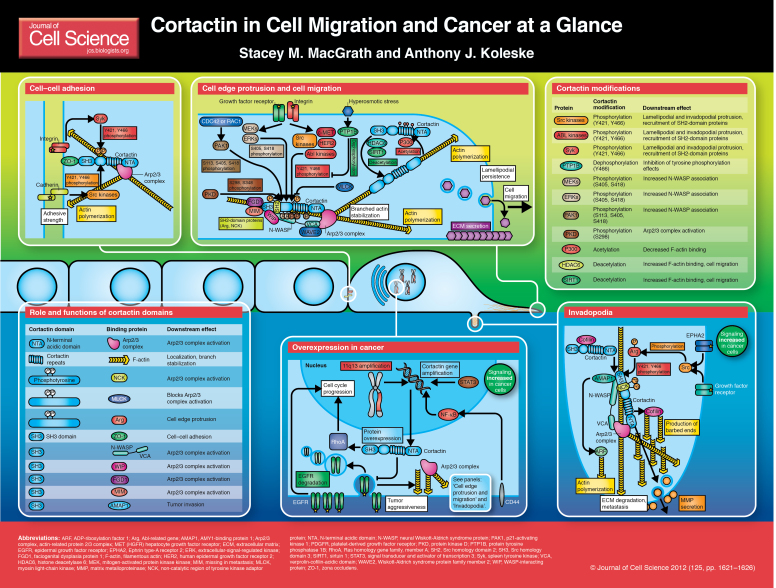
The actin-nucleation-promoting factor cortactin has emerged not only as a crucial regulator of actin cytoskeletal dynamics, but also as a key player in aggressive cancers (Buday and Downward, 2007; Weaver, 2008). Cortactin was first identified as a prominent substrate of the Src nonreceptor tyrosine kinase. Its name was derived from the observation that cortactin localizes to cortical actin structures (Wu et al., 1991). Since then, cortactin has been recognized for its association with cancer progression. Amplification of the gene that encodes cortactin (CTTN) and the resulting overexpression of cortactin protein have been observed in 15% of primary metastatic breast carcinomas and in nearly 30% of head and neck squamous cell carcinomas (Akervall et al., 1995; Buday and Downward, 2007). Overexpression of cortactin has also been linked to invasive cancers, including melanoma, colorectal cancer and glioblastoma, making cortactin an important biomarker for invasive cancers (Hirakawa et al., 2009; Kirkbride et al., 2011; Rothschild et al., 2006; Weaver, 2008; Xu et al., 2010).
Much research on cortactin in the past decade has focused on mechanistically dissecting its role in cellular migration and invasion. Early on, cortactin was shown to bind filamentous (F)-actin (Wu and Parsons, 1993). This was followed by the discovery that cortactin is a nucleation-promoting factor (NPF) that binds and activates the Arp2/3 complex – a seven-subunit complex that is responsible for the formation of branched F-actin – revealing a direct mechanism for regulating the polymerization of actin during cell motility (Goley and Welch, 2006; Higgs and Pollard, 2001; Uruno et al., 2001; Weaver et al., 2001; Weed et al., 2000). The ability of cortactin to bind several cytoskeletal proteins through its C-terminal SH3 domain links this protein to numerous
processes, including endocytosis, lamellipodial protrusion and directed cell migration (Ammer and Weed, 2008). More recent studies have focused on the role of post-translational modifications (PTMs) of cortactin, including phosphorylation and acetylation, as a means to regulate binding to actin, activation of the Arp2/3 complex and recruitment of cortactin-binding partners through interactions with Src homology 2 (SH2) domains. Importantly, cortactin has a central role in the development and maturation of invadopodia, which are actin-driven protrusive structures in invasive cancer cells that degrade the extracellular matrix (Weaver, 2008; Yamaguchi and Condeelis, 2007). This Cell Science at a Glance article focuses on how cortactin functions in cell migration and cancer-cell metastasis, and how it is regulated during these processes.
Cortactin the actin cytoskeleton
Cortactin structure
Cortactin was initially shown to colocalize with cortical F-actin at the cell periphery (hence the name cortactin), suggestive of interactions with the actin cytoskeleton (Wu et al., 1991). Early sequence analysis and binding studies identified six central ‘cortactin repeats’ and demonstrated that the fourth repeat is required for direct binding to F-actin (Weed et al., 2000; Wu and Parsons, 1993). Structure prediction, circular dichroism and crosslinking studies suggest that the cortactin repeats are unstructured in solution and transition to a more stable conformation upon F-actin binding, which is in agreement with electron-microscopy models, but not necessarily with other molecular dynamics modeling (Pant et al., 2006; Shvetsov et al., 2009; Zhang et al., 2007). Regulation of F-actin binding might occur through alternative splicing within this region, as some splice variants show decreased affinity for F-actin (van Rossum et al., 2003). Structural analysis of cortactin so far has yielded diverse information. Single-particle electron microscopy images depict cortactin as having an elongated conformation, whereas small-angle X-ray scattering analysis suggests a more globular shape (Cowieson et al., 2008; Weaver et al., 2002). Sequence and functional analysis have elucidated the basic domain structure of cortactin, including an N-terminal acidic (NTA) region, which binds and activates the Arp2/3 complex, actin-binding repeats, multiple phosphorylation sites and a C-terminal SH3 domain (Ammer and Weed, 2008; Buday and Downward, 2007).
SH3-domain-binding partners regulate cellular processes
The identification of cortactin SH3-domain-binding partners has provided important insights into how cortactin regulates distinct cellular processes. The cortactin SH3 domain interacts with the adhesion-complex protein zona occludens protein 1 (ZO-1) (Hirakawa et al., 2009; Katsube et al., 1998). Cortactin has also been shown to act downstream of E-cadherin activated by Src kinase and is required for actin polymerization at these cell–cell adhesions (Helwani et al., 2004; Ren et al., 2009). Although not shown to be a direct interaction, cortactin colocalizes with Syk kinase at sites of cell–cell contact, where Syk can phosphorylate cortactin (Zhang et al., 2009a). Cortactin-stimulated actin polymerization might increase the strength of cell–cell contacts at sites of adhesion (Weed et al., 2000).
