Abstract
Nongonococcal urethritis (NGU), an inflammation of the urethra not caused by gonorrhea, is the most common urethritis syndrome seen in men in the United States. It is a sexually transmitted infection commonly caused by Chlamydia trachomatis, a pathogen which occurs more frequently in African-American men compared to white men.
The purpose of this study was to investigate factors related to retention of study participants in a randomized, double-blinded clinical trial that evaluated four treatment regimens for the treatment of NGU. After the one-week treatment period, follow-up visits were scheduled during days 15–19 and days 35–45. Participants were phoned prior to scheduled appointments to encourage attendance, and contacted after missed appointments to reschedule their clinic visits.
Of the 305 male study participants, 298 (98%) were African-American, 164 (54%) were 25 years of age or younger, and 80 (31%) had a post-secondary school education. The overall retention rate was 75%. Factors associated with study completion were educational level attained and clinical center. Participants with higher levels of education were more likely to complete the study. Clinical centers with the highest retention rates also provided the highest monetary incentives for participation.
The retention rate for this study suggests that strategies are needed for improving the proportion of study participants that complete a clinical trial among young men with a sexually transmitted disease. These strategies may include increasing contacts with study participants to remind them of scheduled study visits using text messaging or social media and the use of financial incentives.
Keywords: nongonococcal urethritis, clinical trial retention, sexually transmitted infections
Introduction
Non-gonococcal urethritis (NGU), an inflammation of the urethra not caused by gonorrhea, is the most common urethritis syndrome seen in men in the U.S. [1]. It is characterized by urethral discharge, dysuria, and genital itching. A sexually transmitted infection, it is most frequently caused by Chlamydia trachomatis [2]. Mycoplasma genitalium and Trichomonas vaginalis have also been suggested as underlying pathogens [1, 3, 4]. Prevalence of chlamydia, the most frequently reported sexually transmitted infection in the United States, is eight times higher among African-Americans than among white persons, and is highest in the Southeastern United States [5].
Among men, its prevalence is highest in the 20–24 year age range and declines with age [5]. An estimated 16% of men attending sexually transmitted diseases (STD) clinics are diagnosed with NGU [6]. Currently recommended treatments for NGU are azithromycin and doxycycline [1].
Although clinical trials are the gold standard for evaluating new therapeutic regimens, members of minority populations are underrepresented in clinical trials [7–12]. Barriers to clinical trial participation include lack of awareness of clinical trial availability [8, 13], challenges meeting the demands of participation [14, 15], mistrust of research activities [16], fear of participation [17], and concerns regarding side effects [16]. Among those who enrolled in clinical trials, minority participants were more likely than their white counterparts to terminate participation prematurely [7, 18–20]. The burden of study participation may be disproportionately challenging for minority participants due to resources required for transportation and the potential loss of income from spending time on the study. Minority participants may be uncomfortable with the participation in research studies due to mistrust of medical research [15, 17].
Retention of study participants is essential to the estimation and interpretation of the outcome measures of a trial [21]. Retention of men in clinical trials of urethritis ranges from 69% to 87% [22–24]; two of these trials recruited men from STD clinics [23, 24]. Lower retention rates at 10–18 weeks were reported among study participants with recurrent chlamydia or gonorrhea infection [25]. Among minority women with a sexually transmitted infection, 75% completed a clinical trial of a behavioral intervention [26]. In a HIV prevention study of African-American men between 16 and 20 years of age, 77% and 65% returned for the 3-month and 6-month visits, respectively [27]. Attendance at one or more follow-up visits was reported in 76% of minority patients who were positive for the human T-cell lymphotropic virus (HTLV) [19].
Factors associated with retention to clinical trials have been reported in trials directed at the treatment and prevention of human immunodeficiency virus (HIV) [27–32] and cancer treatment [11, 33], but there is little information on patient characteristics associated with retention in a treatment trial of non-HIV sexually transmitted infections. In this paper, we report the retention rate in a multi-center clinical trial that investigated whether the addition of tinidazole to azithromycin or doxycycline improved clinical cure rates in men with NGU, and assess factors related to study retention.
Material and Methods
The design of the trial has been described previously [1] and the trial was conducted in four STD clinics. Participating clinics were selected among members of the Sexually Transmitted Infections Clinical Trials Group (www.stictg.org). The clinics were located in Birmingham, Alabama; New Orleans, Louisiana; Baltimore, Maryland and Durham, North Carolina, and all serve predominantly low-income, minority populations. Briefly, to be eligible for the clinical trial, a participant had to be a heterosexual male between 16 and 45 years of age diagnosed with NGU. Men who were seen at one of the participating clinics with complaints consistent with NGU underwent a urethral Gram stain or Methylene Blue stain as part of their routine evaluation and were referred to the clinic study recruiter or site investigator if the stain results were ≥ 5 polymorphonuclear leucocytes (PMNs) per 3–5 oil immersion fields which is indicative of infection, and there was no evidence of gonorrhea. The study recruiter or site investigator discussed the trial with the potential participant and the informed consent form was signed if the individual agreed to participate. Screening and enrollment occurred on the same visit.
