through spontaneous cell fusion. In addition, we showed that successful somatic reprogramming of cardiomyocytes required both intercellular structures composed of f-actin and microtubules and transfer of functional stem-cell mitochondria into cardiomyocytes.
MATERIALS AND METHODS
Cell isolation and culture
For hMADS cell isolation, adipose tissues were obtained from young donors, with written informed consent of the parents, as surgical scraps from surgical specimens obtained from various surgeries, as approved by the Regional Ethical Committee (“Comité de Protection des Personnes Ile de France IX”). HMADS cells were isolated as previously described [3]. Human bone-marrow mesenchymal stem cells (hBMSCs) were a generous gift from Dr Hélène Rouard (Etablissement Français du Sang, Créteil, France). hBMSCs were isolated from iliac-crest bone-marrow aspirates from patients undergoing standard bone-marrow transplantation (Henri Mondor Hospital, Créteil, France) after receiving their informed consent [31].
The human lung fibroblast MRC5 cell line was a gift from Dr. Jorge Boczkowski (INSERM U955, Créteil). HMADS cells, hBMSCs and MRC5 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM), 1 g/l glucose containing 10% heat inactivated fetal bovine serum (FBS) (Dominique Dutscher), 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 mM HEPES (Invitrogen) in a 5% CO2 atmosphere at 37°C. As described [3], hMADS cells had the following phenotype: CD44+, CD49b+, CD105+, CD90+, CD13+, Stro-1−, CD34−, CD15−, CD117−, Flk-1−, Gly-A−, CD133−, HLA-DR− and HLA-Ilow.
Generation of hMADS cells devoid of functional mitochondria (hMADSρ0 cells)
For mitochondrial DNA depletion, hMADS cells were cultivated in DMEM supplemented with 10% FBS and 0.5 μg/ml ethidium bromide (Sigma-Aldrich), 50 μg/ml uridine and 100 μg/ml pyruvate for at least 1 month [32–34].
Isolation of adult mouse ventricular myocytes
Ventricular cardiomyocytes were isolated from hearts of 2- to 5-month old male mice after intracardiac perfusion of enzymatic solution containing 12.5 μM CaCl2 (Sigma-Aldrich), 0.1 mg/ml liberase blendzyme IV (Roche diagnostics), 0.14 mg/mL trypsine (Sigma-Aldrich), as described [35]. Cardiomyocytes were obtained from several mouse lines, including C57BL/6J mice (Janvier), GCAG-GFP transgenic mice [36](gift of Dr Fabrice Chrétien, Pasteur Institute, Paris, France) and Rosa26R transgenic mice (gift of Dr. Shaharagim Tajbakhsh, Pasteur Institute, Paris, France).
Co-culture with adult mouse cardiomyocytes
Freshly isolated mouse cardiomyocytes were co-cultured with hMADS cells, hBMSCs or MRC5 cells in DMEM supplemented with 10% FBS in a 1:1 ratio, each cell type seeded at 3,500 cells/cm2.
In experiments preventing physical contact between the 2 cell types, cardiomyocytes were seeded on cell-culture inserts containing a polycarbonate membrane with a 0.4-μm pore (Millicell, Millipore) placed in 35-mm dishes plated with hMADS cells.
To assess passive human mitochondria transfer, cells were indirectly co-cultured by use of polycarbonate cell-culture inserts with an 8-μm pore (Millicell, Millipore). For co-culture with hMADSρ0 cells, culture medium was supplemented with 50 μg/ml uridine and 100 μg/ml pyruvate to permit survival of stem cells devoid of functional mitochondria.
Detection of cell fusion
For detection of fused cells, hMADS cells were infected with a human serotype V adenovirus expressing Cre under a cytomegalovirus promoter (gift of Dr Athanassia Sotiropoulos, Cochin Institute, Paris, France) at a multiplicity of infection of 50 at 72 hr before co-culture with Rosa26R cardiomyocytes. Rosa26R mice carried the lacZ gene that encodes for β-galactosidase under the control of an internal stop codon flanked by loxP sites [37]. When Cre-expressing cells fuse with Rosa26R cells, Cre recombinase contacts the genome of Rosa26R cells and excises its floxed stop cassette, thus resulting in expression of LacZ in the fused cells. Consequently, fused cells and their progeny can be detected by Xgal staining (substrate of β-galactosidase) or by immunocytochemistry with antibodies against β-galactosidase.
Xgal staining
For Xgal staining, co-cultured cells were fixed with 2% paraformaldehyde (PFA) before being placed in phosphate buffer containing 10 mM K3Fe(CN)6, 10 mM K4Fe(CN)6 and 1 mg/ml Xgal (Qiagen) at 37°C for 24 hr.
Immunocytochemistry
Cells were fixed with 4% PFA or methanol-acetone (50:50) and stained with antibodies (Abs) against GATA-4 (goat polyclonal Ab [pAb], 1:20, R&D Systems, or rabbit pAb, 1:50, Santa Cruz Biotechnology), β-galactosidase (chicken pAb, 1:500, Abcam), Ki67 (rabbit pAb, 1:500, Abcam), Nkx2.5 (rabbit pAb, 1:50, Santa Cruz Biotechnology), myocyte enhancer factor 2C (MEF-2C; goat pAb, 1:50, Santa Cruz Biotechnology), desmin (rabbit pAb, 1:100, Abcam), cardiac troponin I (cTnI, rabbit pAb, 1:100; Abcam), α-sarcomeric actinin (mouse monoclonal Ab [mAb], 1:500, Sigma-Aldrich), α-smooth actin (rabbit pAb, 1:100, Abcam), prolyl 4 hydroxylase subunit beta (P4HB; mouse mAb, 1:100, Novus Biologicals), GFP (rabbit pAb, 1:100, Gene Tex), Lamin A/C (rabbit pAb, 1:100, Cell Signaling Technology), human Lamin A/C (mouse mAb, 1:100, Novocastra), or human mitochondria (mouse mAb, 1:800, Abcam). Donkey secondary anti-goat, -rabbit, -chicken and -mouse antibodies (FITC-, Cy3- or Cy5-conjugated, 1:100) were purchased from Jackson ImmunoResearch Laboratories. Nuclei were stained with Hoechst 33342 (Sigma-Aldrich). For co-staining with phalloidin-rhodamine (5 μg/ml, Sigma-Aldrich) and FITC-conjugated α-tubulin (mouse mAb, 1:100, Abcam), hMADS cells were fixed with 4% PFA, then with cold acetone. For fluorescence analysis, we used a Zeiss Axioplan 2 Imaging microscope.