The cortactin SH3 domain also binds proteins that promote actin polymerization mediated by the Arp2/3 complex, including the NPFs neural Wiskott-Aldrich syndrome protein (N-WASP) and WASp-interacting protein (WIP) (Kinley et al., 2003; Kowalski et al., 2005; Tehrani et al., 2007). The cortactin SH3 domain activates N-WASP in cells and enhances cell migration (Kowalski et al., 2005). Importantly, in addition to regulating actin polymerization through its SH3 domain, cortactin also binds and activates the Arp2/3 complex directly through its N-terminal acidic domain, as discussed below (Uruno et al., 2001; Weaver et al., 2001; Weed et al., 2000).
Cortactin activates the Arp2/3 complex and stabilizes branched actin networks
The NTA domain of cortactin was first shown to be important for targeting the actin machinery to the cell periphery, leading to the discovery that cortactin also weakly activates the Arp2/3 complex (Uruno et al., 2001; Weaver et al., 2001; Weed et al., 2000). Several cortactin SH3-domain-binding proteins regulate its ability to activate the Arp2/3 complex, including myosin light-chain kinase (MLCK), a protein involved in faciogenital dysplasia (FGD1), and missing in metastasis (MIM, also known as MTSS1) (Bershteyn et al., 2010; Dudek et al., 2002; Hou et al., 2003; Kim et al., 2004; Lin et al., 2005). The cortactin NTA domain binds the Arp3 subunit of the Arp2/3 complex and can synergize with the N-WASP verprolin-cofilin-acidic (VCA) domain in vitro to promote actin polymerization, but the activation of Arp2/3 complex by cortactin and N-WASP might be more complex than originally thought (Uruno et al., 2003; Weaver et al., 2002; Weaver et al., 2001). Unlike VCA, cortactin binds most tightly to the Arp2/3 complex after its incorporation into actin branches, which is likely to be due to interactions between cortactin and F-actin rather than conformational changes within the Arp2/3 complex (Uruno et al., 2003; Weaver et al., 2001). This is consistent with structural data showing that cortactin, but not WASP family members, can associate with the Arp2/3 complex after branch formation (Egile et al., 2005). A recent study by Xu et al. showed that, although cortactin and WASP family proteins bind the Arp2/3 complex at different sites, the conformations of Arp2/3 in complex with different NPFs are similar (Xu et al., 2011). This supports the model that interactions with F-actin stabilize the binding of cortactin to the Arp2/3 complex, rather than conformational changes of the Arp2/3 complex that occur following the release of N-WASP. Cortactin also stabilizes F-actin networks and inhibits de-branching, most probably by strengthening the interactions between the Arp2/3 complex and F-actin (Weaver et al., 2001). The ability of cortactin to promote cell migration requires its NTA domain, which facilitates migration by increasing lamellipodial persistence and promotes adhesion assembly through the binding and activation of the Arp2/3 complex, and through interactions with F-actin (Bryce et al., 2005).
Post-translational modification of cortactin regulates cell migration
Tyrosine phosphorylation of cortactin in cell migration
In addition to interactions with its numerous binding partners, cortactin activity is also regulated by several post-translational modifications. Cortactin is phosphorylated at tyrosine residues in response to signaling downstream of different upstream receptors, which include integrin- and cadherin-adhesion receptors and growth factor receptors (Helwani et al., 2004; Lapetina et al., 2009; Ren et al., 2009; Vuori and Ruoslahti, 1995). Several nonreceptor tyrosine kinases have been implicated in the phosphorylation of cortactin, including Src family kinases, ABL family kinases, FER, and Syk (Boyle et al., 2007; Fan et al., 2004; Maruyama et al., 1996; Sangrar et al., 2007; Zhang et al., 2009a). Activation of the hepatocyte growth factor receptor (HGFR, also known as MET) and human epidermal growth factor receptor 2 (HER2, also known as ERBB2) tyrosine kinases also stimulates cortactin phosphorylation in cancer cells (Crostella et al., 2001; Garcia-Castillo et al., 2009).
Tyrosine phosphorylation occurs primarily on Y421 and Y466, which are located within the proline-rich domain, although mapping of post-translational modifications by mass spectrometry has identified additional targets, such as Y482 (Huang et al., 1998; Martin et al., 2006). Interestingly, Y466 appears to be selectively phosphorylated downstream of fibroblast growth factor receptor 1 (FGFR1), suggesting the presence of different upstream kinases for each site, although this requires further investigation (Hinsby et al., 2003). Phosphorylation of the tyrosine residues stimulates the cleavage of cortactin by calpain, and this cleavage appears to mediate cortactin-regulated protrusion of the cell edge (Perrin et al., 2006). Phosphorylation of cortactin is also required for the endocytosis of several receptors (Luo et al., 2006; Lynch et al., 2003; Smith-Pearson et al., 2010; Zhu et al., 2007). At a mechanistic level, tyrosine phosphorylation triggers recruitment of SH2-domain proteins, including several kinases and NCK1, which links cortactin with N-WASP and WIP, and leads to enhanced activation of the Arp2/3 complex (Lapetina et al., 2009; Okamura and Resh, 1995; Oser et al., 2010; Tehrani et al., 2007). These findings are consistent with the observation that tyrosine phosphorylation of cortactin induces lamellipodial protrusion and cell migration (Huang et al., 1998; Kowalski et al., 2005; Kruchten et al., 2008). Although less is known about the phosphatases that regulate cortactin, protein-tyrosine phosphatase 1B (PTP1B, also known as PTPN1) has been shown to dephosphorylate Y466 on cortactin during hyperosmotic stress (Stuible et al., 2008).
