Abstract
In an attempt to find alternative control methods for stored products insects, extracts of seven plant species (Cassia senna, Caesalpinia gilliesii, Thespesia populnea var. acutiloba, Chrysanthemum frutescens, Euonymus japonicus, Bauhinia purpurea, and Cassia fistula) were evaluated under laboratory conditions for their ability to protect wheat (Triticum spp.) grains against Trogoderma granarium insect. Moreover, gas chromatography-mass spectrometry (GC-MS) analysis was carried to identify the chemical components of the most effective plant extract against T. granarium. Furthermore, the safety of the most effective plant extract was evaluated with respect to biochemical and histological changes in treated rats relative to control. The results revealed that, the tested botanical extracts showed high efficiency against T. granarium with respect to mortality and progeny of the adults. C. senna was the most effective botanical extract against T. granarium. The GC-MS analysis of the most effective plant extract showed the presence of different bioactive compounds that is known by its insecticidal activity. The most effective plant extract showed no toxicity on treated rats relative to control with respect to biochemical and histological changes. The results suggest the ability of using these plant extracts for wheat grains protection as a safe alternative to insecticides.
1. Introduction
Stored products of agricultural and animal origin are attacked by more than 600 species of beetle pests, 70 species of moths, and about 355 species of mites causing quantitative and qualitative losses [1], and insect contamination in food commodities is an important quality control problem of concern for food industries. In industrialized countries like Canada and Australia there is zero tolerance for insects in food grains [2]. T. granarium is among the most serious and of widest occurrence in stores in tropical and subtropical regions of Asia and Africa [3] and is common in geographical areas characterized by high temperature and low humidity [4].
Control of stored-product insect populations is primarily dependent upon continued applications of insecticides [2]. In spite of its efficacy, their repeated use for several decades has disrupted biological control system by natural enemies and led to outbreaks of insect pests, widespread development of resistance, undesirable effects on nontarget organisms, and environmental and human health concerns [2, 5].
These problems have highlighted the need for the development of new types of selective insect-control alternatives. Plants may provide potential alternative to currently used insect-control agents because they constitute a rich source of bioactive chemicals [6]. Since these are often active against a limited number of species including specific target insect, they are often biodegradable to nontoxic products, potentially suitable for use in integrated pest management, and they could lead to the development of new classes of safer insect-control agents. Much effort has, therefore, been focused on plant-derived materials for potentially useful products as commercial insect-control agents. Little work has been done to manage stored-product insects by using aromatic medicinal plants despite their excellent pharmacological actions [7, 8].
Most of the alternatives insecticides substances were tested against insects attacking stored products in order to establish new control practices with lower mammalian toxicity and lower persistence in the environment relative to insecticides. Therefore, studies should conduct not only on the evaluation of botanical extracts against the target pests but also on their safety on human health that are in demand. Although the assessment of enzymes activity in the blood is generally a more sensitive measure of compound toxicity than histopathological changes and can be assessed within a shorter time, the tissue alterations are considered a confirmatory and supporting diagnostic role in the case of certain abnormalities in blood sampling [9].
Therefore, this study attempted to evaluate insecticidal activity of some newly used plant extracts (C. senna, C. gilliesii, T. populnea var. acutiloba, C. frutescens, E. japonicus, B. purpurea, and C. fistula) against T. granarium in wheat grains with respect to progeny and mortality of the insect adults, to identify the chemical components of the most effective plant extract against T. granarium, and finally to evaluate the toxicity of the most effective plant extract on rats with respect to biochemical and histological changes relative to control.
2. Materials and Methods
2.1. The Insect
T. granarium (Everts) was obtained from the Department of Stored Product Pests Control, Research Institute of Plant Protection, Sakha, Kafr El-Shiekh. This strain was reared free of insecticidal contamination for several years at 30 ± 2°C and 70% ± 5 relative to humidity. The cultures were maintained under the same conditions in the Pesticide Department, Faculty of Agriculture, Kafr El-Shiekh University, Egypt. The culture was raised by infesting 30 pairs of newly emerged T. granarium adults into 500 g of wheat grains in large box. After that, 35 d newly emerged (F1) adults were collected and used to infest the wheat samples.
2.2. The Stored Product
Wheat grains were used to culture T. granarium and to evaluate the efficacy of tested plant extracts as well as malathion against the same insect as well. Wheat grains were stored in airtight tins until being required for experiments. The experiments were carried out in a room kept at a constant temperature of 25°C and 70% r.h.