Real-time PCR
Total RNA was extracted from cells at co-culture days 0, 1, 2, 4, and 7 by use of the Qiagen RNeasy Mini Kit (Qiagen), then reverse-transcribed with the Superscript First-Strand Synthesis System (Invitrogen) and Oligo(dT)20. Quantitative RT-PCR reactions were performed in triplicate on a 7900 real-time PCR detection system (Applied Biosystems) with use of Platinium SYBR Green qPCR SuperMix (Invitrogen). PCR conditions were 50°C for 2 min, 95°C for 2 min, 45 cycles at 95°C for 15 s, and 60°C for 45 s, with GAPDH used as the reference gene. Primer sequences are described in supporting information Table 1. Results are reported as mean ± SD. Between-group comparisons for biochemical data involved the two-tailed Student’s t test. P < 0.05 was considered statistically significant.
In situ hybridization
FISH experiments were performed as previously described [38]. Co-cultured cells were hybridized with human and mouse COT-1 DNA probes (Roche Diagnostics and Invitrogen) previously labeled by NICK translation (Roche Diagnostics) with Cy3 and biotin, respectively. Mouse biotin-labeled DNA was detected with a streptavidin-fluorescein conjugate (Sigma-Aldrich). In experiments combining GATA-4 immunocytochemistry and FISH, co-cultured cells were stained with GATA-4 and then labeled with all human or all mouse centromere probes conjugated to FITC (Kreatech Diagnostics). After hybridization, nuclei were counterstained with Hoechst and examined by confocal microscopy (Zeiss LSM 510 Meta).
Electronic microscopy
Co-culture–derived cells were fixed overnight in 3% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.4, post-fixed for 1.5 h with 1% osmium tetraoxide, dehydrated by successive ethanol washes (50%, 70%, 80%, 95%, 100%, 100%), and impregnated with epoxy resin. After polymerization, 70- to 80-nm sections were cut by use of a Reichert Ultracut E ultramicrotome, stained with 2% uranyl acetate plus Reynold’s lead citrate, and visualized under a Philips CM120 transmission electron microscope.
Inhibition of f-actin or microtubule polymerization
After proper cell adhesion (~6 hr), co-cultured cells were treated for 12 hr with 1 μM nocodazole to inhibit α-tubulin polymerization or 0.5 μM cytochalasin D or 0.25 μM latrunculin A, two inhibitors of f-actin polymerization. Inhibitory treatments were repeated 3 days later.
Stem-cell mitochondria transfer
Prior to co-culturing, hMADS cells were labeled with MitoTracker Green FM (200 nM, Invitrogen). Images were obtained by time-lapse video microscopy (Leica DMI6000, Metamorph 7 acquisition software, Universal Imaging) or fluorescence microscopy (Zeiss Axioplan 2 Imaging, Axiovision LE 4.6 software).
RESULTS
Fusion of hMADS cells and adult cardiomyocytes leads to formation of hybrid cells with cardiac progenitor-like features
To investigate whether hMADS cells fused with fully differentiated cardiomyocytes, we used a Cre/loxP system that allows lacZ gene expression only in fused cells. Cre-expressing hMADS cells were co-cultured with cardiomyocytes from Rosa26R transgenic mice. From 2 days after co-culture initiation, we detected colonies of small rounded cells that were lacZ-positive and whose morphology differed from that of both hMADS cells and adult cardiomyocytes (Fig. 1A). Xgal staining was not due to endogenous β-galactosidase activity because co-cultures with non-genetically modified hMADS cells and Rosa26R mouse cardiomyocytes were devoid of blue cells (Fig. 1B). In addition, immunocytochemistry revealed that colony-derived cells co-expressed β-galactosidase with the early cardiac transcription factor GATA-4, which suggests that fused cells belonged to the cardiac lineage (Fig. 1C). Again, the presence of false-positive β-galactosidase cells in co-cultures was ruled out by immunocytochemistry of co-cultures without induction of the Cre/loxP system (Fig. 1D).
Figure 1. Formation of GATA-4+ hybrid cells after fusion of hMADS with cardiomyocytes.
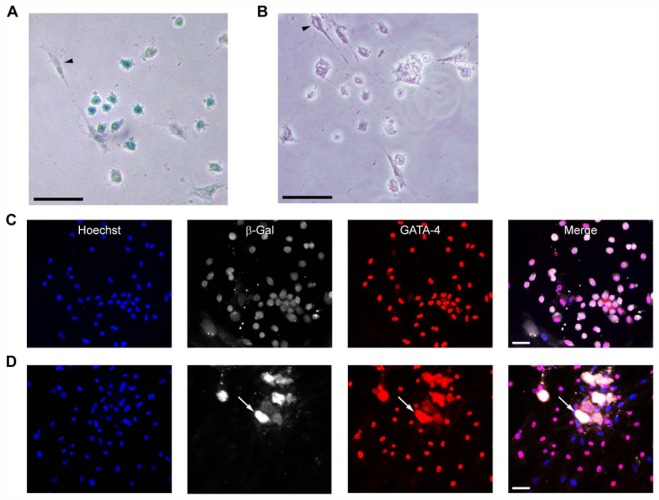
(A) Detection of hybrid cells (blue) by Xgal staining on day 2 of co-culture of Rosa26R cardiomyocytes with Cre-human multipotent adipose-derived stem cells (hMADS) cells (arrowhead). (B) Negative Xgal staining on co-culture with non-transduced hMADS cells (arrowhead). (C–D) Co-immunostaining for β-galactosidase (β-gal, white) and GATA-4 (red) on day 7 of co-culture of Rosa26R cardiomyocytes and (C) Cre- or (D) non-transduced hMADS cells. Arrow: dead cardiomyocyte bodies. Nuclei were counterstained with Hoechst 33342 (blue). (A–D) Scale bars, 50 μm.
To further characterize the phenotype of fused cells, we used immunostaining for GATA-4 with a series of markers directed against cardiac, smooth muscle or fibroblastic cells. GATA-4+ cells expressed early cardiac cell markers such as the transcription factors Nkx2.5 and MEF-2C (Fig. 2A, B). In contrast, later cardiac differentiation markers such as desmin and cardiac troponin I (cTnI) were expressed only in a small proportion (<5%) of GATA-4+ cells (Fig. 2C, D), and α-sarcomeric actinin was never detected (Fig. 2E). Furthermore, GATA-4+ colony-derived cells were negatively stained for α-smooth muscle actin and P4HB, which confirms that these cells did not originate from smooth muscle or fibroblastic lineages, respectively (Fig. 2F, G). Of note, hMADS cells that were cultured alone showed negative staining for all these antibodies, except P4HB. Finally, hybrid GATA-4+ cells expressed Ki67, a cell-cycling and proliferation marker (Fig. 2H and Supporting information Fig. 1A). Counting of such hybrids from day 2 to 5 of co-culture allowed for estimating their population doubling time at about 24 hr. MTT assay performed from day 8 to 14 of co-culture depleted in human stem cells after 5 days of oubain exposure confirmed the proliferative potential of cardiac hybrid cells. Nevertheless, it also indicated that such potential was limited because cell growth decreased with time to become undetectable after 2 weeks of co-culture (Supporting information Fig. 1B, C).