Serine phosphorylation of cortactin in cell migration
Cortactin is also phosphorylated by several serine/threonine kinases. The serine/threonine-protein kinase PAK3 phosphorylates cortactin on S113 in response to growth factor receptor stimulation (Webb et al., 2006). PAK1 phosphorylates cortactin on S405 and S418 downstream of RAC1 and CDC42, which are members of the Rho family of small GTPases that are required for localization of cortactin to the cell edge and increased association of cortactin with N-WASP (Grassart et al., 2010; Vidal et al., 2002; Weed et al., 1998). Mitogen-activated protein kinase kinases (MEKs, also known as MAP2Ks) and extracellular signal-regulated kinases (ERKs, also known as MAPKs) have also been shown to phosphorylate cortactin on residues S405 and S418 (Campbell et al. 1999). ERK-mediated phosphorylation enhances cortactin association with N-WASP, leading to increased actin polymerization mediated by the Arp2/3 complex (Martinez-Quiles et al., 2004). Protein kinase D (PKD) phosphorylates cortactin on S298, which appears to increase cell migration owing to activation of the Arp2/3 complex (Eiseler et al., 2010). In addition to S298, PKD also phosphorylates S348 in invadopodia of breast cancer cells, but the role of this second phosphorylation site is unclear (De Kimpe et al., 2009). Interestingly, it appears that tyrosine and serine phosphorylation can occur simultaneously, suggesting that cortactin integrates signals from diverse upstream signaling cascades (Kelley et al., 2010b; Kelley et al., 2011).
Acetylation of cortactin regulates binding of F-actin
In addition to phosphorylation, cortactin is also regulated through acetylation by histone acetyltransferase p300, and deacetylation by histone deacetylase 6 (HDAC6) and sirtuin-1 (SIRT1) (Zhang et al., 2009b). Acetylation neutralizes charged lysine residues within the F-actin-binding repeats, which abrogates binding of cortactin to F-actin and decreases cell motility (Zhang et al., 2007). Like many regulatory mechanisms of cortactin, acetylation also appears to be important in cancer progression because HDAC6 expression is required for the formation and degradation of invadopodia in breast cancer cells (Rey et al., 2011).
Cortactin in cancer cell invasion
Cortactin is overexpressed in many cancers
In humans, amplification of segment 11q13 on chromosome 11 – a region that includes the CTTN gene – and the associated overexpression of cortactin has been linked to many cancers, including head and neck squamous cell carcinoma (HNSCC), oral squamous cell carcinoma, lung squamous cell carcinoma, gliosarcoma, breast cancer, colorectal cancer and melanoma (Akervall et al., 1995; Cai et al., 2010; Campbell et al., 1996; Chen et al., 2010; Faoro et al., 2010; Schuuring et al., 1993; Schuuring et al., 1992; van Damme et al., 1997; Xu et al., 2010; Yamada et al., 2010). In some cancers, overexpression of cortactin might also result from upregulation of transcription factors that activate transcription of CTTN (Du et al., 2009; Hill et al., 2006). Additionally, there are instances where cortactin is hyperphosphorylated by overexpressed kinases, such as Src and HER2 in cancer cells, leading to increased cell migration (Garcia-Castillo et al., 2009; Wu et al., 1991).
As cortactin is a marker for aggressive cancers, it is assumed that cortactin overexpression alters cell migration. Although this is, indeed, the case, overexpression of cortactin also promotes cell cycle progression through a RhoA-dependent pathway in cancer cells (Croucher et al., 2010). Cortactin overexpression also inhibits the degradation of epidermal growth factor (EGF) receptor, leading to increased pro-mitotic receptor signaling (Patel et al., 1998; Timpson et al., 2005). More recently, cortactin expression has been identified as a key factor in the secretion of extracellular matrix (ECM), which dictates cell migration and might explain diverging theories regarding the importance of cortactin in cell motility (Sung et al., 2011). For example, some studies show that loss of cortactin does not affect cell migration when cells are plated on ECM (Tanaka et al., 2009). However, cells plated in the absence of ECM show a pronounced defect in cell migration, suggesting that cortactin is important for cell migration in experiments in which ECM is not provided exogenously (Sung et al., 2011).
Cortactin is essential for the formation and function of invadopodia
Invadopodia are actin-driven protrusions found in invasive cancer cells and are thought to drive cancer cell migration (Weaver, 2006; Yamaguchi and Condeelis, 2007). The formation of actin-rich puncta, called invadopodial precursors, requires the recruitment of cortactin, possibly through scaffolding proteins such as tyrosine kinase substrate with five SH3 domains (TKS5) (Artym et al., 2006; Ayala et al., 2008; Crimaldi et al., 2009; Oser et al., 2009; Webb et al., 2007). Cortactin also regulates the trafficking and secretion of matrix metalloproteinases (MMPs) to the tips of degrading invadopodia, which is crucial for the formation and function of invadopodia (Clark et al., 2007).
Cortactin phosphorylation by Src and Arg kinases in breast cancer cells leads to extensive polymerization of actin through binding of the adaptor protein NCK1 and N-WASP (Desmarais et al., 2009; Mader et al., 2011; Oser et al., 2010; Smith-Pearson et al., 2010). Cortactin binds and inhibits the actin-severing protein cofilin; phosphorylation of cortactin releases cofilin, leading to its activation and the subsequent increase in polymerizing actin barbed ends (Oser et al., 2009). The extent of cortactin tyrosine phosphorylation correlates strongly with the level of ECM degradation by invasive cancer cells. Interestingly, phosphorylation of Y421 and Y466, but not Y482, is required to promote actin polymerization, which facilitates the stabilization and function of invadopodia by driving membrane protrusion (Kelley et al., 2010a; Oser et al., 2010). The importance of cortactin in the formation and function of invadopodia is emphasized by a reduction in matrix degradation in cells that have been injected with inhibitory cortactin antibodies, as well as cells in which cortactin has been knocked down by small interfering RNAs (siRNAs) (Clark et al., 2007; Oser et al., 2009; Weaver, 2008).