2.3. Plants and Preparation of Crude Extracts
The leaves of seven medicinal plant species (C. senna, C. gilliesii, T. populnea var. acutiloba, C. frutescens, E. japonicus, B. purpurea, and C. fistula) were collected from a local nursery at Kafr El-Sheikh, Monofia, Gharbia, and Alexandria Governorates, Egypt. C. senna (Alexandrian Senna), belonging to the family Fabaceae, is native to tropical Africa and cultivated in Egypt and Sudan. C. gilliesii (bird of paradise), belonging to the family Fabaceae, is native to tropical America, mainly Argentina and Uruguay. T. populnea var. acutiloba (Portia Tree), belonging to the family Malvaceae, is native to South Africa. C. frutescens (marguerite daisy), belonging to the family Asteraceae, is native to the Canary Islands. E. japonicus (Japanese Spindle), belonging to the family Celastraceae, is native to Japan, Korea, and China. B. purpurea (Purple camel's foot), belonging to the family Fabaceae, is native to South China. C. fistula (Cassias), belonging to the family Fabaceae, is native to southern Asia. The different leave samples were oven dried for 24 h at 70°C and, then, finely powdered using a blender. Each sample (25 g) was extracted twice with 300 mL of methanol at room temperature for 2 days. The extracts were filtered through Whatman filter paper (no. 15), Whatman Inc. (North Americ) USA. The combined filtrate was concentrated to dryness by rotary evaporation at 40°C.
2.4. Effect of Tested Plant Extracts and Malathion on Progeny of T. granarium
The tested plant extracts at concentration levels of 100, 300, and 500 ppm were used to evaluate its efficacy against T. granarium. Malathion was used as recommended compound against T. granarium at concentration levels of 5, 10, and 20 mg/L. Each concentration was applied in three replicates, and each replicate contained 20 g of wheat grains. The treatment of wheat grains was carried out by dipping wheat grains in water solution of malathion and botanical extracts at tested concentration levels twice consecutively for 5 seconds and subsequently spread on top of plastic sheets to dry for 90 min. The control treatment was carried using water only and replicated three times. Then, 10 adults of T. granarium were transferred to treated wheat grains which were put in a 85 × 75 mm plastic jar and kept at 30 ± 2°C and 70% ± 5 r.h, according to the method described by Kestenholz et al. [10]. The emerged adults from the hatched eggs were recorded after 6 weeks of treatment. These adults were used to calculate the reduction percentages in T. granarium progeny from the use of the tested plant extracts as well as malathion compared to the control as shown in the following equation as described by El-Lakwah et al. [11]:
| (1) |
2.5. Efficiency of the Tested Plant Extracts and Malathion on Adults, Pupae, and Larvae of T. granarium Beetle by Mean Mortality
Wheat grains were treated with the tested plant extracts and malathion for protection against larvae, pupae, and adults of T. granarium at concentration levels mentioned before. Each concentration was applied in three replicates and in each replicate contained 20 gm of wheat grains. The treatment of wheat grains was carried out by dipping wheat grains in aqueous solution of malathion and botanical extracts at the tested concentration levels twice consecutively for 5 seconds and subsequently spread on top of plastic sheets to dry for 90 min. The control treatment was carried using water only and replicated three times. Then, 10 adults, pupae, and larvae of T. granarium were transferred to treated wheat grains which were put in a 85 × 75 mm glass jar and kept at 30 ± 2°C and 70% ± 5 r.h, according to the method described by Kestenholz et al. [10]. The glass jars were covered with cotton cloths held on with rubber bands. The number of dead adults, pupae, and larvae in each jar was counted after one and two weeks and the percentage of insect mortality was recorded.
2.6. Chemical Composition of the Most Effective Plant Extract
GC/MS analysis was carried to identify the components of the most effective plant extract (C. senna) according to the method described by Durate-Almeida et al. [12]. The samples were injected three times for confirmation. The analysis was conducted on HP 6890 GC system coupled with a 5973 network mass selective detector with a capillary column of HP-5MS (60 m × 0.25 mm, film thickness 0.25 m). The oven temperature program was turned on at 50°C, held for 2 min, and then raised up to 200°C at a rate of 5°C·min−1. Helium was used as the carrier gas at a flow rate 1.0 mL·min−1, with a split ratio equal to 1/50. The detector and injector temperatures were 250 and 200°C, respectively. Some of the detected compounds in the tested plant extracts were identified by comparison of their retention indices (RIs) and mass spectra fragmentation with the available analytical standards (1,8 Cineole, Linalool, and Butanoic acid). They were also identified by comparison of their RIs and mass spectra fragmentation with those stored in the Wiley and NIST libraries associated with GC-MS. Several other compounds could be identified only through the second method. The samples were analyzed by the Central Laboratory for Pesticides, Agriculture Research Centre, Cairo, Egypt.
2.7. Toxicity Assessments
2.7.1. Animal Treatment
The used adult Wistar male rats (Rattus norvegicus) with 8 weeks old and 80–100 gm in weight were obtained from Faculty of Medicine, Tanta University. Wister rats were housed in wire cages under standard conditions with free access to drinking water and food. The rats were kept in temperature-controlled room with 14 hours light and 10 hrs dark cycles. The rats were given a standard diet as described by Romestaing et al. [13]. Before treatment, rats were left two weeks for adaptation. The animals were randomly divided into two groups each comprising of three animals one group for the treatment with the most effective plant extract (C. senna) till 21 days and the second group for control. The most effective plant extract was administered to rats orally at concentration level of 500 mg/kg body weight. Control group rats were orally administrated with equal amount of almond oil. After 21 days the rats were sacrificed under anesthesia. Then, the blood samples were taken by cardiac puncture in vials containing heparin. Moreover, specimens from kidney and liver were taken from each treatment and kept in neutral buffered formalin 10% for histopathological test.