Figure 2. Phenotypic characterization of hybrid cells.
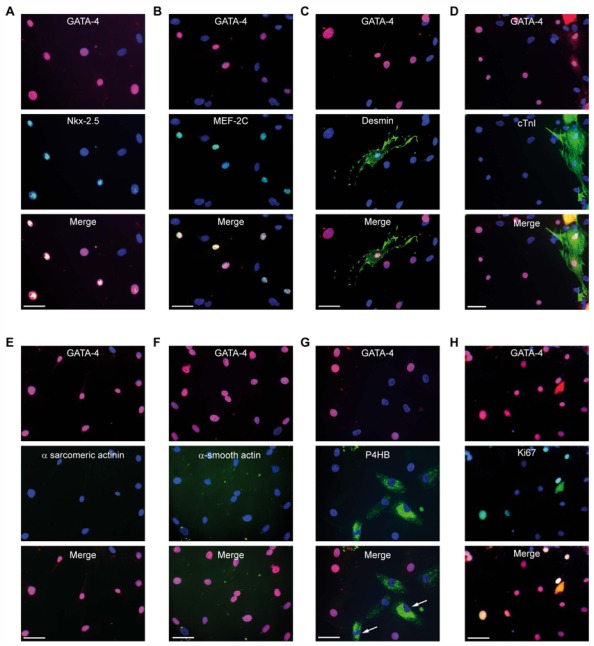
Immunohistochemistry on day 7 of co-culture showed that colony-derived GATA-4+ cells expressed (A) Nkx2.5 and (B) myocyte enhancer factor 2C (MEF-2C) but rarely (C) desmin and (D) cardiac troponin I (cTnI) and were negatively stained for (E) α-sarcomeric actinin, (F) α-smooth actin and (G) prolyl 4 hydroxylase subunit beta (P4HB) fibroblastic markers. HMADS cells were positive for P4HB staining (arrow). (H) Day-7 co-culture-derived GATA-4+ cells also expressed Ki67. (A–H) Antibodies were conjugated with FITC (green), except for GATA-4, which was conjugated with Cy3 (red). Nuclei were counterstained with Hoechst 33342 (blue). Scale bars, 50 μm.
According to these results, cell fusion between stem cells and post-mitotic cardiomyocytes led to the generation of hybrid cells exhibiting proliferative potential, the expression of early cardiac markers and absence of an organized contractile cytoskeleton. These features were reminiscent of a cardiac progenitor cell type. Therefore, hybrid cells are hereafter designated cardiac progenitor-like cells.
Cardiac progenitor-like cells originate from somatic reprogramming of cardiomyocytes, not from stem-cell transdifferentiation
To determine the parental origin of cardiac genes expressed in hybrid cells, we performed real-time PCR analysis with species-specific primers on cells co-cultured from day 0 to 7. As compared with mouse cardiomyocytes alone, co-cultured cells exhibited transcriptional activation of mouse genes involved in early cardiac commitment and proliferation, including GATA-4, MEF-2C, p300 (a histone acetyltransferase implicated in cardiac precursor cell induction [39]) and Ki67, whereas expression of later cardiac mouse genes such as desmin and cardiac troponin T (cTnT) appeared unaffected (Fig. 3A). In contrast, co-culture cells and hMADS cells cultivated alone did not differ in human gene expression (Fig. 3B).
Figure 3. Cell-fusion induction of somatic reprogramming of mouse cardiomyocytes.

(A–B) Mouse and human gene expression changes during co-culture assessed by quantitative RT-PCR from day 0 to 7 of co-culture. (C–D) Immunocytochemistry of mouse phenotype of colony GATA-4+ cells at day 7 of co-culture. (C) A GATA-4+ (Cy3, red) colony expressing murine (Cy5, white) but not human (FITC, green) Lamin A/C. Arrow: hMADS cells expressing human Lamin A/C but not GATA-4. (D) Co-culture with GFP+ mouse cardiomyocytes leading to GATA-4+ (Cy3, red)/GFP+ (FITC, green) cells. (C–D) Nuclei were counterstained with Hoechst 33342 (blue). Scale bars, 50 μm. (E–F) RT-PCR showing no modification of mouse- or human-cardiac gene expression when human and mouse cells were separated by permeable membrane. (G–J) Real-time PCR analysis of mouse and human gene expression on co-culture with (G–H) human bone-marrow–derived stem cells or (I–J) human MRC5 fibroblasts. (A, B, E–J) Data represent the mean±SD of at least 3 independent experiments *P<0.05; ** P<0.01; ***P<0.001. MEF-2C: myocyte enhancer factor 2C; cTnT: cardiac troponin T
Real-time PCR results suggesting a mouse phenotype of cardiac progenitor-like cells were further confirmed by immunostaining for GATA-4 with both human/mouse and human -specific Lamin A/C antibodies and by co-culturing GFP+ transgenic mouse cardiomyocytes. As expected, progenitor-like GATA-4+ cells expressed only mouse Lamin A/C, as shown by the negative stain with human-specific antibody (Fig. 3C). In agreement with this finding, cardiac progenitor-like cells were GFP+ when coculture was performed with mouse GFP+ cardiomyocytes (Fig. 3D).
The mouse phenotype of hybrid cells highlighted that cellular fusion between stem cells and cardiomyocytes did not trigger cardiomyogenic conversion of stem cells but, rather, promoted somatic reprogramming of cardiomyocytes toward a progenitor-like state. To show that somatic reprogramming resulted from cell fusion, co-cultures were performed with (i) a cell-culture insert to impede physical interactions between the 2 cell types without affecting the diffusion of soluble factors or (ii) living cardiomyocytes and hMADS cells previously killed with PFA to inhibit cellular fusion [40]. Indeed, PFA pre-fixation prevented fusion of live sperm cells or neural stem cells with fixed sea urchin eggs or endothelial cells, respectively, but did not disrupt receptor-mediated recognition and association of the co-cultivated cell types [41, 42]. Reprogramming did not occur in either condition, which indicates that soluble signaling molecules or cell-to-cell interaction through a receptor had little or no effect on the generation of mouse cardiac progenitor-like cells (Fig. 3E, F and not shown).