Cortactin is crucial for cancer cell invasion and metastasis
Elevated levels of cortactin expression are associated with increased aggressiveness of HNSCC cells, even under harsh growth conditions (Clark et al., 2009; van Rossum et al., 2006). In particular, tyrosine phosphorylation of cortactin correlates strongly with aggressiveness of cancer cells and is required for metastasis in a mouse model, whereas inhibition of phosphorylation decreases cancer cell metastasis (Li et al., 2001). Increased phosphorylation of cortactin and cellular invasion is also observed in cells, in which activation of the ephrin type-A receptor 2 (EPHA2) is increased through activation of Src (Faoro et al., 2010). Recently, cortactin has also been shown to bind the AMY1-binding protein 1 (AMAP1, also known as MYCBP), an ADP-ribosylation factor 1 (ARF1) GTPase-activating protein (GAP) involved in cytoskeletal rearrangement, which acts downstream of the vascular endothelial growth factor (VEGF) receptor. Furthermore, if binding of AMAP1 to cortactin is inhibited, invasion, metastasis and tumor cell angiogenesis are blocked (Hashimoto et al., 2011; Hashimoto et al., 2006). Together, these studies highlight the importance of cortactin in both normal cell motility, and cancer cell invasion and metastasis.
Perspectives
Great progress has been made in understanding how cortactin regulates cell migration, invadopodia formation and function, and cancer cell invasion and metastasis. However, studies on the importance of cortactin in cell migration have been inconsistent, with some pointing to a role in different stages of migration, whereas others find no role at all (Ammer and Weed, 2008; Lai et al., 2009; Tanaka et al., 2009). The recent results discussed above might shed some light on how cortactin affects migration – depending on the in vitro conditions used – by regulating ECM secretion (Sung et al., 2011). There is evidence that also suggests a role for cortactin in trafficking and secretion of MMPs in cancer cells, although the underlying mechanism for this is unclear (Clark et al., 2007). Although this might prove to be a new function of cortactin, it might also be an example of secondary processes that are affected by stabilization of the actin network by cortactin.
The exact functions of cortactin phosphorylation remain unclear. For example, disruption of the tyrosine phosphorylation sites of cortactin prevents cell edge protrusion, cancer cell invasion and actin polymerization in invadopodia (Kelley et al., 2011; Lapetina et al., 2009; Mader et al., 2011). However, other in vitro studies show that, if phosphorylation is disrupted, interactions between cortactin and N-WASP are promoted and activation of the Arp2/3 complex is increased (Martinez-Quiles et al., 2004). It is important to note that many of these studies were performed using different cell types and cortactin expression constructs, and it is possible that certain modes of regulation are dependent on the stimulation conditions and environmental context. Additionally, cortactin has numerous binding partners, many of which bind its SH3 domain. It is impossible for all of them to bind cortactin simultaneously, particularly when many of these proteins localize to different areas of the cell or are found in different cell types. Thus, it is probable that these interactions are influenced greatly by cell type, by the specific protein composition of the subcellular niche, and also by specific PTMs. Although the context of these protein–protein interactions and PTMs requires further study, they also provide viable targets for cancer treatment through inhibition of binding or disruption of upstream signaling pathways. For example, inhibition of kinases that act upstream of cortactin but downstream of Src kinase might provide the required specificity to selectively target highly invasive cancer cells, while minimizing off-target effects. Furthermore, exploration of the use of small molecules that disrupt protein interactions with the cortactin SH3 domain is underway (Hashimoto et al., 2006).
Supplementary Material
Footnotes
Funding
A.J.K. was funded by an NIH grant [grant number NS39475 CA133346]. Deposited in PMC for release after 12 months.
A high-resolution version of the poster is available for downloading in the online version of this article at jcs.biologists.org. Individual poster panels are available as JPEG files at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.093781/-/DC1.
References
- Akervall J. A., Jin Y., Wennerberg J. P., Zatterstrom U. K., Kjellen E., Mertens F., Willen R., Mandahl N., Heim S., Mitelman F. (1995). Chromosomal abnormalities involving 11q13 are associated with poor prognosis in patients with squamous cell carcinoma of the head and neck. Cancer 76, 853-859 [DOI] [PubMed] [Google Scholar]
- Ammer A. G., Weed S. A. (2008). Cortactin branches out: roles in regulating protrusive actin dynamics. Cell Motil. Cytoskeleton 65, 687-707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artym V. V., Zhang Y., Seillier-Moiseiwitsch F., Yamada K. M., Mueller S. C. (2006). Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 66, 3034-3043 [DOI] [PubMed] [Google Scholar]
- Ayala I., Baldassarre M., Giacchetti G., Caldieri G., Tete S., Luini A., Buccione R. (2008). Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J. Cell Sci. 121, 369-378 [DOI] [PubMed] [Google Scholar]
- Bershteyn M., Atwood S. X., Woo W. M., Li M., Oro A. E. (2010). MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Dev. Cell 19, 270-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden E. T., Barth M., Thomas D., Glazer R. I., Mueller S. C. (1999). An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene 18, 4440-4449 [DOI] [PubMed] [Google Scholar]