2.7.2. Enzymes Assays
Blood samples were centrifuged at 4500 rpm for 15 min at 4°C and the blood serum was used to determine the Glutamate Pyruvate Transaminase (GPT), creatinine, and alkaline phosphatase (ALP) according to the methods described by Barham and Trinder [14], Reitman and Frankel [15], and Wilkinson et al. [16], respectively.
2.7.3. Histopathological Test
The histopathology test was carried out at Histopathology Laboratory, Department of Histopathology, Faculty of Veterinary Medicine, Kafr El-Sheikh University, according to the method described by Bancroft and Stevens [17].
2.8. Statistical Analysis
Data from the experiments were statistically analyzed using one-way repeated measurement analysis of variance. For mortality experiments, the statistical analysis was carried out after mortality percentages were corrected. Newman-Keuls's multiple range test using a computer program SAS (Version 6.12, SAS Institute Inc., Cary, NC, USA) was used to separate means.
3. Results
3.1. Effect of Tested Plant Extracts and Malathion on Progeny of T. granarium
The numbers of emerged adults of T. granarium were significantly decreased in all treatments (the tested plant extracts and malathion) relative to the control, as shown in Table 1. Moreover, the tested plant extracts delayed the progeny of the tested insect three weeks relative to control treatment. Increasing the concentration level of all tested treatments reduced the emergence of T. granarium even more (concentration dependent). C. senna extract followed by malathion and B. purpurea was the most effective treatment while C. furtescens extract was the least effective one.
Table 1.
Effect of the tested plant extracts and malathion on progeny of T. granarium.
| Treatments | Concentration level (mg/L) | % Reduction |
|---|---|---|
| Cassia senna | 100 | 95bc |
| 300 | 100a | |
| 500 | 100a | |
| 100 | 82g | |
| Caesalpinia gilliesii | 300 | 92.5cd |
| 500 | 97.5ab | |
| 100 | 70i | |
| Thespesia populnea var. acutiloba | 300 | 90ed |
| 500 | 92.5cd | |
| Chrysanthemum frutescens | 100 | 62j |
| 300 | 70i | |
| 500 | 85f | |
| 100 | 70i | |
| Euonymus japonicus | 300 | 90ed |
| 500 | 95bc | |
| Bauhinia purpurea | 100 | 97.5ab |
| 300 | 97.5ab | |
| 500 | 100a | |
| Cassia fistula | 100 | 80hg |
| 300 | 92.5cd | |
| 500 | 95bc | |
| Malathion | 5 | 80hg |
| 10 | 97ab | |
| 20 | 100a | |
| Control | 0.0 | 0.00k |
a,b,c,d,e,f,g,h,i,j,kSeparation of means according to the Student Newman Keuls multiple range test (P < 0.05).
3.2. Efficiency of Tested Plant Extracts and Malathion on T. granarium Adults Determined by Mortality Values
The efficacy of the tested plant extracts and malathion against T. granarium adults by means of mortality was presented in Table 2. The results showed that C. senna was the most effective treatment against T. granarium adults followed by B. purpurea, C. gilliesii, E. japonicus, T. populnea var. acutiloba, C. fistula, malathion, and C. frutescens, respectively. The morality percentages of T. granarium were significantly increased in the second week relative to the first week at all tested treatments. Increasing the concentration level of all tested treatments increased the mortality of T. granarium adults even more (concentration dependent).
Table 2.
Effect of the tested plant extracts and malathion on adult's mortality of T. granarium.
| Treatments | Concentration level (mg/L) | % Mortality after one week | % Mortality after two week |
|---|---|---|---|
| Cassia senna | 100 | 86.7abc | 100a |
| 300 | 90abc | 100a | |
| 500 | 93abc | 100a | |
| 100 | 43de | 100a | |
| Caesalpinia gilliesii | 300 | 60abc | 100a |
| 500 | 86.7abc | 100a | |
| 100 | 40c | 90a | |
| Thespesia populnea var. acutiloba | 300 | 63d | 93a |
| 500 | 83bc | 100a | |
| Chrysanthemum frutescens | 100 | 50de | 70b |
| 300 | 60de | 97a | |
| 500 | 83bc | 100a | |
| 100 | 60de | 90a | |
| Euonymus japonicus | 300 | 80c | 97a |
| 500 | 96.7ab | 100a | |
| Bauhinia purpurea | 100 | 83bc | 100a |
| 300 | 90abc | 100a | |
| 500 | 93abc | 100a | |
| Cassia fistula | 100 | 57e | 93a |
| 300 | 63d | 100a | |
| 500 | 80c | 100a | |
| Malathion | 5 | 60de | 90a |
| 10 | 80a | 93a | |
| 2 0 | 87a | 100a | |
| Control | 0.00 | 0.00f | 0.00c |
a,b,c,d,e,f,g,h,i,j,kSeparation of means according to the Student Newman Keuls multiple range test (P < 0.05).