Finally, we addressed the frequency of cardiomyocyte reprogramming by counting the number of immunostained cardiac βgal+/GATA-4+ hybrid cells resulting from co-cultures rendered mitotically inactive by mitomycin C treatment. We detected a mean of 10 hybrid cells per 100 000 cardiomyocytes initially seeded. However, because propidium iodide staining and flow cytometry revealed that more than 90% of cardiomyocytes were suffering before co-culture (Supporting information Fig. 2), the rate of cardiomyocytes efficiently reprogrammed might be about 0.1% when considering the fraction of healthy cardiomyocytes at the start of co-culture.
In addition, we tried to improve reprogramming efficiency by increasing cardiomyocyte survival in vitro because living cardiomyocytes were found to have limited lifespan (< 24 hr) under our co-culture conditions. We performed co-cultures on laminin-coated dishes in the presence of medium containing insulin-transferrin-selenium (ITS) and 2,3butanedione monoxime (BDM) as previously reported [43]. Nevertheless, under these conditions, the yield of reprogrammed cardiomyocytes seemed not affected.
Human bone-marrow derived stem cells (hBMSC) but not human fibroblasts can reprogram mature cardiomyocytes
To determine whether reprogramming of cardiomyocytes was specific to hMADS cells, we created co-cultures with hBMSCs as another model of multipotent stem cells and with human fibroblasts (MRC5 cell line) as an example of proliferative cells devoid of plasticity potential. Real-time PCR of BMSC co-cultures revealed results similar to those with hMADS cells: increased transcription of mouse but not human genes involved in proliferation and early cardiac commitment such as Ki67, GATA-4, MEF-2C and p300, as well as unchanged expression of both mouse and human later cardiac differentiation genes such as desmin and cardiac troponin T (Fig. 3G, H). In contrast, co-culture with MRC5 fibroblasts resulted in different mouse and human gene expression patterns. Even fibroblasts promoted enhanced transcription of mouse GATA-4; they failed to enhance that of mouse p300 and MEF-2C, which indicates that reprogramming of mouse cardiomyocytes was incomplete (Fig. 3I). In On the other hand, coculture with human fibroblasts exhibited increased transcription of human GATA-4 gene, which suggests that these cells initiate a process of cardiomyogenic conversion (Fig. 3J). Accordingly, somatic reprogramming seems to be a feature of multipotent stem cells that is not shared by fibroblastic cells.
Cardiomyocyte reprogramming is mainly mediated by a partial cell-fusion process
To gain additional insights into the mechanisms underlying somatic reprogramming, we investigated whether hybrid cells originated from permanent or partial cell fusion processes. The occurrence of permanent cell fusion was tested by FISH nuclear staining with species-specific DNA probes. Rare synkaryons (1:200 hybrid cells) whose nuclei exhibited both human and mouse DNA were detected by this method (Fig. 4A), whereas colony-derived GATA-4+ cells contained only mouse DNA (Fig. 4B–D). Such observations argued against permanent cell fusion as the principle mechanism of the formation of cardiac progenitor-like hybrid cells. Further supporting this hypothesis, live cell imaging and electron microscopy revealed partial cell fusion events between co-cultured hMADS cells and cardiomyocytes. Live cell imaging microscopy revealed intercellular structures resembling tunneling nanotubes that ensure membrane continuity between stem and cardiac cells (Fig. 5A, B). Electron microscopy revealed 2 other types of heterocellular interactions: one connecting distant cells and consisting in long slender protrusions differing from nanotubes in that they adhered to the substratum [44](Fig. 5C, D), and the other consisting in focal, broad, tight cell-to-cell contact between neighboring cells (Fig. 5F). Membrane disruptions suggestive of partial cell fusion seemed to occur focally in both cases (Fig. 5E, G). Cytoskeletal composition of intercellular structures connecting hMADS cells and cardiomyocytes was then analysed by staining f-actin and microtubules with phalloidin and anti-α-tubulin antibody, respectively; most of the intercellular communications between the 2 cultivated cell types contained both microtubule and f-actin filaments (Fig. 6A, B).
Figure 4. Analysis of nuclear fusion events during co-culture.

(A–B) FISH with mouse (FITC) and human (Cy3) COT-1 DNA probes. (A) Right panel: detection of synkaryons containing human (red) and mouse (green) DNA (arrows) 2 days after co-culture initiation. Arrowhead: hMADS cells labeled with only human COT-1 probe. Left and middle panels: double FISH performed on mouse cardiomyocytes or hMADS alone, respectively. Star: cardiomyocyte body aggregate. (B) Nuclei of colony cells expressing mouse but not human COT-1 DNA. Arrow: human nucleus (red). (C–D) Immunocytochemistry on day 2 of co-culture with GATA-4 combined with FISH labeling with FITC-conjugated human or mouse all centromere probes (green) confirmed that GATA-4+ nuclei (Cy3, red) contained (C) mouse but (D) not human DNA. Human nuclei devoid of GATA-4 staining are also shown. (A–D) Nuclei were counterstained with Hoechst 33342 (blue). Scale bars, 20 μm.
Figure 5. Occurrence of partial cell fusion events during co-culture.
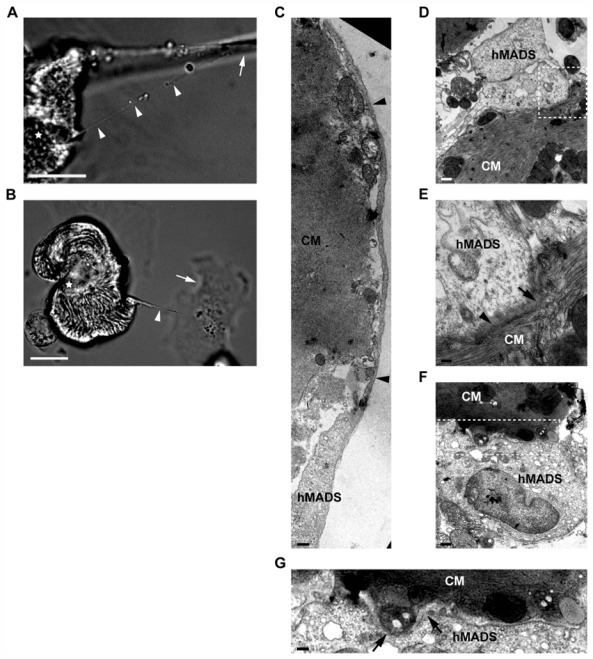
(A–B) Live cell imaging at 6 hr after co-culture initiation revealed thin membranous channels (arrowheads) connecting hMADS cells (arrow) to cardiomyocytes (stars). Scale bars, 20 μm. (C–G) Electron microscopy images illustrating various types of intracellular interactions between hMADS cells and mouse cardiomyocytes (CM) in 24-hr co-cultures. (C–E) Adherent long slender protrusions connecting hMADS to distant cardiomyocytes. (C) Partial view of a long hMADS protrusion surrounding a cardiomyocyte membrane (arrowheads). Scale bar, 378 nm. (D) Terminal portion of a protrusion. Scale bar, 644 nm. (E) Higher magnification of the contact area shown in (D) with blurring (arrowhead) and suspected focal loss (arrow) of the 2 plasma membranes. Scale bar, 275 nm. (F) Close membrane apposition between stem cells and cardiac cells. Scale bar, 863 nm. (G) Higher magnification of the contact site shown in (F) suggesting membrane disruption (arrows). Scale bar, 410 nm.