- Boyle S. N., Michaud G. A., Schweitzer B., Predki P. F., Koleske A. J. (2007). A critical role for cortactin phosphorylation by Abl-family kinases in PDGF-induced dorsal-wave formation. Curr. Biol. 17, 445-451 [DOI] [PubMed] [Google Scholar]
- Bryce N. S., Clark E. S., Leysath J. L., Currie J. D., Webb D. J., Weaver A. M. (2005). Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr. Biol. 15, 1276-1285 [DOI] [PubMed] [Google Scholar]
- Buday L., Downward J. (2007). Roles of cortactin in tumor pathogenesis. Biochim. Biophys. Acta. 1775, 263-273 [DOI] [PubMed] [Google Scholar]
- Cai J. H., Zhao R., Zhu J. W., Jin X. L., Wan F. J., Liu K., Ji X. P., Zhu Y. B., Zhu Z. G. (2010). Expression of cortactin correlates with a poor prognosis in patients with stages II-III colorectal adenocarcinoma. J. Gastrointest. Surg. 14, 1248-1257 [DOI] [PubMed] [Google Scholar]
- Campbell D. H., deFazio A., Sutherland R. L., Daly R. J. (1996). Expression and tyrosine phosphorylation of EMS1 in human breast cancer cell lines. Int. J. Cancer 68, 485-492 [DOI] [PubMed] [Google Scholar]
- Campbell D. H., Sutherland R. L., Daly R. J. (1999). Signaling pathways and structural domains required for phosphorylation of EMS1/cortactin. Cancer Res. 59, 5376-2385 [PubMed] [Google Scholar]
- Chen J. H., Chen K. Y., Ma H. I., Yu C. P., Nieh S., Lee H. S., Jin J. S. (2010). Cortactin, fascin and survivin expression associated with clinicopathological parameters in brain gliosarcoma. Chin. J. Physiol 53, 234-244 [DOI] [PubMed] [Google Scholar]
- Clark E. S., Whigham A. S., Yarbrough W. G., Weaver A. M. (2007). Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 67, 4227-4235 [DOI] [PubMed] [Google Scholar]
- Clark E. S., Brown B., Whigham A. S., Kochaishvili A., Yarbrough W. G., Weaver A. M. (2009). Aggressiveness of HNSCC tumors depends on expression levels of cortactin, a gene in the 11q13 amplicon. Oncogene 28, 431-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowieson N. P., King G., Cookson D., Ross I., Huber T., Hume D. A., Kobe B., Martin J. L. (2008). Cortactin adopts a globular conformation and bundles actin into sheets. J. Biol. Chem. 283, 16187-16193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimaldi L., Courtneidge S. A., Gimona M. (2009). Tks5 recruits AFAP-110, p190RhoGAP, and cortactin for podosome formation. Exp. Cell Res. 315, 2581-2592 [DOI] [PubMed] [Google Scholar]
- Crostella L., Lidder S., Williams R., Skouteris G. G. (2001). Hepatocyte Growth Factor/scatter factor-induces phosphorylation of cortactin in A431 cells in a Src kinase-independent manner. Oncogene 20, 3735-3745 [DOI] [PubMed] [Google Scholar]
- Croucher D. R., Rickwood D., Tactacan C. M., Musgrove E. A., Daly R. J. (2010). Cortactin modulates RhoA activation and expression of Cip/Kip cyclin-dependent kinase inhibitors to promote cell cycle progression in 11q13-amplified head and neck squamous cell carcinoma cells. Mol. Cell Biol. 30, 5057-5070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kimpe L., Janssens K., Derua R., Armacki M., Goicoechea S., Otey C., Waelkens E., Vandoninck S., Vandenheede J. R., Seufferlein T., et al. (2009). Characterization of cortactin as an in vivo protein kinase D substrate: interdependence of sites and potentiation by Src. Cell Signal 21, 253-263 [DOI] [PubMed] [Google Scholar]
- Desmarais V., Yamaguchi H., Oser M., Soon L., Mouneimne G., Sarmiento C., Eddy R., Condeelis J. (2009). N-WASP and cortactin are involved in invadopodium-dependent chemotaxis to EGF in breast tumor cells. Cell Motil. Cytoskeleton 66, 303-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X. L., Yang H., Liu S. G., Luo M. L., Hao J. J., Zhang Y., Lin D. C., Xu X., Cai Y., Zhan Q. M., et al. (2009). Calreticulin promotes cell motility and enhances resistance to anoikis through STAT3-CTTN-Akt pathway in esophageal squamous cell carcinoma. Oncogene 28, 3714-3722 [DOI] [PubMed] [Google Scholar]
- Dudek S. M., Birukov K. G., Zhan X., Garcia J. G. (2002). Novel interaction of cortactin with endothelial cell myosin light chain kinase. Biochem. Biophys. Res. Commun. 298, 511-519 [DOI] [PubMed] [Google Scholar]
- Egile C., Rouiller I., Xu X. P., Volkmann N., Li R., Hanein D. (2005). Mechanism of filament nucleation and branch stability revealed by the structure of the Arp2/3 complex at actin branch junctions. PLoS Biol. 3, e383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiseler T., Hausser A., De Kimpe L., Van Lint J., Pfizenmaier K. (2010). Protein kinase D controls actin polymerization and cell motility through phosphorylation of cortactin. J. Biol. Chem. 285, 18672-18683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Di Ciano-Oliveira C., Weed S. A., Craig A. W., Greer P. A., Rotstein O. D., Kapus A. (2004). Actin depolymerization-induced tyrosine phosphorylation of cortactin: the role of Fer kinase. Biochem. J. 380, 581-591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faoro L., Singleton P. A., Cervantes G. M., Lennon F. E., Choong N. W., Kanteti R., Ferguson B. D., Husain A. N., Tretiakova M. S., Ramnath N., et al. (2010). EphA2 mutation in lung squamous cell carcinoma promotes increased cell survival, cell invasion, focal adhesions, and mammalian target of rapamycin activation. J. Biol. Chem. 285, 18575-18585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Castillo J., Pedersen K., Angelini P. D., Bech-Serra J. J., Colome N., Cunningham M. P., Parra-Palau J. L., Canals F., Baselga J., Arribas J. (2009). HER2 carboxyl-terminal fragments regulate cell migration and cortactin phosphorylation. J. Biol. Chem. 284, 25302-25313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goley E. D., Welch M. D. (2006). The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 7, 713-726 [DOI] [PubMed] [Google Scholar]