3.3. Efficiency of Tested Plant Extracts and Malathion on T. granarium Pupae Determined by Mortality Values
The efficacy of the tested plant extracts and malathion against T. granarium pupae by means of mortality was presented in Table 3. The results showed that B. purpurea was the most effective treatment against T. granarium pupae followed by C. senna, C. gilliesii, E. japonicus, T. populnea var. acutiloba, C. fistula, malathion, and C. frutescens, respectively. The morality percentages of T. granarium pupae were significantly increased in the second week relative to the first week at all tested treatments. Increasing the concentration level of all tested treatments increased the mortality of T. granarium pupae even more (concentration dependent).
Table 3.
Effect of the tested plant extracts and malathion on mortality of T. granarium pupae.
| Treatments | Concentration level (mg/L) | % Mortality after one week | % Mortality after two week |
|---|---|---|---|
| Cassia senna | 100 | 66.7def | 100a |
| 300 | 86.7abc | 100a | |
| 500 | 93bc | 100a | |
| 100 | 43hi | 97ab | |
| Caesalpinia gilliesii | 300 | 47ghi | 100a |
| 500 | 53fgh | 100a | |
| 100 | 33ig | 100a | |
| Thespesia populnea var. acutiloba | 300 | 60efg | 100a |
| 500 | 80bcd | 100a | |
| Chrysanthemum frutescens | 100 | 33jg | 80de |
| 300 | 47ghi | 87bc | |
| 500 | 53fgh | 100a | |
| 100 | 73cde | 80e | |
| Euonymus japonicas | 300 | 80bcd | 93cd |
| 500 | 83bcd | 100a | |
| Bauhinia purpurea | 100 | 80bcd | 100a |
| 300 | 93ab | 100a | |
| 500 | 100a | 100a | |
| Cassia fistula | 100 | 33g | 93bc |
| 300 | 40hi | 95bc | |
| 500 | 47gh | 100a | |
| Malathion | 5 | 47ghi | 67f |
| 10 | 80bcd | 93abc | |
| 20 | 93ab | 100a | |
| Control | 0.00 | 0.00k | 0.00g |
a,b,c,d,e,f,g,h,i,j,kSeparation of means according to the Student Newman Keuls multiple range test (P < 0.05).
3.4. Efficiency of Tested Plant Extracts and Malathion on T. granarium Larvae Determined by Mortality Values
The efficacy of the tested plant extracts and malathion against T. granarium larvae by means of mortality was presented in Table 4. The results showed that C. gilliesii was the most effective treatment against T. granarium larvae followed by E. japonicus, C. senna, B. purpurea, T. populnea var. acutiloba, malathion, C. furtescens, and C. fistula, respectively. Among the tested plant extracts, C. gilliesii extract was the most effective one and C. fistula extract recorded the lowest efficacy against the larvae of T. granarium. The mortality percentages of T. granarium larvae were significantly increased in the second week relative to the first week at all tested treatments. Increasing the concentration level of all tested treatments increased the mortality of T. granarium larvae even more (concentration dependent).
Table 4.
Effect of tested plant extracts and malathion on mortality of T. granarium larvae.
| Treatments | Concentration level (mg/L) | % Mortality after one week | % Mortality after two week |
|---|---|---|---|
| Cassia senna | 100 | 80bc | 93a |
| 300 | 93ab | 100a | |
| 500 | 97ab | 100a | |
| 100 | 80bc | 100a | |
| Caesalpinia gilliesii | 300 | 93abc | 100a |
| 500 | 94ab | 100a | |
| 100 | 46.6ef | 87bc | |
| Thespesia populnea var. acutiloba | 300 | 80bc | 100a |
| 500 | 93ab | 100a | |
| Chrysanthemum frutescens | 100 | 47ef | 80c |
| 300 | 53e | 87bc | |
| 500 | 60de | 93ab | |
| 100 | 60de | 100a | |
| Euonymus japonicus | 300 | 73cd | 100a |
| 500 | 93ab | 100a | |
| Bauhinia purpurea | 100 | 33fg | 93ab |
| 300 | 53e | 100a | |
| 500 | 60de | 100a | |
| Cassia fistula | 100 | 37g | 87bc |
| 300 | 47ef | 93ab | |
| 500 | 60de | 100a | |
| Malathion | 5 | 50ef | 87c |
| 10 | 80bc | 93ab | |
| 20 | 93a | 100a | |
| Control | 0.00 | 0.00h | 0.00d |
a,b,c,d,e,f,g,h,i,j,kSeparation of means according to the Student Newman Keuls multiple range test (P < 0.05).
3.5. Composition of the Most Effective Botanical Extract
The identified chemical components of the most effective botanical extract (C. senna) against T. granarium were presented in Table 5. Eighteen compounds were identified from C. senna extract, separately with different percentages as shown in Table 5.The identified compounds were belonging to different fatty acids and their derivatives (eldyhydes, esters, and alcohols).
Table 5.