Figure 6. Involvement of f-actin and microtubule filaments in cardiomyocyte reprogramming.

(A) Six hours after co-culture initiation, thin membrane channels interconnecting stem (arrow) to cardiac (arrowhead) cells contained both f-actin (rhodamine-phalloidin staining, red) and microtubules (FITC-conjugated α-tubulin, green). (B) Close membrane apposition between stem and cardiac cells showing concentration of microtubule (green) and f-actin (red) filaments at the contact site. Scale bars (A, B), 10 μm.
(C–D) Impaired somatic reprogramming by inhibitors of f-actin (cytochalasin D 0.5 μM, latrunculin A 0.25 μM) or microtubule polymerization (nocodazole 1 μM) assessed by real-time PCR at 7 days of co-culture. No statistical differences between inhibitory effects of nocodazole, cytochalasin D or latrunculin A were observed. Relative expression was compared to day-7 untreated co-cultures. Data represent the mean±SD of at least 3 independent experiments *P<0.05; ** P<0.01; ***P<0.001.
To assess the involvement of such connections in somatic reprogramming, co-cultured cells were transiently treated to not alter cell viability with cytochalasin D or latrunculin A, 2 inhibitors of f-actin polymerization, or with nocodazole, which inhibits α-tubulin polymerization. The expression of mouse and human cardiac genes was analyzed in day-7 co-cultures by real-time PCR. As compared with untreated co-cultures, nocodazole-, cytochalasin D- or latrucunlin A-treated co-cultures showed decreased transcriptional expression of mouse early cardiac GATA-4 gene with relative abundances of 0.7, 0.6 and 0.82, respectively. This effect was associated with increased expression of mouse later cardiac genes, with relative abundances of 3, 3.8 and 2 for desmin and 2.7, 3.1 and 2.8 for cardiac troponin T, respectively (Fig. 6C). This cardiac gene expression pattern reminiscent of that of fully differentiated cardiomyocytes, these results indicated that transient disruption of f-actin filaments or microtubules prevented cardiomyocyte reprogramming back to a progenitor-like state. In addition, we observed an increased level of transcripts encoding for human GATA-4 and desmin, whereas the expression of human MEF-2C remained quite similar to that in untreated co-cultures (Fig. 6D). These data indicated that transient disruption of f-actin or microtubule networks favored transdifferentiation of hMADS cells into a cardiac lineage. The phenotype of transdifferentiated hMADS cells was further characterized by immunohistochemistry. We detected the protein expression of GATA-4 but no desmin, cardiac troponin I or MEF2C, which supports that cardiomyogenic conversion of hMADS cells was incomplete (Supporting information Fig. 3 and data not shown). Interestingly, the effect of nocodazole on mouse and human cardiac gene transcription was comparable to that of cytochalasin D and latrunculin A (no significant statistical differences between drugs)(Fig. 6C, D). Thus, f-actin and microtubules may act in concert and hence, somatic reprogramming might be mainly mediated by intercellular structures containing both f-actin and α-tubulin filaments.
Transfer of functional stem cell mitochondria into cardiomyocytes is essential for somatic reprogramming
Because the transfer of functional mitochondria from stem cells was suggested to rescue cardiomyocytes in co-culture [21], we analysed whether mitochondria of hMADS cells labelled with mitotracker before co-culture were transferred to mouse cardiomyocytes. As soon as 6 hr after co-culture initiation, live cell imaging revealed some mouse cardiomyocytes containing fluorescent human mitochondria (Fig. 7A). Passive transfer of human mitochondria toward cardiomyocytes was tested in indirect co-cultures in which hMADS cells previously labeled with mitotracker dye were separated from cardiomyocytes by an 8-μm–pore cell-culture insert. Under these conditions, cardiomyocytes were unable to capture mitotracker dye from hMADS cells, which supports that stem-cell mitochondria were mainly transferred through intercellular structures and not from extracellular medium (data not shown). Accordingly, human mitochondria were detected inside intercellular structures connecting hMADS cells to cardiomyocytes (Fig. 7B).
Figure 7. Role of functional stem cell mitochondria transfer towards cardiomyocytes for somatic reprogramming.
