Abstract
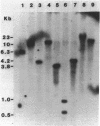
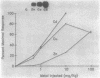
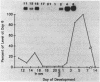
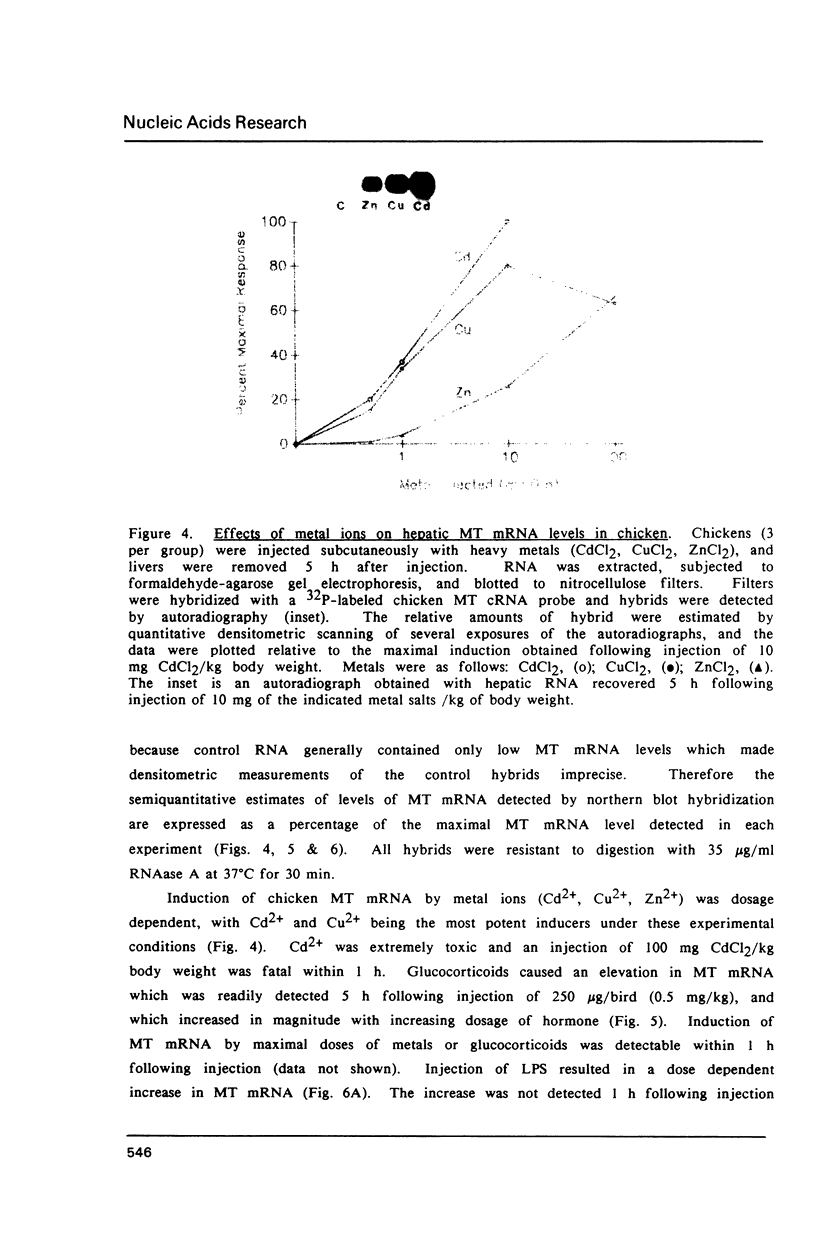
A cDNA library was constructed using RNA isolated from the livers of chickens which had been treated with zinc. This library was screened with a RNA probe complementary to mouse metallothionein-I (MT), and eight chicken MT cDNA clones were obtained. All of the cDNA clones contained nucleotide sequences homologous to regions of the longest (376 bp) cDNA clone. The latter contained an open reading frame of 189 bp, and the deduced amino acid sequence indicates a protein of 63 amino acids of which 20 are cysteine residues. Amino acid composition and partial amino acid sequence analyses of purified chicken MT protein agreed with the amino acid composition and sequence deduced from the cloned cDNA. Amino acid sequence comparisons establish that chicken MT shares extensive homology with mammalian MTs, but is more closely related to the MT-II than to the MT-I isoforms from various mammals. The nucleotide sequence of the coding region of chicken MT shares approximately 70% homology with the consensus sequence for the mammalian MTs. Southern blot analysis of chicken DNA indicates that the chicken MT gene is not a part of a large family of related sequences, but rather is likely to be a unique gene sequence. In the chicken liver, levels of chicken MT mRNA were rapidly induced by metals (Cd2+, Zn2+, Cu2+), glucocorticoids and lipopolysaccharide. MT mRNA was present in low levels in embryonic liver and increased to high levels during the first week after hatching before decreasing again to the basal levels found in adult liver. The results of this study establish that MT is highly conserved between birds and mammals and is regulated in the chicken by agents which also regulate expression of mammalian MT genes. However, in contrast to the mammals, the results suggest the existence of a single isoform of MT in the chicken.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. D., Birren B. W., Taplitz S. J., Herschman H. R. Rat metallothionein-1 structural gene and three pseudogenes, one of which contains 5'-regulatory sequences. Mol Cell Biol. 1986 Jan;6(1):302–314. doi: 10.1128/mcb.6.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen R. D., Piletz J. E., Birren B. W., Herschman H. R. Levels of metallothionein messenger RNA in foetal, neonatal and maternal rat liver. Eur J Biochem. 1983 Apr 5;131(3):497–500. doi: 10.1111/j.1432-1033.1983.tb07289.x. [DOI] [PubMed] [Google Scholar]
- Andrews G. K., Adamson E. D., Gedamu L. The ontogeny of expression of murine metallothionein: comparison with the alpha-fetoprotein gene. Dev Biol. 1984 Jun;103(2):294–303. doi: 10.1016/0012-1606(84)90317-8. [DOI] [PubMed] [Google Scholar]
- Andrews G. K., Dziadek M., Tamaoki T. Expression and methylation of the mouse alpha-fetoprotein gene in embryonic, adult, and neoplastic tissues. J Biol Chem. 1982 May 10;257(9):5148–5153. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. U., Lopez J. M. Isolation and partial characterization of a cadmium-binding protein from the liver of alligators exposed to cadmium. Comp Biochem Physiol C. 1985;82(1):123–128. doi: 10.1016/0742-8413(85)90218-x. [DOI] [PubMed] [Google Scholar]