The main constituents of C. senna plant extract identified by GC-MS analysis.
| No. | Name | Retention time (min) | % Area |
|---|---|---|---|
| 1 | Beta phellandrene | 4.33 | 1.5 |
| 2 | Mone inositol | 4.37 | 2.75 |
| 3 | 1,8 Cineole | 4.98 | 26.55 |
| 4 | Linalool | 5.70 | 10.28 |
| 5 | 3 cyclohexen-1-ol 4-methyl-1-(1-methylethyl) | 6.57 | 2.69 |
| 6 | 3cyclohexene-1-methanol-alpha 4-trimethyl-p-menth 1-en-8-ol | 6.71 | 2.48 |
| 7 | Butanoic acid | 7.2 | 20.02 |
| 8 | α-Terpineol acetate | 8.16 | 21.06 |
| 9 | 9-Octadecanoic acid (z)2, 6 octadien-1-ol 3, 7 dimethyl acetate | 8.37 | 2.2 |
| 10 | Caryophyllene | 8.85 | 0.75 |
| 11 | Cycloheptasiloxane tetradecamethyl | 9.25 | 1.7 |
| 12 | Croweacin | 9.72 | 20.3 |
| 13 | 1, 6 dodecatrien-3-ol 3, 7, 11 titramethyl | 9.95 | 1.12 |
| 14 | Cyclononosiloxane octadecanmethyl | 11.97 | 2.23 |
| 15 | Hexadecanoic acid methyl ester | 13.07 | 0.94 |
| 16 | Tetratetracontane | 15.04 | 1.19 |
| 17 | Tetra cosamethyl cyclododecasiloxane | 19.19 | 2.6 |
| 18 | Iron monocarbonyl 1,3 butadiene 1,4, dicarbonic acid diethyl ether | 20.13 | 1.83 |
3.6. Toxicity Evaluation
3.6.1. Effect of the Most Effective Plant Extract on Liver Enzymes
The Alkaline phosphatase and GPT activities are known as cytosolic marker enzymes reflecting hepatocellular necrosis as they are released into the blood after cell membrane damage. In the present study, therefore, both enzyme activities were used as indicators of hepatic damage. The obtained data in Table 6 showed that there were no significant differences in the activity of ALT and GPT after 21 days of rat's administration with the most effective plant extract at dose level of 500 mg/kg body weight relative to control treatment.
Table 6.
Effect of the most effective plant extract (C. senna) on serum GPT, ALT, and creatinine of treated rats at dose level of 500 mg/kg body weight.
| Treatments | SGPT U/L | ALP U/L | Creatinine mg/dL |
|---|---|---|---|
| Control | 65 ± 1.39 | 101 ± 5.57 | 0.205 ± 0.06 |
| C. senna | 67 ± 3.57 | 111 ± 5.1 | 0.197 ± 0.08 |
3.6.2. Effect of the Most Effective Plant Extract on Kidney Function
Regarding the kidney function, there were no significant differences in creatinine level in rats administration with the most effective plant extract at dose level of 500 mg/kg relative to control (Table 6). The normal creatinine in rats treated with the most effective plant extract relative to control treatment was assumed to be the normal kidney function. Moreover, the histology of kidney tissue treated with the most effective plant extract relative to control supports this explanation.
3.6.3. The Histopathological Changes in the Kidney
The normal structure of kidney tissue in control treatment was shown in Figure 1(a). However, for the rats treated with C. senna extract at dose level of 500 mg/kg, the tissue was some what like control with a small vaculation and degeneration in renal tubules (Figure 1(b)).
Figure 1.
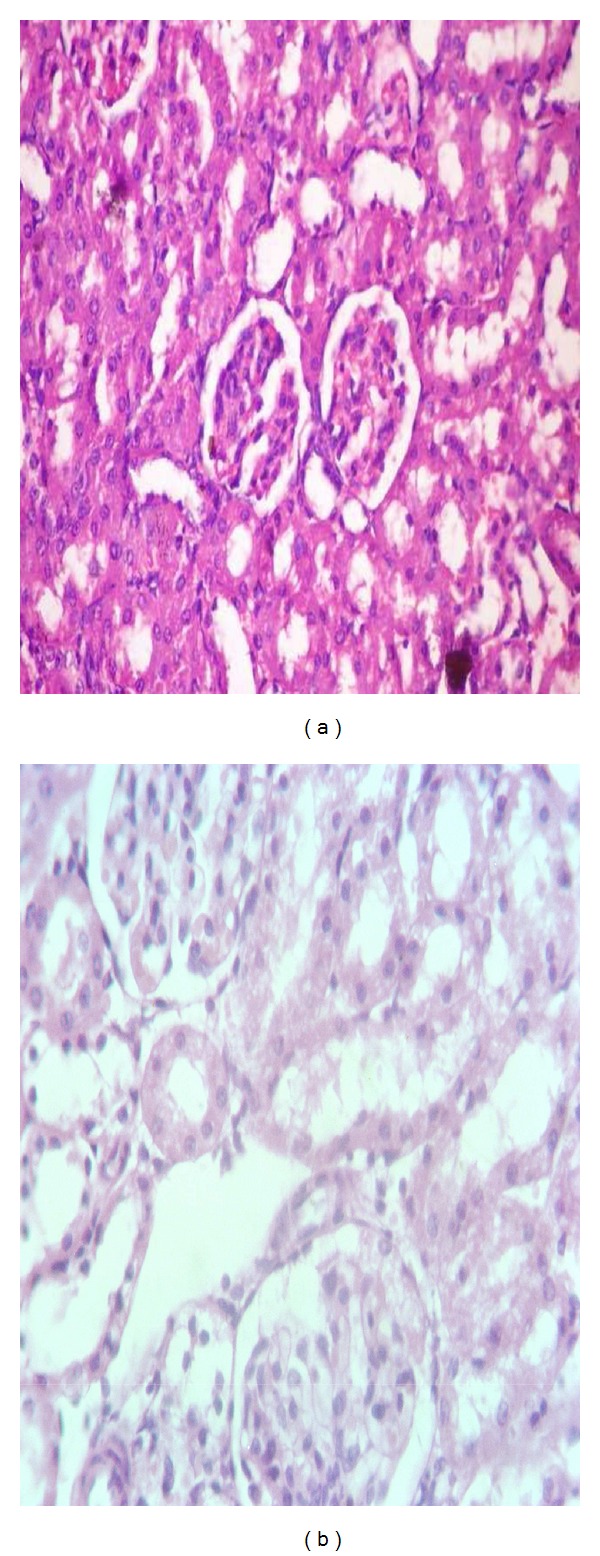
Sections in kidney of rats treated with C. senna extract (b) at dose level of 500 mg/kg after 21 days of treatment relative to control (a).