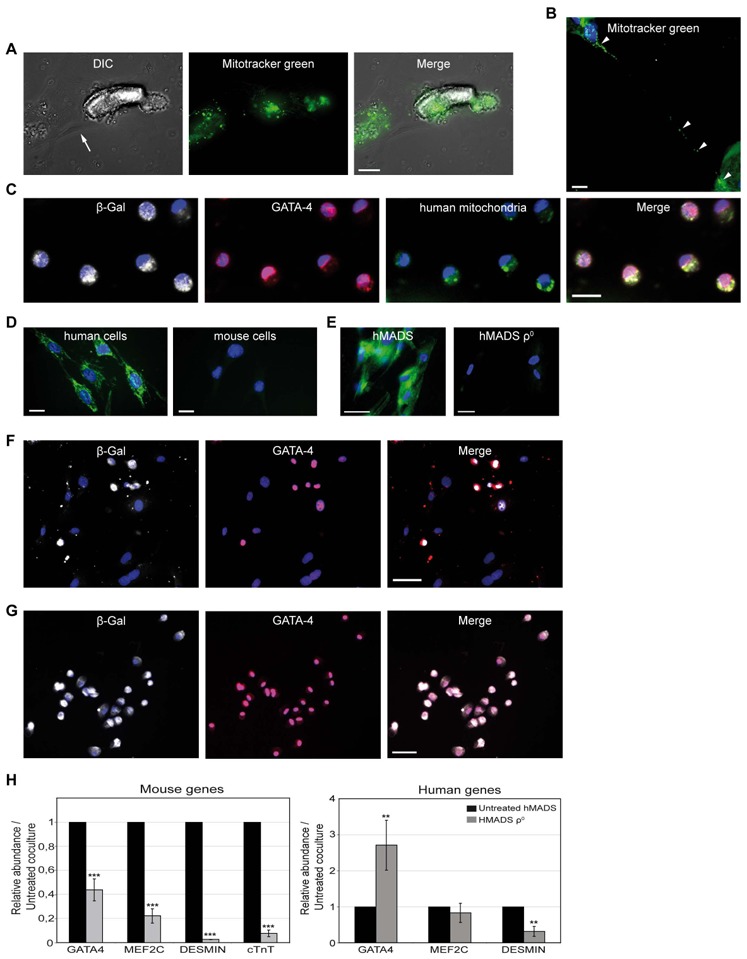
(A) Microscopy live-cell imaging in a 6-hr co-culture showing hMADS mitochondria (MitoTracker Green FM) in cardiomyocyte (arrowhead). Arrow points to an hMADS cell. (B) Presence of human mitochondria (MitoTracker Green FM) inside a tunneling nanotube-like structure connecting a stem cell to a cardiac cell and into cardiomyocyte cytoplasm (arrowhead; 12-hr co-culture, paraformaldehyde-fixed cells). (C) Despite their mouse phenotype, β-gal+ (Cy5, white)/GATA-4+ (Cy3, red) hybrid cells show human stem-cell mitochondria in their cytoplasm (FITC, green). (D) Human mitochondria antibody used in (C) stained human but not mouse cells (left and right panels, respectively. (E) MitoTracker green staining showing mitochondrial DNA depletion in hMADSρ0 (ethidium bromide-treated, right panel) compared to untreated hMADS cells (left panel). (F) Day 7 co-culture of Cre-hMADSρ0 cells and Rosa26R mouse cardiomyocytes shows a marked decrease in number of β-gal+/GATA-4+ hybrid cells (Cy5, white and Cy3 red staining, respectively) as compared with (G) untreated co-cultures. Scale bars, (A–D) 20 μm, (C–G) 50 μm. (H) Real-time PCR assays showing human (right panel) and mouse (left panel) cardiac gene expression on co-culture with hMADSρ0 cells compared to untreated co-cultures. Data represent the mean±SD of at least 3 independent experiments. MEF-2C: myocyte enhancer factor 2C; cTnT: cardiac troponin T
To determine whether transferred human mitochondria persisted in mouse progenitor-like cells derived from cell fusion, day-7 co-cultures of Rosa26R mouse cardiomyocytes and hMADS cells expressing Cre recombinase underwent immunocytochemistry for GATA-4, β-gal and human mitochondria. Human mitochondria were revealed inside the cytoplasm of mouse-reprogrammed GATA-4+/β-gal+ cells (Fig. 7C). As expected, the human mitochondria antibody stained human but not mouse cells (Fig. 7D).
To assess the role of stem-cell mitochondria transfer in cardiomyocyte reprogramming, hMADS cells were pre-treated for a long time (>1 month) with low doses of ethidium bromide. Such a treatment allows mutations and depletion of mitochondrial DNA without altering nuclear function, as previously reported for other cell types [32–34]. After 1 month of ethidium bromide exposure, more than 95% of treated cells, commonly designated as hMADSρ0 cells, exhibited greatly decreased mitochondrial DNA as compared with untreated hMADS cells (Fig. 7E). To investigate their cell fusion ability, hMADSρ0 cells were genetically modified to express Cre recombinase before co-culture with Rosa26R mouse cardiomyocytes. The proportion of cardiac GATA-4+/β-gal+ progenitor-like cells was 70% to 90% lower in treated (Fig. 7F) than untreated co-cultures (Fig. 7G), indicating that functional stem cell mitochondria transfer is essential for successful achievement of somatic reprogramming. The detection of a low amount of fused cardiac progenitor-like cells was likely due to the presence in the hMADSρ0 population of a few human cells whose mitochondria were still unaltered. The requirement of functional stem-cell mitochondria for reprogramming was confirmed by real-time PCR analysis. As compared with untreated co-cultures, hMADSρ0 co-cultures showed significantly decreased mouse early but also late cardiac differentiation transcripts, with relative abundances of 0.43 for GATA-4, 0.22 for MEF-2C, 0.02 for desmin and 0.07 for cTnT (Fig. 7H, left panel). The relative transcription level of mouse GATA-4 and MEF-2C genes should reflect the presence of a few cardiac hybrid cells previously identified by immunocytochemistry, whereas the marked decrease of mouse desmin and cTnT transcripts strongly suggests that in the absence of functional stem-cell mitochondria, adult cardiomyocytes died, which explains why they were unable to be reprogrammed. Again, concomitant with somatic reprogramming inhibition, we observed enhanced transcription of human GATA-4 gene, with a relative abundance of 2.71 as compared with that in untreated co-cultures, which suggests the entry of hMADS cells into the cardiomyogenic lineage (Fig. 7H, right panel).
DISCUSSION
To further characterize stem cell fusion as tissue repair mechanism, our study provides the first evidence that hMADS cells can rescue post-mitotic cardiomyocytes in vitro via cell fusion and reprogramming to a rejuvenated progenitor-like state. Interestingly, hBMSCs but not human fibroblasts exhibited similar reprogramming ability, which suggests that this feature is restricted to some types of multipotent stem cells. Another important consideration concerns the frequency with which somatic reprogramming occurs. According to our results, the rate of this phenomenon is rare and might be underestimated because of the poor viability of cardiomyocytes after dissociation from the heart and their low survival potential on culture. Consequently, future efforts to optimize reprogramming efficiency might focus on improving methods for dissociation from the heart and culture of mouse cardiomyocytes.
Additionally, our study reveals information on the phenotype of hybrid cardiac cells, which was until now largely unexplored. We found that fused cells are in fact an intermediate state between stem and fully differentiated cells because they look like a type of cardiac progenitor in terms of their proliferative potential, expression of early cardiac transcription factors and lack of contractile proteins. Therefore, whether such cells resemble or differ from cardiac progenitors or cardiac stem cells reported to reside in heart tissue [15, 45–47]remains a question that requires further knowledge of cardiac hybrid cells. In particular, whether co-culture–derived hybrid cells can differentiate into beating cardiomyocytes needs to be determined to show whether they are truly cardiac progenitors. Although this phenomenon was never observed in our co-culture conditions, we cannot exclude that such a differentiation could occur in experimental settings more faithfully reproducing the heart environment. In strengthening this hypothesis, recent unpublished data from our lab suggest that cardiac hybrid cells generated by co-culture differentiate into mature cardiomyocytes in vivo when engrafted into mouse infarcted myocardium.