Using a blocked randomization scheme with stratification for clinical center, participants were randomized equally to one of four treatment arms: azithromycin, azithromycin with tinidazole, doxycycline, or doxycycline with tinidazole. Participants were required to take their tinidazole/placebo medication in the clinic under direct observation of study staff. The treatment period was one week.
The first follow-up visit was scheduled for one week after completion of therapy (Day 15–19) and the final follow-up visit was scheduled between days 35 and 45. At each follow-up visit, an interim medical and sexual history was taken, a genital examination was performed, and two urethral swabs and a urine specimen were obtained.
To facilitate retention, participants were contacted by phone by clinic staff on the business day preceding each scheduled follow-up visit. If a participant missed a scheduled follow-up visit, the clinic called the participant on the next business day to reschedule the appointment. If a participant could not be reached by phone or did not return a call to reschedule within 2 business days, the clinic mailed a letter to the participant requesting that he contact the clinic to reschedule the appointment. Incentives were provided for attendance at the conclusion of the initial and follow-up visits: $75 per visit at sites B and C; and $50 per visit in sites A and D. At the conclusion of each visit, participants were provided with a check or gift certificate. Protocol-defined reasons for study termination were: baseline positive gonorrhea test result reported after enrollment; participant vomited within one hour of direct observed therapy; direct observed therapy not performed; participant required systemic antibiotics, lithium, anticoagulant therapy or antabase; clinical failure at first follow-up visit; investigator decision; adverse event; participant withdrew consent, and participant died. Definition of clinical failure varied slightly according to the study visit. At the first follow-up visit, clinical failure was defined as persistent symptoms and > 5 PMNs per 3–5 oil immersion fields on the urethral smear, or persistent urethral discharge on examination. At the final study visit, failure was defined as > 5 PMNs per 3–5 oil immersion fields or persistent urethral discharge on examination.
The study retention rate was defined as the proportion of study participants who completed the final follow-up visit or discontinued from the study due to a protocol-defined reason. The binomial proportion and its 95% confidence interval were used to estimate the retention rate. Fisher’s exact test and Pearson’s chi-square test were used to determine the univariate associations between retention and demographic factors, sexual history, and treatment. Factors that were statistically significant at the 0.10 level were incorporated into a multivariate logistic model to assess each factor’s independent effect on the retention rate at the 0.05 level. Odds ratios were estimated by their point estimates and Wald’s 95% confidence interval.
Results
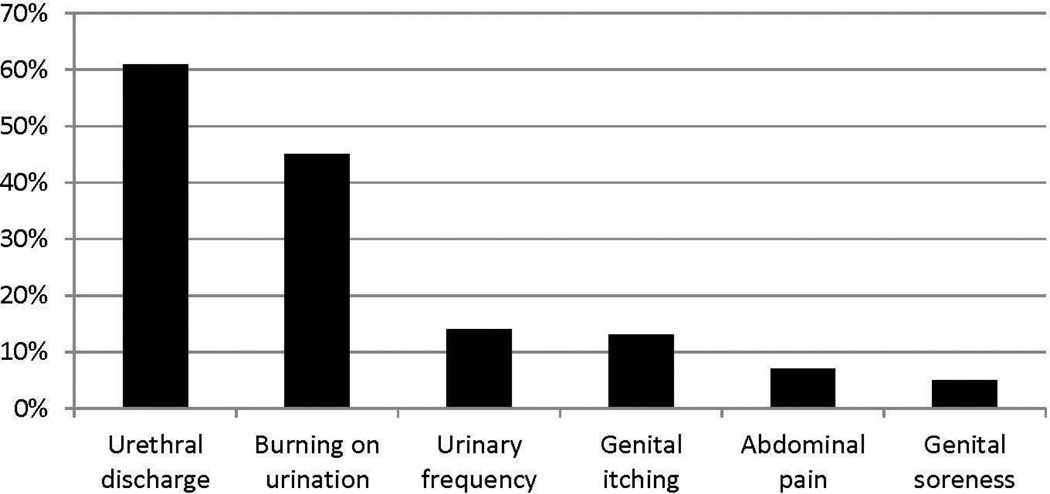
The study enrolled 305 participants. The study population was predominantly African-American, had never been married, and only 30% had continued their education after high school (Table 1). On average, participants had their initial sexual experience at 14 years of age. The most frequently reported symptoms at study entry were urethral discharge and burning on urination (Figure 1).
Table 1.