3.6.4. The Histopathological Changes in the Liver
The normal structure of liver tissue in control treatment was shown in Figure 2(a). However, the liver of rats treated with C. senna at dose level of 500 mg/kg showed that blood vessels engorged and hepatocyte contain vacuolated cytoplasm (Figure 2(b)).
Figure 2.
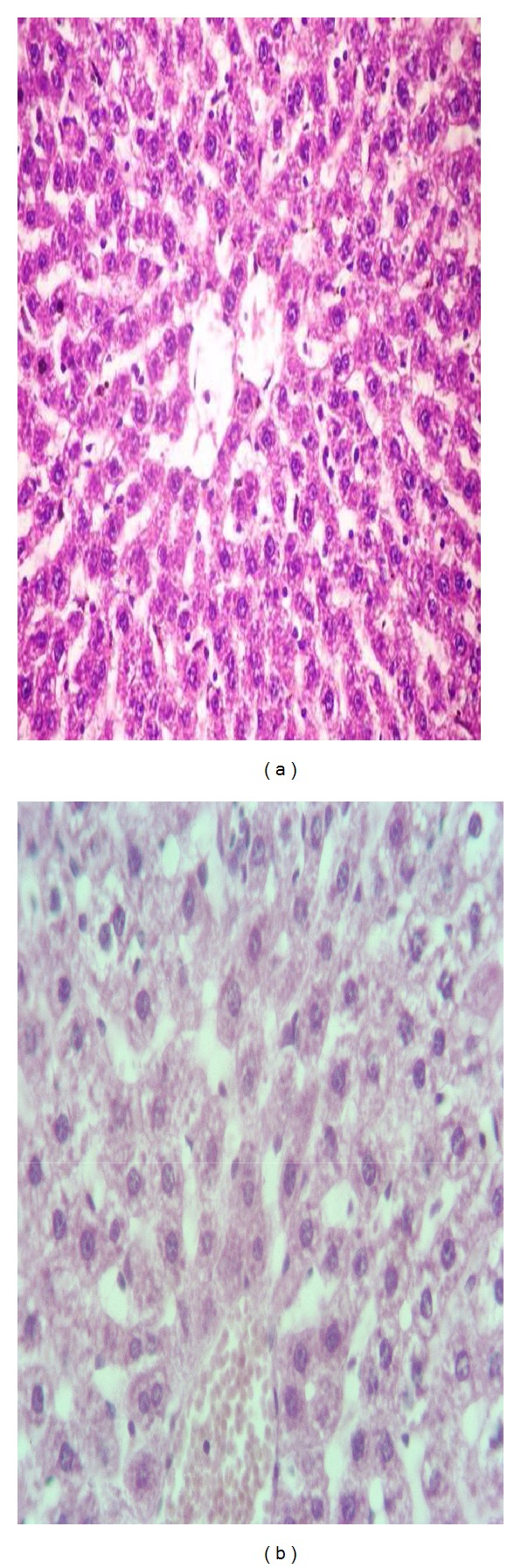
Sections in liver of rats treated with C. senna extract (b) at dose level of 500 mg/kg after 21 days of treatment relative to control (a).
4. Discussion
The results of the present study implied that the tested plant extracts were effective against T. granarium in stored wheat with respect to progeny of adults and mortality of all its stages (larvae, pupae, and adult). The efficacy of the plant extracts against T. granarium insect in stored wheat with respect to progeny and adult mortality has been reported by many researchers [10, 18, 19]. However, the efficacy of the tested plant extracts, especially the most effective ones, has not been reported against T. granarium and considered first report.