Besides assessing the hybrid phenotype, our study also provides insights into mechanisms underlying cell fusion processes leading to cardiomyocyte reprogramming. Despite the occurrence of permanent cell fusion during co-culture, this process fails to fully explain the generation of hybrid cells in terms of its frequency, which is extremely rare, and the fact that we never detected colonies of synkaryons expressing GATA-4. Rather, our observations favor a role of partial cell fusion in the emergence of cardiac hybrid cells. Evidence of partial cell fusion events in vitro was reported from co-culture of neonatal cardiomyocytes and endothelial progenitor cells or bone-marrow–derived stem cells [21, 48]. These studies revealed thin membranous connections between stem and cardiac cells, but did not consider their cytoskeletal composition. Here, we report the possibility of partial cell fusion by transient thin channels resembling tunneling nanotubes or by close cell-to-cell membrane apposition accompanied by focal membrane disruption. Our results also highlight the involvement of both f-actin and microtubule filaments in this process. These 2 cytoskeletal components may act in concert inside the same intercellular connections. Interestingly, intercellular channels containing both f-actin and microtubules have been described to transfer mitochondria between macrophages in vitro [29]and, according to our observations, may also participate in conveyance of stem-cell mitochondria toward cardiomyocytes.
Despite previous evidence of cell-to-cell connections and organelle transfer between stem and cardiac cells [20, 21], the formation of cardiac progenitor-like cells has never been reported in co-culture studies. The fact is probably ascribable to differences in developmental stage of cardiomyocytes used for co-culture. Indeed, other investigators used neonatal cardiac cells capable of division [7, 49, 50], whereas we used non-replicative adult cardiomyocytes. Finally, one of the most exciting outcomes of our study relates to the role of stem-cell mitochondria in cardiomyocyte reprogramming. Here, we provide the first evidence to our knowledge that human stem-cell mitochondria transferred toward mature cardiomyocytes persisted over time in mouse cardiac progenitor-like cells derived from cell fusion.
Importantly, we found that depletion of stem-cell mitochondrial DNA did not alter the cell fusion ability of stem cells but, rather, led to a dramatic decrease in somatic reprogramming frequency. Thus, delivery of stem-cell–intact mitochondria to distressed cardiomyocytes whose mitochondria are rapidly altered after isolation may prolong the survival of such cells and maintain their nuclear nuclear integrity, which is a pre-requisite for reprogramming.
In support of our findings, Spees and collaborators [34]recently described that transfer of stem-cell mitochondria can rescue aerobic respiration in mammalian cells with non-functional mitochondria. However, whether stem-cell mitochondria may affect somatic reprogramming by favoring cardiomyocyte transcriptional machinery remodeling through conveyance of key factors from stem cells or by modulating transcription factor activities into cardiomyocytes through mitochondria-to-nucleus retrograde signaling is yet unclear[51]. Whatever the fine mechanisms by which stem-cell mitochondria sustain somatic reprogramming, these findings may reconcile current controversies about mechanisms underlying the cardiac benefit of stem cells by proposing an alternative regenerative pathway to other well-accepted candidates including transdifferentiation, permanent cell fusion and paracrine effects. In particular, transfer of stem-cell mitochondria may well explain why heart functional improvements are observed despite the fact that few of the donor cells are engrafted at long term. Nevertheless, determining whether partial cell fusion, mitochondria transfer and cardiomyocyte reprogramming take place in vivo is currently difficult. However, several recent studies have strengthened the hypothesis that such processes occur in living tissues. For instance, the existence of tunneling nanotubes has been confirmed in cornea following injury [52], and heart regeneration in zebrafish has been shown to be mediated by cardiomyocyte dedifferentiation and proliferation[53].
Future research into partial cell fusion will contribute to a better understanding of the regenerative properties of stem cells and lead to the development of more efficient cell therapies to treat heart degenerative diseases.
CONCLUSIONS
Our study provides the first evidence that adult stem cells can reprogram cardiomyocytes back to a more immature state through partial cell fusion processes and transfer of functional mitochondria. The timeline of cardiomyocyte reprogramming is summarized in supporting information Fig. 4. In contrast, permanent cell fusion and the formation of synkaryons were rare in our co-culture conditions and therefore do not seem to be the principle mechanisms of hybrid cardiac cell generation.
Supplementary Material
Acknowledgments
We are grateful to M. Goossens, A. d’Anglemont de Tassigny, T Piolot and X Decrouy for their helpful advice regarding FISH, cardiomyocyte dissociation, time-lapse videomicroscopy and confocal microscopy experiments, respectively. This work was financially supported by INSERM, the Association Française contre les Myopathies and the Association pour la Recherche et l’Etude des Maladies Cardiovasculaires.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors declare they have no potential conflicts of interests.
Author contributions: A.A. conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; T.B. conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; P.F.L. collection and assembly of data, data analysis and interpretation; F.F. collection and assembly of data, data analysis and interpretation; A.E.C. collection and assembly of data, data analysis and interpretation; O.L.C. collection and assembly of data, data analysis and interpretation; C.C. collection and assembly of data; X.B. collection and assembly of data; F.A. provision of study material or patients; R.Y. conception and design, final approval of manuscript; J.L.D. financial support, conception and design, final approval of manuscript; A.M.R. conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing.