- Grassart A., Meas-Yedid V., Dufour A., Olivo-Marin J. C., Dautry-Varsat A., Sauvonnet N. (2010). Pak1 phosphorylation enhances cortactin-N-WASP interaction in clathrin-caveolin-independent endocytosis. Traffic 11, 1079-1091 [DOI] [PubMed] [Google Scholar]
- Hashimoto A., Hashimoto S., Ando R., Noda K., Ogawa E., Kotani H., Hirose M., Menju T., Morishige M., Manabe T., et al. (2011). GEP100-Arf6-AMAP1-Cortactin Pathway Frequently Used in Cancer Invasion Is Activated by VEGFR2 to Promote Angiogenesis. PLoS ONE 6, e23359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Hirose M., Hashimoto A., Morishige M., Yamada A., Hosaka H., Akagi K., Ogawa E., Oneyama C., Agatsuma T., et al. (2006). Targeting AMAP1 and cortactin binding bearing an atypical src homology 3/proline interface for prevention of breast cancer invasion and metastasis. Proc. Natl. Acad. Sci. USA 103, 7036-7041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helwani F. M., Kovacs E. M., Paterson A. D., Verma S., Ali R. G., Fanning A. S., Weed S. A., Yap A. S. (2004). Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J. Cell Biol. 164, 899-910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs H. N., Pollard T. D. (2001). Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 70, 649-676 [DOI] [PubMed] [Google Scholar]
- Hill A., McFarlane S., Mulligan K., Gillespie H., Draffin J. E., Trimble A., Ouhtit A., Johnston P. G., Harkin D. P., McCormick D., et al. (2006). Cortactin underpins CD44-promoted invasion and adhesion of breast cancer cells to bone marrow endothelial cells. Oncogene 25, 6079-6091 [DOI] [PubMed] [Google Scholar]
- Hinsby A. M., Olsen J. V., Bennett K. L., Mann M. (2003). Signaling initiated by overexpression of the fibroblast growth factor receptor-1 investigated by mass spectrometry. Mol. Cell Proteomics 2, 29-36 [DOI] [PubMed] [Google Scholar]
- Hirakawa H., Shibata K., Nakayama T. (2009). Localization of cortactin is associated with colorectal cancer development. Int. J. Oncol. 35, 1271-1276 [DOI] [PubMed] [Google Scholar]
- Hou P., Estrada L., Kinley A. W., Parsons J. T., Vojtek A. B., Gorski J. L. (2003). Fgd1, the Cdc42 GEF responsible for Faciogenital Dysplasia, directly interacts with cortactin and mAbp1 to modulate cell shape. Hum. Mol. Genet. 12, 1981-1993 [DOI] [PubMed] [Google Scholar]
- Huang C., Liu J., Haudenschild C. C., Zhan X. (1998). The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J. Biol. Chem. 273, 25770-25776 [DOI] [PubMed] [Google Scholar]
- Katsube T., Takahisa M., Ueda R., Hashimoto N., Kobayashi M., Togashi S. (1998). Cortactin associates with the cell-cell junction protein ZO-1 in both Drosophila and mouse. J. Biol. Chem. 273, 29672-29677 [DOI] [PubMed] [Google Scholar]
- Kelley L. C., Ammer A. G., Hayes K. E., Martin K. H., Machida K., Jia L., Mayer B. J., Weed S. A. (2010a). Oncogenic Src requires a wild-type counterpart to regulate invadopodia maturation. J. Cell Sci. 123, 3923-3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley L. C., Hayes K. E., Ammer A. G., Martin K. H., Weed S. A. (2010b). Cortactin phosphorylated by ERK1/2 localizes to sites of dynamic actin regulation and is required for carcinoma lamellipodia persistence. PLoS ONE 5, e13847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley L. C., Hayes K. E., Ammer A. G., Martin K. H., Weed S. A. (2011). Revisiting the ERK/Src cortactin switch. Commun. Integr. Biol. 4, 205-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Hou P., Gorski J. L., Cooper J. A. (2004). Effect of Fgd1 on cortactin in Arp2/3 complex-mediated actin assembly. Biochemistry 43, 2422-2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinley A. W., Weed S. A., Weaver A. M., Karginov A. V., Bissonette E., Cooper J. A., Parsons J. T. (2003). Cortactin interacts with WIP in regulating Arp2/3 activation and membrane protrusion. Curr. Biol. 13, 384-393 [DOI] [PubMed] [Google Scholar]
- Kirkbride K. C., Sung B. H., Sinha S., Weaver A. M. (2011). Cortactin: a multifunctional regulator of cellular invasiveness. Cell Adh. Migr. 5, 187-198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski J. R., Egile C., Gil S., Snapper S. B., Li R., Thomas S. M. (2005). Cortactin regulates cell migration through activation of N-WASP. J. Cell Sci. 118, 79-87 [DOI] [PubMed] [Google Scholar]
- Kruchten A. E., Krueger E. W., Wang Y., McNiven M. A. (2008). Distinct phospho-forms of cortactin differentially regulate actin polymerization and focal adhesions. Am. J. Physiol. Cell Physiol. 295, C1113-C1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F. P., Szczodrak M., Oelkers J. M., Ladwein M., Acconcia F., Benesch S., Auinger S., Faix J., Small J. V., Polo S., et al. (2009). Cortactin promotes migration and platelet-derived growth factor-induced actin reorganization by signaling to Rho-GTPases. Mol. Biol. Cell 20, 3209-3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina S., Mader C. C., Machida K., Mayer B. J., Koleske A. J. (2009). Arg interacts with cortactin to promote adhesion-dependent cell edge protrusion. J. Cell Biol. 185, 503-519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Tondravi M., Liu J., Smith E., Haudenschild C. C., Kaczmarek M., Zhan X. (2001). Cortactin potentiates bone metastasis of breast cancer cells. Cancer Res. 61, 6906-6911 [PubMed] [Google Scholar]
- Lin J., Liu J., Wang Y., Zhu J., Zhou K., Smith N., Zhan X. (2005). Differential regulation of cortactin and N-WASP-mediated actin polymerization by missing in metastasis (MIM) protein. Oncogene 24, 2059-2066 [DOI] [PubMed] [Google Scholar]