Among the identified compounds from C. senna extract, some compounds such as 1,8 cineole, linalool, butanoic acid, α-terpineol acetate, and croweacin were detected with high percentages relative to other detected compounds. The insecticidal activity of C. senna extract against T. granarium may be due to the presence of the previous fatty acids and its derivatives [20–24]. Moreover, the efficacy of the most effective plant extract at higher concentrations might actually have efficacy comparable to the chemical pesticides. In fact, the actual dosage of any one compound identified in this extract could be relatively low, safe, and economically feasible.
Although the insecticidal activity of the most effective plant extract is attributed mainly to its major compounds mentioned before, the synergistic or antagonistic effect of some compounds in the mixture has to be considered [25]. Each of the plant extract components has its own contribution on biological activity of the extract against the tested insect.
The mode of action of the bioactive natural monoter-penoids (hydrocarbons, alcohols, and ketones) isolated from plant extracts oils may be due to inhibition of acetylcholinesterase [26–28]. Since Lee et al. [28] reported that 1, 8-Cineole was the most potent inhibitor of AChE among the monoterpenes tested. This inhibition may be a mode of action for essential oils and monoterpenes against stored grain insects as well. Also, the mode of action of the tested botanical extracts may be largely attributable to its fumigant action [20, 29].
The botanical extracts as pest control agents present two main characters: the first is their safety to the people and the environment, and the second is the less resistance development against it by the tested insect. Regarding the safety, the toxicity evaluation of the most effective plant extract revealed that there were some slight variations that occurred sporadically in treated rats relative to control with respect to enzyme markers and histopathology of treated organs. Moreover, the observed changes in the tissues were mostly uncorrelated with the dosages which reflect the safety of the tested plant extract on human health. With referring to resistance development, it is believed that it is difficult for the insect to develop resistance to such a mixture of bioactive components with, apparently, different mechanisms of insecticidal activity [30].
This study is considered the first step toward more investigation and concern about using these effective botanical extracts as alternative for controlling of stored product pests. This will help to reduce the environmental pollution and the adverse effect on human health resulted from using insecticides since these botanical extracts revealed nonsignificant toxicity relative to the high dosage that were given orally and will not reach human by this dose as a residue under any conditions.
5. Conclusions
The insecticidal activity of the tested plant extracts against T. granarium indicated the potential of some plant species (C. senna, B. purpurea, and C. gilliesii) as a natural source of insecticidal material. Insecticidal activity was confirmed in all the tested plant species, although the results showed variation in their effectiveness against T. granarium insect. The ability of using botanical products as alternative of chemical control of T. granarium is possible if the problem of cost-effective commercial production can be solved. Moreover, some of these botanical extracts could find a place in IPM strategies, especially where the emphasis is on environmental, food safety and on replacing the more dangerous toxic insecticides. Work in this regard should continue to obtain information regarding its practical effectiveness under natural conditions to protect the stored products without any side effects.
References
- 1.Rajendran S, Sriranjini V. Plant products as fumigants for stored-product insect control. Journal of Stored Products Research. 2008;44(2):126–135. [Google Scholar]
- 2.White NDG, Leesch JG. Chemical control. In: Subramanyam B, Hagstrum DW, editors. Integrated Management of Insects in Stored Products. New York, NY, USA: Marcel Dekker; 1995. pp. 287–330. [Google Scholar]
- 3.Viljoen JH. The occurrence of Trogoderma (Coleoptera: Dermestidae) and related species in southern Africa with special reference to T. granarium and its potential to become established. Journal of Stored Products Research. 1990;26(1):43–51. [Google Scholar]
- 4.Ghanem I, Shamma M. Effect of non-ionizing radiation (UVC) on the development of Trogoderma granarium Everts. Journal of Stored Products Research. 2007;43(4):362–366. [Google Scholar]
- 5.Subramanyam B, Hagstrum DW. Resistance measurement and management. In: Subramanyam B, Hagstrum DW, editors. Integrated Management of Insects in Stored Products. New York, NY, USA: Marcel Dekker; 1995. pp. 331–397. [Google Scholar]
- 6.Wink M. Production and application of phytochemicals from an agricultural perspective. In: van Beek TA, Breteler H, editors. Phytochemistry and Agriculture. Vol. 34. Oxford, UK,: Clarendon; 1993. pp. 171–213. [Google Scholar]
- 7.Tang W, Eisenbrand G. Chinese Drugs of Plant Origin. New York, NY, USA: Springer; 1992. [Google Scholar]