References
- 1.D’Ippolito G, Diabira S, Howard GA, et al. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 2.Reyes M, Lund T, Lenvik T, et al. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez AM, Pisani D, Dechesne CA, et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 5.Kawada H, Fujita J, Kinjo K, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 6.Rota M, Kajstura J, Hosoda T, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon J, Shim WJ, Ro YM, et al. Transdifferentiation of mesenchymal stem cells into cardiomyocytes by direct cell-to-cell contact with neonatal cardiomyocyte but not adult cardiomyocytes. Ann Hematol. 2005;84:715–721. doi: 10.1007/s00277-005-1068-7. [DOI] [PubMed] [Google Scholar]
- 8.Mirotsou M, Jayawardena TM, Schmeckpeper J, et al. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2010 doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 10.Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 11.Ying QL, Nichols J, Evans EP, et al. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa F, Shimazu H, Shultz LD, et al. Purified human hematopoietic stem cells contribute to the generation of cardiomyocytes through cell fusion. Faseb J. 2006;20:950–952. doi: 10.1096/fj.05-4863fje. [DOI] [PubMed] [Google Scholar]
- 13.Dedja A, Zaglia T, Dall’Olmo L, et al. Hybrid cardiomyocytes derived by cell fusion in heterotopic cardiac xenografts. Faseb J. 2006;20:2534–2536. doi: 10.1096/fj.06-6586fje. [DOI] [PubMed] [Google Scholar]
- 14.Nygren JM, Jovinge S, Breitbach M, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 15.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Payne TR, Oshima H, Sakai T, et al. Regeneration of dystrophin-expressing myocytes in the mdx heart by skeletal muscle stem cells. Gene Ther. 2005;12:1264–1274. doi: 10.1038/sj.gt.3302521. [DOI] [PubMed] [Google Scholar]
- 17.Spees JL, Whitney MJ, Sullivan DE, et al. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. Faseb J. 2008;22:1226–1236. doi: 10.1096/fj.07-8076com. [DOI] [PubMed] [Google Scholar]
- 18.Kajstura J, Rota M, Whang B, et al. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127–137. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 19.Pijnappels DA, Schalij MJ, Ramkisoensing AA, et al. Forced alignment of mesenchymal stem cells undergoing cardiomyogenic differentiation affects functional integration with cardiomyocyte cultures. Circ Res. 2008;103:167–176. doi: 10.1161/CIRCRESAHA.108.176131. [DOI] [PubMed] [Google Scholar]
- 20.Koyanagi M, Brandes RP, Haendeler J, et al. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res. 2005;96:1039–1041. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- 21.Plotnikov EY, Khryapenkova TG, Vasileva AK, et al. Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J Cell Mol Med. 2008;12:1622–1631. doi: 10.1111/j.1582-4934.2007.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rustom A, Saffrich R, Markovic I, et al. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 23.Gerdes HH, Carvalho RN. Intercellular transfer mediated by tunneling nanotubes. Curr Opin Cell Biol. 2008;20:470–475. doi: 10.1016/j.ceb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Levchenko A, Mehta BM, Niu X, et al. Intercellular transfer of P-glycoprotein mediates acquired multidrug resistance in tumor cells. Proc Natl Acad Sci U S A. 2005;102:1933–1938. doi: 10.1073/pnas.0401851102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bukoreshtliev NV, Wang X, Hodneland E, et al. Selective block of tunneling nanotube (TNT) formation inhibits intercellular organelle transfer between PC12 cells. FEBS Lett. 2009;583:1481–1488. doi: 10.1016/j.febslet.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 26.Plotnikov EY, Khryapenkova TG, Galkina SI, et al. Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Exp Cell Res. 2010;316:2447–2455. doi: 10.1016/j.yexcr.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Tavi P, Korhonen T, Hanninen SL, et al. Myogenic skeletal muscle satellite cells communicate by tunnelling nanotubes. J Cell Physiol. 2010;223:376–383. doi: 10.1002/jcp.22044. [DOI] [PubMed] [Google Scholar]
- 28.Gousset K, Schiff E, Langevin C, et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11:328–336. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- 29.Onfelt B, Nedvetzki S, Benninger RK, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476–8483. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- 30.Sowinski S, Jolly C, Berninghausen O, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 31.Chevallier N, Anagnostou F, Zilber S, et al. Osteoblastic differentiation of human mesenchymal stem cells with platelet lysate. Biomaterials. 2010;31:270–278. doi: 10.1016/j.biomaterials.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 32.King MP, Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989;246:500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 33.Miller SW, Trimmer PA, Parker WD, Jr, et al. Creation and characterization of mitochondrial DNA-depleted cell lines with “neuronal-like” properties. J Neurochem. 1996;67:1897–1907. doi: 10.1046/j.1471-4159.1996.67051897.x. [DOI] [PubMed] [Google Scholar]
- 34.Spees JL, Olson SD, Whitney MJ, et al. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitra R, Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985;249:H1056–1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- 36.Okabe M, Ikawa M, Kominami K, et al. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 37.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 38.Brown KE, Baxter J, Graf D, et al. Dynamic repositioning of genes in the nucleus of lymphocytes preparing for cell division. Mol Cell. 1999;3:207–217. doi: 10.1016/s1097-2765(00)80311-1. [DOI] [PubMed] [Google Scholar]
- 39.Yanazume T, Hasegawa K, Morimoto T, et al. Cardiac p300 is involved in myocyte growth with decompensated heart failure. Mol Cell Biol. 2003;23:3593–3606. doi: 10.1128/MCB.23.10.3593-3606.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon J, Choi SC, Park CY, et al. Bone marrow-derived side population cells are capable of functional cardiomyogenic differentiation. Mol Cells. 2008;25:216–223. [PubMed] [Google Scholar]
- 41.Wurmser AE, Nakashima K, Summers RG, et al. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature. 2004;430:350–356. doi: 10.1038/nature02604. [DOI] [PubMed] [Google Scholar]
- 42.Vacquier VD. The fertilizing capacity of sea urchin sperm rapidly decreases after induction of the acrosome reaction. Dev Growth Differ. 1979;21:61–69. doi: 10.1111/j.1440-169X.1979.00061.x. [DOI] [PubMed] [Google Scholar]
- 43.Sambrano GR, Fraser I, Han H, et al. Navigating the signalling network in mouse cardiac myocytes. Nature. 2002;420:712–714. doi: 10.1038/nature01306. [DOI] [PubMed] [Google Scholar]
- 44.Gurke S, Barroso JF, Gerdes HH. The art of cellular communication: tunneling nanotubes bridge the divide. Histochem Cell Biol. 2008;129:539–550. doi: 10.1007/s00418-008-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 46.Dawn B, Stein AB, Urbanek K, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 48.Cselenyak A, Pankotai E, Horvath EM, et al. Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections. BMC Cell Biol. 2010;11:29. doi: 10.1186/1471-2121-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishiyama N, Miyoshi S, Hida N, et al. The significant cardiomyogenic potential of human umbilical cord blood-derived mesenchymal stem cells in vitro. Stem Cells. 2007;25:2017–2024. doi: 10.1634/stemcells.2006-0662. [DOI] [PubMed] [Google Scholar]
- 50.Rose RA, Jiang H, Wang X, et al. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26:2884–2892. doi: 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]
- 51.Liu Z, Butow RA. Mitochondrial retrograde signaling. Annu Rev Genet. 2006;40:159–185. doi: 10.1146/annurev.genet.40.110405.090613. [DOI] [PubMed] [Google Scholar]
- 52.Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: Membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol. 2008;180:5779–5783. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jopling C, Sleep E, Raya M, et al. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