- Luo C., Pan H., Mines M., Watson K., Zhang J., Fan G. H. (2006). CXCL12 induces tyrosine phosphorylation of cortactin, which plays a role in CXC chemokine receptor 4-mediated extracellular signal-regulated kinase activation and chemotaxis. J. Biol. Chem. 281, 30081-30093 [DOI] [PubMed] [Google Scholar]
- Lynch D. K., Winata S. C., Lyons R. J., Hughes W. E., Lehrbach G. M., Wasinger V., Corthals G., Cordwell S., Daly R. J. (2003). A Cortactin-CD2-associated protein (CD2AP) complex provides a novel link between epidermal growth factor receptor endocytosis and the actin cytoskeleton. J. Biol. Chem. 278, 21805-21813 [DOI] [PubMed] [Google Scholar]
- Mader C. C., Oser M., Magalhaes M. A., Bravo-Cordero J. J., Condeelis J., Koleske A. J., Gil-Henn H. (2011). An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 71, 1730-1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K. H., Jeffery E. D., Grigera P. R., Shabanowitz J., Hunt D. F., Parsons J. T. (2006). Cortactin phosphorylation sites mapped by mass spectrometry. J. Cell Sci. 119, 2851-2853 [DOI] [PubMed] [Google Scholar]
- Martinez-Quiles N., Ho H. Y., Kirschner M. W., Ramesh N., Geha R. S. (2004). Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol. Cell Biol. 24, 5269-5280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama S., Kurosaki T., Sada K., Yamanashi Y., Yamamoto T., Yamamura H. (1996). Physical and functional association of cortactin with Syk in human leukemic cell line K562. J. Biol. Chem. 271, 6631-6635 [DOI] [PubMed] [Google Scholar]
- Okamura H., Resh M. D. (1995). p80/85 cortactin associates with the Src SH2 domain and colocalizes with v-Src in transformed cells. J. Biol. Chem. 270, 26613-26618 [DOI] [PubMed] [Google Scholar]
- Oser M., Yamaguchi H., Mader C. C., Bravo-Cordero J. J., Arias M., Chen X., Desmarais V., van Rheenen J., Koleske A. J., Condeelis J. (2009). Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J. Cell Biol. 186, 571-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser M., Mader C. C., Gil-Henn H., Magalhaes M., Bravo-Cordero J. J., Koleske A. J., Condeelis J. (2010). Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia. J. Cell Sci. 123, 3662-3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant K., Chereau D., Hatch V., Dominguez R., Lehman W. (2006). Cortactin binding to F-actin revealed by electron microscopy and 3D reconstruction. J. Mol. Biol. 359, 840-847 [DOI] [PubMed] [Google Scholar]
- Patel A. S., Schechter G. L., Wasilenko W. J., Somers K. D. (1998). Overexpression of EMS1/cortactin in NIH3T3 fibroblasts causes increased cell motility and invasion in vitro. Oncogene 16, 3227-3232 [DOI] [PubMed] [Google Scholar]
- Perrin B. J., Amann K. J., Huttenlocher A. (2006). Proteolysis of cortactin by calpain regulates membrane protrusion during cell migration. Mol. Biol. Cell 17, 239-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G., Helwani F. M., Verma S., McLachlan R. W., Weed S. A., Yap A. S. (2009). Cortactin is a functional target of E-cadherin-activated Src family kinases in MCF7 epithelial monolayers. J. Biol. Chem. 284, 18913-18922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey M., Irondelle M., Waharte F., Lizarraga F., Chavrier P. (2011). HDAC6 is required for invadopodia activity and invasion by breast tumor cells. Eur. J. Cell Biol. 90, 128-135 [DOI] [PubMed] [Google Scholar]
- Rothschild B. L., Shim A. H., Ammer A. G., Kelley L. C., Irby K. B., Head J. A., Chen L., Varella-Garcia M., Sacks P. G., Frederick B., et al. (2006). Cortactin overexpression regulates actin-related protein 2/3 complex activity, motility, and invasion in carcinomas with chromosome 11q13 amplification. Cancer Res. 66, 8017-8025 [DOI] [PubMed] [Google Scholar]
- Sangrar W., Gao Y., Scott M., Truesdell P., Greer P. A. (2007). Fer-mediated cortactin phosphorylation is associated with efficient fibroblast migration and is dependent on reactive oxygen species generation during integrin-mediated cell adhesion. Mol. Cell Biol. 27, 6140-6152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuuring E., Verhoeven E., Mooi W. J., Michalides R. J. (1992). Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene 7, 355-361 [PubMed] [Google Scholar]
- Schuuring E., Verhoeven E., Litvinov S., Michalides R. J. (1993). The product of the EMS1 gene, amplified and overexpressed in human carcinomas, is homologous to a v-src substrate and is located in cell-substratum contact sites. Mol. Cell Biol. 13, 2891-2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvetsov A., Berkane E., Chereau D., Dominguez R., Reisler E. (2009). The actin-binding domain of cortactin is dynamic and unstructured and affects lateral and longitudinal contacts in F-actin. Cell Motil. Cytoskeleton 66, 90-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Pearson P. S., Greuber E. K., Yogalingam G., Pendergast A. M. (2010). Abl kinases are required for invadopodia formation and chemokine-induced invasion. J. Biol. Chem. 285, 40201-40211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuible M., Dube N., Tremblay M. L. (2008). PTP1B regulates cortactin tyrosine phosphorylation by targeting Tyr446. J. Biol. Chem. 283, 15740-15746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung B. H., Zhu X., Kaverina I., Weaver A. M. (2011). Cortactin controls cell motility and lamellipodial dynamics by regulating ECM secretion. Curr. Biol. 21, 1460-1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Kunii M., Harada A., Okabe S. (2009). Generation of cortactin floxed mice and cellular analysis of motility in fibroblasts. Genesis 47, 638-646 [DOI] [PubMed] [Google Scholar]