- Bonham K., Gedamu L. Induction of metallothionein and metallothionein mRNA in rainbow-trout liver following cadmium treatment. Biosci Rep. 1984 Aug;4(8):633–642. doi: 10.1007/BF01121016. [DOI] [PubMed] [Google Scholar]
- Bryan S. E., Hidalgo H. A., Koppa V., Smith H. A. Cadmium, an effector in the synthesis of thionein. Environ Health Perspect. 1979 Feb;28:281–285. doi: 10.1289/ehp.7928281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compere S. J., Palmiter R. D. DNA methylation controls the inducibility of the mouse metallothionein-I gene lymphoid cells. Cell. 1981 Jul;25(1):233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J Biol Chem. 1981 Jun 10;256(11):5712–5716. [PubMed] [Google Scholar]
- Durnam D. M., Perrin F., Gannon F., Palmiter R. D. Isolation and characterization of the mouse metallothionein-I gene. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6511–6515. doi: 10.1073/pnas.77.11.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flos R., Bas J., Hidalgo J. Metallothionein in the liver of the small lizard Podarcis muralis. Comp Biochem Physiol C. 1986;83(1):93–98. doi: 10.1016/0742-8413(86)90018-6. [DOI] [PubMed] [Google Scholar]
- Gallant K. R., Cherian M. G. Influence of maternal mineral deficiency on the hepatic metallothionein and zinc in newborn rats. Biochem Cell Biol. 1986 Jan;64(1):8–12. doi: 10.1139/o86-002. [DOI] [PubMed] [Google Scholar]
- Griffith B. B., Walters R. A., Enger M. D., Hildebrand C. E., Griffith J. K. cDNA cloning and nucleotide sequence comparison of Chinese hamster metallothionein I and II mRNAs. Nucleic Acids Res. 1983 Feb 11;11(3):901–910. doi: 10.1093/nar/11.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Heguy A., West A., Richards R. I., Karin M. Structure and tissue-specific expression of the human metallothionein IB gene. Mol Cell Biol. 1986 Jun;6(6):2149–2157. doi: 10.1128/mcb.6.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker P. E., Kägi J. H. Isolation and characterization of six human hepatic isometallothioneins. Biochem J. 1985 Oct 15;231(2):375–382. doi: 10.1042/bj2310375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Heguy A., Dietlin T., Cooke T. Metal-responsive elements act as positive modulators of human metallothionein-IIA enhancer activity. Mol Cell Biol. 1987 Feb;7(2):606–613. doi: 10.1128/mcb.7.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Imbra R. J., Heguy A., Wong G. Interleukin 1 regulates human metallothionein gene expression. Mol Cell Biol. 1985 Oct;5(10):2866–2869. doi: 10.1128/mcb.5.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Imbra R. J., Heguy A., Wong G. Interleukin 1 regulates human metallothionein gene expression. Mol Cell Biol. 1985 Oct;5(10):2866–2869. doi: 10.1128/mcb.5.