- 8.Namba T. The Encyclopedia of Wakan-Yaku (Traditional Sino-Japanese Medicines) with Color Pictures, Vol I. Osaka, Japan: Hoikusha; 1993. [Google Scholar]
- 9.Cronelius CE, Charles W, Arhode E. Serum and tissue transaminase activities in domestic animals. The Cornell Veterinarian. 1959;49(1):116–126. [PubMed] [Google Scholar]
- 10.Kestenholz C, Stevenson PC, Belmain SR. Comparative study of field and laboratory evaluations of the ethnobotanical Cassia sophera L. (Leguminosae) for bioactivity against the storage pests Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) and Sitophilus oryzae (L.) (Coleoptera: Curculionidae) Journal of Stored Products Research. 2007;43(1):79–86. [Google Scholar]
- 11.El-Lakwah FA, Darwish AA, Khaled OM. Effectiveness of Dill seed powder on stored products insects. Annals of Agricultural Science, Moshtohor. 1992;34:2031–2037. [Google Scholar]
- 12.Duarte-Almeida JM, Negri G, Salatino A. Volatile oils in leaves of Bauhinia (Fabaceae Caesalpinioideae) Biochemical Systematics and Ecology. 2004;32(8):747–753. [Google Scholar]
- 13.Romestaing C, Piquet MA, Bedu E, et al. Long term highly saturated fat diet does not induce NASH in Wistar rats. Nutrition and Metabolism. 2007;4, article 4 doi: 10.1186/1743-7075-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barham D, Trinder P. A colorimetric method for the determination of Creatinine in serum. Analyst. 1972;97:142–145. doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- 15.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. American Journal of Clinical Pathology. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson JH, Boutwell JH, Winsten S. Evaluation of a new system for the kinetic measurement of serum alkaline phosphatase. Clinical Chemistry. 1969;15(6):487–495. [PubMed] [Google Scholar]
- 17.Bancroft JD, Stevens A. Theory and Practice of Histopathological Techniques. 4th edition 1996. [Google Scholar]
- 18.Tapondjou LA, Adler C, Bouda H, Fontem DA. Efficacy of powder and essential oil from Chenopodium ambrosioides leaves as post-harvest grain protectants against six-stored product beetles. Journal of Stored Products Research. 2002;38(4):395–402. [Google Scholar]
- 19.Ketoh GK, Koumaglo HK, Glitho IA. Inhibition of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) development with essential oil extracted from Cymbopogon schoenanthus L. Spreng. (Poaceae), and the wasp Dinarmus basalis (Rondani) (Hymenoptera: Pteromalidae) Journal of Stored Products Research. 2005;41(4):363–371. [Google Scholar]
- 20.Park IK, Lee SG, Choi DH, Park JD, Ahn YJ. Insecticidal activities of constituents identified in the essential oil from leaves of Chamaecyparis obtusa against Callosobruchus chinensis (L.) and Sitophilus oryzae (L.) Journal of Stored Products Research. 2003;39(4):375–384. [Google Scholar]
- 21.Negahban M, Moharramipour S, Sefidko F. Chemical composition and insecticidal activity of Artemisiascoperte essential oil against three Coleoptera stored-product insects. Journal of Asia-Pacific Entomology. 2006;9:381–388. [Google Scholar]
- 22.Rozman V, Kalinovic I, Korunic Z. Toxicity of naturally occurring compounds of Lamiaceae and Lauraceae to three stored-product insects. Journal of Stored Products Research. 2007;43(4):349–355. [Google Scholar]
- 23.Ogendo JO, Kostyukovsky M, Ravid U, et al. Bioactivity of Ocimum gratissimum L. oil and two of its constituents against five insect pests attacking stored food products. Journal of Stored Products Research. 2008;44(4):328–334. [Google Scholar]
- 24.López MD, Jordán MJ, Pascual-Villalobos MJ. Toxic compounds in essential oils of coriander, caraway and basil active against stored rice pests. Journal of Stored Products Research. 2008;44(3):273–278. [Google Scholar]
- 25.Ragasa CY, Hofileña JG, Rideout JA. New furanoid diterpenes from Caesalpinia pulcherrima . Journal of Natural Products. 2002;65(8):1107–1110. doi: 10.1021/np0201523. [DOI] [PubMed] [Google Scholar]
- 26.Gordon WP, Forte AJ, McMurtry RJ. Hepatotoxicity and pulmonary toxicity of pennyroyal oil and its constituent terpenes in the mouse. Toxicology and Applied Pharmacology. 1982;65(3):413–424. doi: 10.1016/0041-008x(82)90387-8. [DOI] [PubMed] [Google Scholar]
- 27.Miyazawa M, Watanabe H, Kameoka H. Inhibition of acetylcholinesterase activity by monoterpenoids with a p-menthane skeleton. Journal of Agricultural and Food Chemistry. 1997;45(3):677–679. [Google Scholar]
- 28.Lee SE, Choi WS, Lee HS, Park BS. Cross-resistance of a chlorpyrifos-methyl resistant strain of Oryzaephilus surinamensis (Coleoptera: Cucujidae) to fumigant toxicity of essential oil extracted from Eucalyptus globulus and its major monoterpene, 1,8-cineole. Journal of Stored Products Research. 2000;36(4):383–389. doi: 10.1016/s0022-474x(99)00059-4. [DOI] [PubMed] [Google Scholar]
- 29.Shaaya E, Kostjukovski M, Eilberg J, Sukprakarn C. Plant oils as fumigants and contact insecticides for the control of stored-product insects. Journal of Stored Products Research. 1997;33(1):7–15. [Google Scholar]
- 30.Wei LW, Wei M, Bing-yu Z, You-chen D, Feng I. Antagonistic activities of volatiles from four strains of Bacillus spp. and Paenibacillus spp. against soil-borne plant pathogens. Agricultural Sciences in China. 2008;7(9):1104–1114. [Google Scholar]