- Tehrani S., Tomasevic N., Weed S., Sakowicz R., Cooper J. A. (2007). Src phosphorylation of cortactin enhances actin assembly. Proc. Natl. Acad. Sci. USA 104, 11933-11938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpson P., Lynch D. K., Schramek D., Walker F., Daly R. J. (2005). Cortactin overexpression inhibits ligand-induced down-regulation of the epidermal growth factor receptor. Cancer Res. 65, 3273-3280 [DOI] [PubMed] [Google Scholar]
- Uruno T., Liu J., Zhang P., Fan Y., Egile C., Li R., Mueller S. C., Zhan X. (2001). Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 3, 259-266 [DOI] [PubMed] [Google Scholar]
- Uruno T., Liu J., Li Y., Smith N., Zhan X. (2003). Sequential interaction of actin-related proteins 2 and 3 (Arp2/3) complex with neural Wiscott-Aldrich syndrome protein (N-WASP) and cortactin during branched actin filament network formation. J. Biol. Chem. 278, 26086-26093 [DOI] [PubMed] [Google Scholar]
- van Damme H., Brok H., Schuuring-Scholtes E., Schuuring E. (1997). The redistribution of cortactin into cell-matrix contact sites in human carcinoma cells with 11q13 amplification is associated with both overexpression and post-translational modification. J. Biol. Chem. 272, 7374-7380 [DOI] [PubMed] [Google Scholar]
- van Rossum A. G., de Graaf J. H., Schuuring-Scholtes E., Kluin P. M., Fan Y. X., Zhan X., Moolenaar W. H., Schuuring E. (2003). Alternative splicing of the actin binding domain of human cortactin affects cell migration. J. Biol. Chem. 278, 45672-45679 [DOI] [PubMed] [Google Scholar]
- van Rossum A. G., Moolenaar W. H., Schuuring E. (2006). Cortactin affects cell migration by regulating intercellular adhesion and cell spreading. Exp. Cell Res. 312, 1658-1670 [DOI] [PubMed] [Google Scholar]
- Vidal C., Geny B., Melle J., Jandrot-Perrus M., Fontenay-Roupie M. (2002). Cdc42/Rac1-dependent activation of the p21-activated kinase (PAK) regulates human platelet lamellipodia spreading: implication of the cortical-actin binding protein cortactin. Blood 100, 4462-4469 [DOI] [PubMed] [Google Scholar]
- Vuori K., Ruoslahti E. (1995). Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J. Biol. Chem. 270, 22259-22262 [DOI] [PubMed] [Google Scholar]
- Weaver A. M. (2006). Invadopodia: specialized cell structures for cancer invasion. Clin. Exp. Metastasis 23, 97-105 [DOI] [PubMed] [Google Scholar]
- Weaver A. M. (2008). Cortactin in tumor invasiveness. Cancer Lett. 265, 157-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver A. M., Karginov A. V., Kinley A. W., Weed S. A., Li Y., Parsons J. T., Cooper J. A. (2001). Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 11, 370-374 [DOI] [PubMed] [Google Scholar]
- Weaver A. M., Heuser J. E., Karginov A. V., Lee W. L., Parsons J. T., Cooper J. A. (2002). Interaction of cortactin and N-WASp with Arp2/3 complex. Curr. Biol. 12, 1270-1278 [DOI] [PubMed] [Google Scholar]
- Webb B. A., Zhou S., Eves R., Shen L., Jia L., Mak A. S. (2006). Phosphorylation of cortactin by p21-activated kinase. Arch. Biochem. Biophys. 456, 183-193 [DOI] [PubMed] [Google Scholar]
- Webb B. A., Jia L., Eves R., Mak A. S. (2007). Dissecting the functional domain requirements of cortactin in invadopodia formation. Eur. J. Cell Biol. 86, 189-206 [DOI] [PubMed] [Google Scholar]
- Weed S. A., Du Y., Parsons J. T. (1998). Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J. Cell Sci. 111, 2433-2443 [DOI] [PubMed] [Google Scholar]
- Weed S. A., Karginov A. V., Schafer D. A., Weaver A. M., Kinley A. W., Cooper J. A., Parsons J. T. (2000). Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J. Cell Biol. 151, 29-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Parsons J. T. (1993). Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J. Cell Biol. 120, 1417-1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Reynolds A. B., Kanner S. B., Vines R. R., Parsons J. T. (1991). Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol. Cell Biol. 11, 5113-5124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. P., Rouiller I., Slaughter B. D., Egile C., Kim E., Unruh J. R., Fan X., Pollard T. D., Li R., Hanein D., et al. (2011). Three-dimensional reconstructions of Arp2/3 complex with bound nucleation promoting factors. EMBO J. 31, 236-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. Z., Garcia M. V., Li T. Y., Khor L. Y., Gajapathy R. S., Spittle C., Weed S., Lessin S. R., Wu H. (2010). Cytoskeleton alterations in melanoma: aberrant expression of cortactin, an actin-binding adapter protein, correlates with melanocytic tumor progression. Mod. Pathol. 23, 187-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Yanamoto S., Kawasaki G., Mizuno A., Nemoto T. K. (2010). Overexpression of cortactin increases invasion potential in oral squamous cell carcinoma. Pathol. Oncol. Res. 16, 523-531 [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Condeelis J. (2007). Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta. 1773, 642-652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Yuan Z., Zhang Y., Yong S., Salas-Burgos A., Koomen J., Olashaw N., Parsons J. T., Yang X. J., Dent S. R., et al. (2007). HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol. Cell 27, 197-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Shrikhande U., Alicie B. M., Zhou Q., Geahlen R. L. (2009a). Role of the protein tyrosine kinase Syk in regulating cell-cell adhesion and motility in breast cancer cells. Mol. Cancer Res. 7, 634-644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang M., Dong H., Yong S., Li X., Olashaw N., Kruk P. A., Cheng J. Q., Bai W., Chen J., et al. (2009b). Deacetylation of cortactin by SIRT1 promotes cell migration. Oncogene 28, 445-460 [DOI] [PubMed] [Google Scholar]
- Zhu J., Yu D., Zeng X. C., Zhou K., Zhan X. (2007). Receptor-mediated endocytosis involves tyrosine phosphorylation of cortactin. J. Biol. Chem. 282, 16086-16094 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.