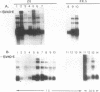
Abstract
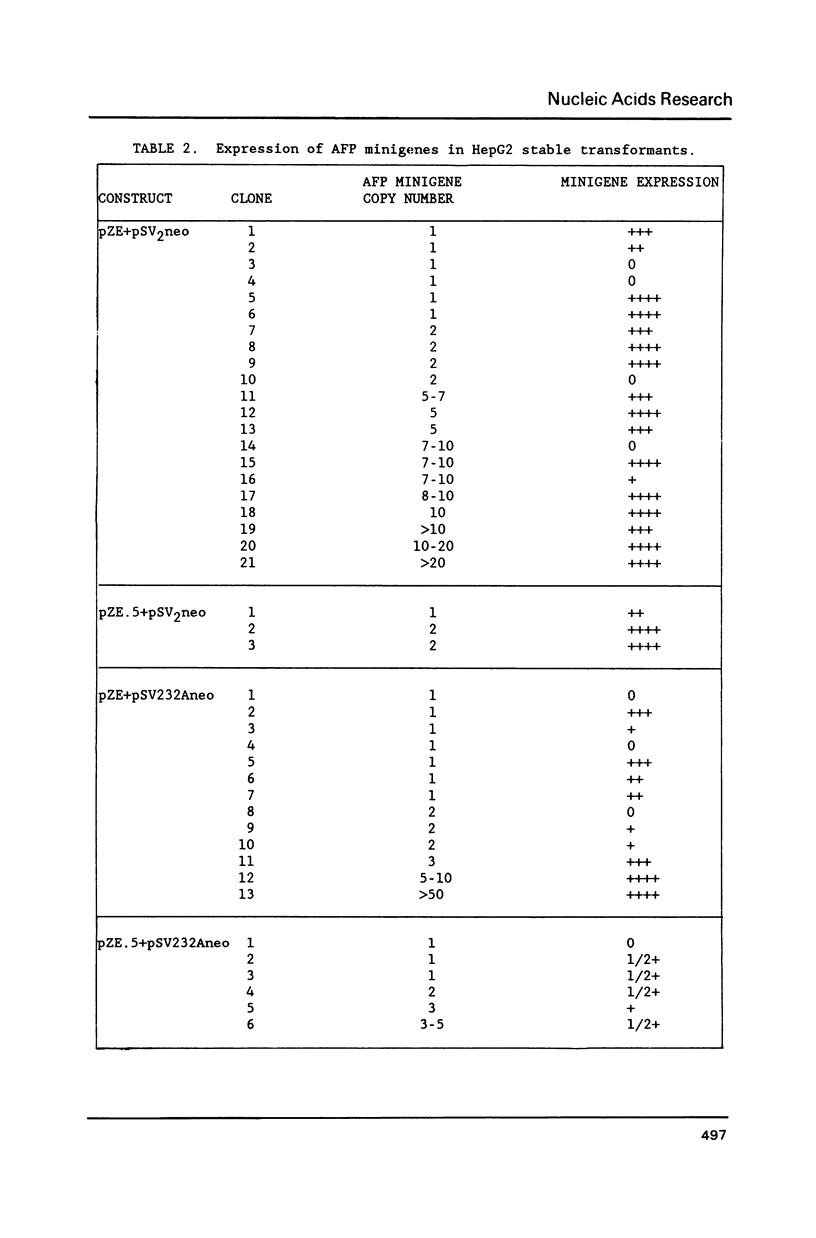
Multiple cis-acting regulatory elements consisting of three cellular enhancers and a proximal promoter element have been identified in the region upstream of the mouse alpha-fetoprotein (AFP) gene. We examined the role of these sequences during differentiation by the introduction of modified AFP genes into cells at different stages of commitment to its expression. Modified AFP genes introduced stably into F9 embryonal carcinoma stem cells by DNA transfection were silent until activated by treatment with retinoic acid to form visceral endoderm. Their activation required the presence of both the enhancer and proximal promoter domains. The introduced genes activated simultaneously with the endogenous AFP genes, but reached maximal levels of expression more rapidly, suggesting a greater initial accessibility to transcription factors. In contrast, when modified AFP genes were stably introduced into HepG2 cells, a human hepatoma cell line that constitutively expresses the AFP gene, the proximal promoter sequences were sufficient to direct a low level of expression. The absolute requirement for the AFP enhancers in F9 cells but not in HepG2 cells supports a model by which there is an obligate requirement for enhancers during differentiation in addition to their role in enhancing gene expression after differentiation.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay P., Treisman R., Mellon P., Chao M., Axel R., Maniatis T. Differences in human alpha- and beta-globin gene expression in mouse erythroleukemia cells: the role of intragenic sequences. Cell. 1984 Aug;38(1):251–263. doi: 10.1016/0092-8674(84)90547-6. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Lai E., Darnell J. E., Jr Transcriptional control of the mouse prealbumin (transthyretin) gene: both promoter sequences and a distinct enhancer are cell specific. Mol Cell Biol. 1986 Dec;6(12):4697–4708. doi: 10.1128/mcb.6.12.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Vande Woude G. F., Wagner M., Smiley J. R., Summers W. C. Construction and characterization of a recombinant plasmid encoding the gene for the thymidine kinase of Herpes simplex type 1 virus. Gene. 1979 Nov;7(3-4):335–342. doi: 10.1016/0378-1119(79)90052-0. [DOI] [PubMed] [Google Scholar]
- Godbout R., Ingram R., Tilghman S. M. Multiple regulatory elements in the intergenic region between the alpha-fetoprotein and albumin genes. Mol Cell Biol. 1986 Feb;6(2):477–487. doi: 10.1128/mcb.6.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Krumlauf R., Camper S. A., Brinster R. L., Tilghman S. M. Diversity of alpha-fetoprotein gene expression in mice is generated by a combination of separate enhancer elements. Science. 