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Metallothioneins: proteins in search of function. Cell. 1985 May;41(1):9–10. doi: 10.1016/0092-8674(85)90051-0. [DOI] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes--primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982 Oct 28;299(5886):797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- Karin M., Richards R. I. The human metallothionein gene family: structure and expression. Environ Health Perspect. 1984 Mar;54:111–115. doi: 10.1289/ehp.8454111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay J., Thomas D. G., Brown M. W., Cryer A., Shurben D., Solbe J. F., Garvey J. S. Cadmium accumulation and protein binding patterns in tissues of the rainbow trout, Salmo gairdneri. Environ Health Perspect. 1986 Mar;65:133–139. doi: 10.1289/ehp.8665133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito H., Ose Y., Mizuhira V., Sato T., Ishikawa T., Tazawa T. Separation and purification of (Cd, Cu, Zn)-metallothionein in carp hepato-pancreas. Comp Biochem Physiol C. 1982;73(1):121–127. doi: 10.1016/0306-4492(82)90178-2. [DOI] [PubMed] [Google Scholar]
- Klauser S., Kägi J. H., Wilson K. J. Characterization of isoprotein patterns in tissue extracts and isolated samples of metallothioneins by reverse-phase high-pressure liquid chromatography. Biochem J. 1983 Jan 1;209(1):71–80. doi: 10.1042/bj2090071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Mechanism of mRNA recognition by eukaryotic ribosomes during initiation of protein synthesis. Curr Top Microbiol Immunol. 1981;93:81–123. doi: 10.1007/978-3-642-68123-3_5. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Maroni G., Otto E., Lastowski-Perry D. Molecular and cytogenetic characterization of a metallothionein gene of Drosophila. Genetics. 1986 Mar;112(3):493–504. doi: 10.1093/genetics/112.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McCormick C. C. Induction and accumulation of metallothionein in liver and pancreas of chicks given oral zinc: a tissue comparison. J Nutr. 1984 Jan;114(1):191–203. doi: 10.1093/jn/114.1.191. [DOI] [PubMed] [Google Scholar]
- McCormick C. C. The tissue-specific accumulation of hepatic zinc metallothionein following parenteral iron loading. Proc Soc Exp Biol Med. 1984 Sep;176(4):392–402. doi: 10.3181/00379727-176-41888. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. H., Nakaue H., Deagen J. T., Whanger P. D., Arscott G. H. Accumulation and depletion of zinc in chick tissue metallothioneins. J Nutr. 1979 Oct;109(10):1720–1729. doi: 10.1093/jn/109.10.1720. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette A. J. Metallothionein mRNA expression in fetal mouse organs. Dev Biol. 1982 Jul;92(1):240–246. doi: 10.1016/0012-1606(82)90168-3. [DOI] [PubMed] [Google Scholar]
- Panemangalore M., Banerjee D., Onosaka S., Cherian M. G. Changes in the intracellular accumulation and distribution of metallothionein in rat liver and kidney during postnatal development. Dev Biol. 1983 May;97(1):95–102. doi: 10.1016/0012-1606(83)90067-2. [DOI] [PubMed] [Google Scholar]
