Abstract
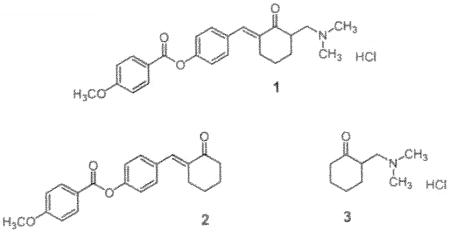
2-[4-(4-Methoxyphenylcarbonyloxy)benzylidene]-6-dimethylaminomethyl cyclohexanone hydrochloride 1 has a MIC value of 0.78 μg/mL towards Mycobacterium tuberculosis H37Rv and displays similar or identical MIC figures towards various drug-resistant strains of this microorganism. The enone 1 along with a partial structure 2-dimethylaminomethylcyclohexanone hydrochloride 3 affected respiration in isolated rat liver mitochondria differently which may contribute to the variation in toxicity to both normal cells and M. tuberculosis.
Several years ago the discovery of the growth-inhibiting properties of various Mannich bases of 2-benzylidenecyclohexanones towards Mycobacterium tuberculosis H37Rv was disclosed (Dimmock et al. 2004). From this study, 1 emerged as a lead molecule having a minimum inhibitory concentration (MIC) value of 0.78 μg/mL. Bearing in mind that the MIC figure of the highly potent antitubercular drug rifampicin is 0.25 μg/mL (Guillon et al. 1998), 1 is considered to be an important lead molecule which is structurally divergent from the contemporary medication used in treating tuberculosis. In an attempt to find the contribution of the groups at positions 2 and 6 of 1 to antitubercular properties, the related partial structures 2 and 3 were evaluated. At a concentration of 12.5 μg/mL, 2 caused a 51% inhibition of the growth of M. tuberculosis (Dimmock et al. 2004) and was not considered further. However the MIC value of 3 is 12.5 μg/mL and further investigations were conducted with 1 and 3.
Ideally an antitubercular agent will have not only significant growth-inhibiting properties towards M. tuberculosis but will be well tolerated by mammalian cells. In other words, a greater toxicity to the microorganism than to normal tissues should be displayed. Both 1 and 3 were evaluated against Vero cells and the IC50 values for 1 and 3 are 16.4 and 0.05 μg/mL, respectively. The selectivity index (SI) figures, i.e., IC50/MIC, for 1 and 3 are therefore 21 and 0.004, respectively. Consideration was given with a view to understanding the reason for the difference in toxicity between 1 and 3 towards normal cells. A number of Mannich bases interfere with respiration in mitochondria (Dimmock et al. 1983; Hamon et al. 1982). The MIC figure of 1 is 0.78 μg/mL or 1.81 μM. Hence concentrations of 2, 4 and 8 μM were employed to detect any differences in the effect on respiration of rat liver mitochondria. Using concentrations of 2 and 4 μM of 1, stimulation of respiration by 13 and 107%, respectively, was noted. However at 8 μM inhibition of respiration by 13% occurred. This bimodal effect was in contrast to 3 in which a concentration-dependent increase in stimulation of respiration took place, i.e., by 30, 45 and 65% when 2, 4 and 8 μM, respectively, of 3 was employed. Thus a possible reason for the variation in mammalian toxicity between 1 and 3 is the difference in effects on mitochondrial respiration.
A compound with a SI value of 10 or greater is considered a useful lead molecule (TAACF, 2009). Hence 1, with a SI value of 21, was investigated further. An enormous clinical problem in treating tuberculosis patients is the emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains of M. tuberculosis (Lenaerts et al. 2008). The enone 1 was evaluated against seven strains of M. tuberculosis (the MIC figures in μg/mL are in parentheses) which are resistant to isoniazid (1.56), rifampin (1.56), ethionamide (1.56), thioacetazone (0.78), ciprofloxacin (0.78), p-aminosalicylic acid (1.56) and kanamycin sulphate (3.13). These MIC values are identical or very similar to the figure generated using the H37Rv strain of M. tuberculosis, namely 0.78 μg/mL. Thus these drug-resistant strains do not possess cross resistance to 1 implying that this Mannich base has a different mode of action than a number of antimycobacterial drugs.
In some series of antimycobacterials an increase in potencies is found with a rise in logP values (Patole et al. 2006). The logP value of 1 is 1.84 which is substantially greater than the figure of −1.86 for the related Mannich base 3. Thus in the future, the insertion of lipophilic groups into the aryl rings of 1 will increase the logP figures which may lead to analogues displaying greater antitubercular potencies than 1.
In conclusion, 2-[4-(4- Methoxyphenylcarbonyloxy)benzylidene]-6-dimethylaminomethyl cyclohexanone hydrochloride: 1 displays potent growth-inhibiting properties towards M. tuberculosis H37Rv as well as a number of drug-resistant strains of this microorganism. The enone 1 has a good SI figure. A related Mannich base 3 has moderate antitubercular potency but is more toxic to Vero cells than 1. This variation in bioactivity may be due to differences in the effects on respiration in mitochondria and possibly the divergent logP values of 1 and 3.