1987 Jan 2;235(4784):53–58. doi: 10.1126/science.2432657. [DOI] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Fromental C., Sassone-Corsi P., Chambon P. A mutated polyoma virus enhancer which is active in undifferentiated embryonal carcinoma cells is not repressed by adenovirus-2 E1A products. Nature. 1986 May 15;321(6067):249–251. doi: 10.1038/321249a0. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Taylor A., Adamson E. Cell interactions modulate embryonal carcinoma cell differentiation into parietal or visceral endoderm. Nature. 1981 May 21;291(5812):235–237. doi: 10.1038/291235a0. [DOI] [PubMed] [Google Scholar]
- Janzen R. G., Andrews G. K., Tamaoki T. Synthesis of secretory proteins in developing mouse yolk sac. Dev Biol. 1982 Mar;90(1):18–23. doi: 10.1016/0012-1606(82)90207-x. [DOI] [PubMed] [Google Scholar]
- Kadesch T., Berg P. Effects of the position of the simian virus 40 enhancer on expression of multiple transcription units in a single plasmid. Mol Cell Biol. 1986 Jul;6(7):2593–2601. doi: 10.1128/mcb.6.7.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussis D., Eiferman F., van de Rijn P., Gorin M. B., Ingram R. S., Tilghman S. M. The evolution of alpha-fetoprotein and albumin. II. The structures of the alpha-fetoprotein and albumin genes in the mouse. J Biol Chem. 1981 Feb 25;256(4):1960–1967. [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Krumlauf R., Hammer R. E., Tilghman S. M., Brinster R. L. Developmental regulation of alpha-fetoprotein genes in transgenic mice. Mol Cell Biol. 1985 Jul;5(7):1639–1648. doi: 10.1128/mcb.5.7.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz D. T. Hormonal inducibility of rat alpha 2u globulin genes in transfected mouse cells. Nature. 1981 Jun 25;291(5817):629–631. doi: 10.1038/291629a0. [DOI] [PubMed] [Google Scholar]
- Latchman D. S., Brzeski H., Lovell-Badge R., Evans M. J. Expression of the alpha-fetoprotein gene in pluripotent and committed cells. Biochim Biophys Acta. 1984 Nov 22;783(2):130–136. doi: 10.1016/0167-4781(84)90004-6. [DOI] [PubMed] [Google Scholar]
- Leff S. E., Evans R. M., Rosenfeld M. G. Splice commitment dictates neuron-specific alternative RNA processing in calcitonin/CGRP gene expression. Cell. 1987 Feb 13;48(3):517–524. doi: 10.1016/0092-8674(87)90202-9. [DOI] [PubMed] [Google Scholar]
- Linney E., Davis B., Overhauser J., Chao E., Fan H. Non-function of a Moloney murine leukaemia virus regulatory sequence in F9 embryonal carcinoma cells. 1984 Mar 29-Apr 4Nature. 308(5958):470–472. doi: 10.1038/308470a0. [DOI] [PubMed] [Google Scholar]