- Price-Haughey J., Bonham K., Gedamu L. Heavy metal-induced gene expression in fish and fish cell lines. Environ Health Perspect. 1986 Mar;65:141–147. doi: 10.1289/ehp.8665141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Quaife C., Hammer R. E., Mottet N. K., Palmiter R. D. Glucocorticoid regulation of metallothionein during murine development. Dev Biol. 1986 Dec;118(2):549–555. doi: 10.1016/0012-1606(86)90025-4. [DOI] [PubMed] [Google Scholar]
- Richards M. P. Synthesis of a metallothionein-like protein by developing turkey embryos maintained in long-term, shell-less culture. J Pediatr Gastroenterol Nutr. 1984;3(1):128–136. doi: 10.1097/00005176-198401000-00025. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C. J., Hamer D. H. Cloning and sequence analysis of two monkey metallothionein cDNAs. Gene. 1983 Sep;24(1):137–146. doi: 10.1016/0378-1119(83)90139-7. [DOI] [PubMed] [Google Scholar]
- Searle P. F., Davison B. L., Stuart G. W., Wilkie T. M., Norstedt G., Palmiter R. D. Regulation, linkage, and sequence of mouse metallothionein I and II genes. Mol Cell Biol. 1984 Jul;4(7):1221–1230. doi: 10.1128/mcb.4.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin C., Hamer D. H. Regulation in vitro of metallothionein gene binding factors. Science. 1987 Mar 13;235(4794):1383–1387. doi: 10.1126/science.3103216. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suzuki K. T., Akitomi H., Kawamura R. Cadmium, copper and zinc-binding protein (metallothionein) in the liver of the water lizard Triturus pyrrhogaster. Toxicol Lett. 1984 May;21(2):179–184. doi: 10.1016/0378-4274(84)90203-0. [DOI] [PubMed] [Google Scholar]
- Suzuki K. T., Ebihara Y. Distribution of cadmium, copper and zinc in the liver of spot salamander, Ambystoma maculatum and their binding to metallothionein. Comp Biochem Physiol C. 1984;78(1):35–38. doi: 10.1016/0742-8413(84)90043-4. [DOI] [PubMed] [Google Scholar]
- Suzuki K. T., Itoh N., Ohta K., Sunaga H. Amphibian metallothionein. Induction in the frogs Rana japonica, R. nigromaculata and Rhacophorus schlegelii. Comp Biochem Physiol C. 1986;83(2):253–259. doi: 10.1016/0742-8413(86)90119-2. [DOI] [PubMed] [Google Scholar]
- Suzuki K. T., Kawamura R. Metallothionein present or induced in the three species of frogs Bombina orientalis, Bufo bufo japonicus and Hyla arborea japonica. Comp Biochem Physiol C. 1984;79(2):255–260. doi: 10.1016/0742-8413(84)90195-6. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornalley P. J., Vasák M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta. 1985 Jan 21;827(1):36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- Waalkes M. P., Bell J. U. Depression of metallothionein in fetal rat liver following maternal cadmium exposure. Toxicology. 1980;18(2):103–110. doi: 10.1016/0300-483x(80)90073-6. [DOI] [PubMed] [Google Scholar]
- Weser U., Rupp H., Donay F., Linnemann F., Voelter W., Voetsch W., Jung G. Characterization of Cd, Zn-thionein (metallothionein) isolated from rat and chicken liver. Eur J Biochem. 1973 Nov 1;39(1):127–140. doi: 10.1111/j.1432-1033.1973.tb03111.x. [DOI] [PubMed] [Google Scholar]
- Wong K. L., Klaassen C. D. Isolation and characterization of metallothionein which is highly concentrated in newborn rat liver. J Biol Chem. 1979 Dec 25;254(24):12399–12403. [PubMed] [Google Scholar]