Experimental
Both 1 and 2 were prepared by a literature method (Dimmock et al. 2004) while the synthesis of 3 has been reported previously (Dimmock et al. 1993). A literature procedure was followed in the isolation of mitochondria from rat liver (kowaltowski et al. 1996). The effect of 1 and 3 on mitochondrial respiration was determined polarographically (Estabrook 1967). In this experiment mitochondria (1 mg protein/mL) were incubated at 30° C in an aqueous buffer pH 7.2 containing succinate (5 mM), magnesium chloride (1 mM), potassium phosphate (5 mM), HEPES (10 mM) and sucrose (125 mM). In the case of 1, the percentage stimulation of mitochondrial respiration (concentration in μM in parentheses) was 13.06 ± 1.87 (2) and 107.2 ± 4.57 (4) while at 8 μM, respiration was inhibited by 12.50 ± 1.96%. The percentage stimulation of respiration by 3 is 29.89 ±5.21 (2), 44.77 ±4.06 (4) and 64.58 ±0.80 (8).
The evaluation of 1 towards the drug-resistant strains of M. tuberculosis was undertaken using the microplate alamar blue assay (Collins and Franzblau, 1997). The cytotoxicity towards Vero cells was carried out using serial dilutions commencing with ten times the concentration of the MIC figure towards M. tuberculosis H37Rv.
The logP values of the free bases of 1 and 3 were obtained using the Molinspiration WebME Editor 1.16 (Molinspiration Chemoinformatics).
Acknowledgments
The authors thank the Canadian Institutes of Health Research for an operating grant to J. R. Dimmock. In addition, appreciation is expressed to the Tuberculosis Antimicrobial Acquisition and Coordinating Facility who, through a research and development contract with the U.S. National Institute of Allergy and Infectious Diseases, undertook the antimycobacterial and Vero cell assays.
References
- Collins LA, Franzblau SG. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother. 1997;41:1004–1009. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmock JR, Kandepu NM, Das U, Zello GA, Nienaber KH. Antimycobacterial arylidenecyclohexanones and related Mannich bases. Pharmazie. 2004;59:502–505. doi: 10.1002/chin.200447216. [DOI] [PubMed] [Google Scholar]
- Dimmock JR, Raghavan SK, Logan BM, Bigam GE. Antileukemic evaluation of some Mannich bases derived from 2-arylidene-1,3-diketones. Eur J Med Chem. 1983;18:248–254. [Google Scholar]
- Dimmock JR, Sidhu KK, Chen M, Reid RS, Allen TM, Kao GY, Truitt GA. Evaluation of some Mannich bases of cycloalkanones and related compounds. Eur J Med Chem. 1993;28:313–322. [Google Scholar]
- Estabrook RW. Mitochondrial respiratory control and the polarographic measurement of ADP:O ratios. Methods Enzymol. 1967;10:41–47. [Google Scholar]
- Guillen J, Dumoulin H, Dallemagne P, Reynolds R, Roult S. Synthesis and antituberculosis activity of new phenylpyrrolo[1,2-a]pyrrole carboxylic acid derivatives. Pharm Pharmacol Commun. 1998;4:33–38. [Google Scholar]
- Hamon NW, Kirkpatrick DL, Chow EWK, Dimmock JR. Effect of 4-dimethylaminomethyl-1-(3-hydroxyphenyl)-1-nonen-3-one hydrochloride and related compounds on respiration in rat liver mitochondria. J Pharm Sci. 1982;71:25–29. doi: 10.1002/jps.2600710106. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Castilho RF, Grijalba MT, Bechara EJ, Vercesi AE. Effect of inorganic phosphate concentration on the nature of inner mitochondrial membrane alterations mediated by Ca2+ ions: A proposed model for phosphate-stimulated lipid peroxidation. J Biol Chem. 1996;271:2929–2934. doi: 10.1074/jbc.271.6.2929. [DOI] [PubMed] [Google Scholar]
- Lenaerts AJ, DeGroote MA, Orme IM. Preclinical testing of new drugs for tuberculosis: current challenges. Trends Microbiol. 2008;16:48–54. doi: 10.1016/j.tim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Molinspiration Chemoinformatics. Bratislava, Slovak Republic: http://www.molinspiration.com. [Google Scholar]
- Patole J, Shingnapurkar D, Padhye S, Ratledge C. Schiff base conjugates of p-aminosalicylic acid as antimycobacterial agents. Bioorg Med Chem Lett. 2006;16:1514–1517. doi: 10.1016/j.bmcl.2005.12.035. [DOI] [PubMed] [Google Scholar]
- TAACF. Tuberculosis Antimicrobial Acquisition and Coordinating Facility. Southern Research Institute; Birmingham, Alabama, U.S.A: 2009. [Google Scholar]