- Lovell-Badge R. H., Evans M. J. Changes in protein synthesis during differentiation of embryonal carcinoma cells, and a comparison with embryo cells. J Embryol Exp Morphol. 1980 Oct;59:187–206. [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas and mammalian embryogenesis. Science. 1980 Aug 15;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Minty A., Blau H., Kedes L. Two-level regulation of cardiac actin gene transcription: muscle-specific modulating factors can accumulate before gene activation. Mol Cell Biol. 1986 Jun;6(6):2137–2148. doi: 10.1128/mcb.6.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki J., Appella E., Ozato K. Negative regulation of the major histocompatibility class I gene in undifferentiated embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9537–9541. doi: 10.1073/pnas.83.24.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muglia L., Rothman-Denes L. B. Cell type-specific negative regulatory element in the control region of the rat alpha-fetoprotein gene. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7653–7657. doi: 10.1073/pnas.83.20.7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel R., Kovesdi I., Nevins J. R. Developmental control of a promoter-specific factor that is also regulated by the E1A gene product. Cell. 1987 Feb 13;48(3):501–506. doi: 10.1016/0092-8674(87)90200-5. [DOI] [PubMed] [Google Scholar]
- Robins D. M., Paek I., Seeburg P. H., Axel R. Regulated expression of human growth hormone genes in mouse cells. Cell. 1982 Jun;29(2):623–631. doi: 10.1016/0092-8674(82)90178-7. [DOI] [PubMed] [Google Scholar]
- Roginski R. S., Skoultchi A. I., Henthorn P., Smithies O., Hsiung N., Kucherlapati R. Coordinate modulation of transfected HSV thymidine kinase and human globin genes. Cell. 1983 Nov;35(1):149–155. doi: 10.1016/0092-8674(83)90217-9. [DOI] [PubMed] [Google Scholar]
- Scott R. W., Tilghman S. M. Transient expression of a mouse alpha-fetoprotein minigene: deletion analyses of promoter function. Mol Cell Biol. 1983 Jul;3(7):1295–1309. doi: 10.1128/mcb.3.7.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. W., Vogt T. F., Croke M. E., Tilghman S. M. Tissue-specific activation of a cloned alpha-fetoprotein gene during differentiation of a transfected embryonal carcinoma cell line. Nature. 1984 Aug 16;310(5978):562–567. doi: 10.1038/310562a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Belayew A. Transcriptional control of the murine albumin/alpha-fetoprotein locus during development. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5254–5257. doi: 10.1073/pnas.79.17.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M. The structure and regulation of the alpha-fetoprotein and albumin genes. Oxf Surv Eukaryot Genes. 1985;2:160–206. [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985 Oct;42(3):705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]
- Wright S., Rosenthal A., Flavell R., Grosveld F. DNA sequences required for regulated expression of beta-globin genes in murine erythroleukemia cells. Cell. 1984 Aug;38(1):265–273. doi: 10.1016/0092-8674(84)90548-8. [DOI] [PubMed] [Google Scholar]