Demographic Characteristics, Treatment Assignment and Retention Rate (N=305)
| N (%) | Retention Rate (%) |
P-value | |
|---|---|---|---|
| Age in years | |||
| <= 25 years of age | 164 (54%) | 71% | 0.081 |
| > 25 years of age | 141 (46%) | 79% | |
| Race | |||
| African-American (%) | 299 (98%) | 75% | 0.899 |
| White (%) | 3 (1%) | 67% | |
| Other (%) | 3 (1%) | 67% | |
| Marital Status | |||
| Single, never married | 258 (85%) | 73% | 0.076 |
| Married, Separated or Divorced | 47 (15%) | 85% | |
| Educational Level | |||
| Less than high school | 69 (23%) | 58% | <0.001 |
| High school graduate or equivalent | 142 (47%) | 76% | |
| Some college, bachelor’s degree or higher | 94 (31%) | 85% | |
| Age at Sexual Debut* | |||
| <=14 years of age | 163 (54%) | 69% | 0.020 |
| > 14 years of age | 141 (46%) | 81% | |
| Clinical Center | |||
| A | 79 (26%) | 67% | <0.001 |
| B | 130 (43%) | 85% | |
| C | 39 (13%) | 79% | |
| D | 57 (19%) | 58% | |
| Treatment Assignment | |||
| Azithromycin | 77 (25%) | 69% | 0.335 |
| Azithromycin + Tinidazole | 79 (26%) | 73% | |
| Doxycycline | 76 (25%) | 82% | |
| Doxycycline + Tinidazole | 73 (24%) | 75% |
missing information for one participant
Figure 1.
Symptoms at Baseline
Of the 305 participants who enrolled in the study, 172 attended the second follow-up visit and 56 discontinued study participation due to a protocol-defined reason: clinical failure at first follow-up (N=39), vomited within one hour of direct observed therapy (N=1), positive baseline gonorrhea test results (N=4), withdrew consent after protocol treatment (N=3) and other reason (N=9), for an overall retention rate of 75% (95% confidence interval: 69.5% to 79.5%). Of the 77 participants who did not complete the study, 49 (64%) did not return after the initial visit.
Demographic and sexual history factors that were significantly associated with retention at the 0.10 level were educational level, clinical site, and age at sexual debut (Table 1). No difference in retention rate was detected between treatment arms. The retention rate did not vary by reported symptoms at baseline: urethral discharge (P=0.685), urinary frequency (P=0.250), burning on urination (P=1.00), genital soreness (P=0.388), abdominal pain (P=0.435) or genital itching (P=0.441). The multivariate analysis showed that educational level and clinical center retained statistical significance (Table 2). Participants who had not completed high school had a lower retention rate than high school graduates and those who had received post-secondary education. Both the A and D clinical centers had lower retention rates than clinical center B. Age at sexual debut was not an independent factor associated with retention, probably due to its correlation with educational level (P<0.001). The proportion of participants who were 14 years of age or younger when they had sex for the first time was 75% among those who did not complete high school, 53% among high school graduates, and 39% among those who went to college.
Table 2.
Multivariate Analysis Retention Rate
| Factor | Odds Ratio (95% CI) |
P-value |
|---|---|---|
| Age in Years | ||
| <=25 years of age | Reference | |
| >25 years of age | 1.61 (0.88, 2.94) | 0.120 |
| Marital Status | ||
| Single, never married | Reference | |
| Married, Separated, or Divorced | 1.43 (0.58, 4.12) | 0.463 |
| Educational Level | ||
| Less than high school | Reference | |
| High school graduate or equivalent | 2.23 (1.15, 4.32) | 0.019 |
| Some college, bachelor’s degree or higher | 3.25 (1.45, 7.27) | 0.003 |
| Clinical Center | ||
| A | 0.35 (0.17, 0.71) | 0.004 |
| B | Reference | |
| C | 0.49 (0.19, 1.27) | 0.158 |
| D | 0.25 (0.12, 0.53) | <0.001 |
| Age at Sexual Debut | ||
| <= 14 years old | Reference | |
| > 14 years old | 1.65 (0.91,2.97) | 0.130 |
Discussion
The retention rate shown in this study of 75% reflects the challenges of retaining NGU patients shown in similar populations recruited at STD clinics [23, 24]. The treatment regimens, source of study participants and study design for this study are similar to other clinical trials of NGU [23, 24]. Much higher retention rates have been reported in microbicide trials to prevent HIV infection in women [34, 35]. Participants in the microbicide trials may have been more highly motivated by the prospect of preventing a life-threatening disease. There are mixed reports on the role of gender on retention to clinical trials which enrolled both men and women [7, 19, 30, 32]. Retention rates did not vary by gender in studies of HIV-infected individuals and persons at high risk for HIV [7, 30, 32]. In a trial of HTLV patients, men were more likely to be lost to follow-up than women [19].
The correlation between educational level and study completion has been reported in a longitudinal study of minorities infected with HTLV [19], a microbicide trial of HIV-seronegative women in Africa [27], and among cervical cancer survivors [33]. Higher educational level may reflect a greater knowledge about clinical trials [11] or to ensuring adequate treatment for their disease [36]. Educational level has been positively associated with willingness to participate in sexual health research [14] which may imply greater propensity to complete research projects.
The difference in retention rates between clinical centers may reflect the difference in financial incentives provided at each site. The two sites with the highest retention rate provided monetary incentives that were 50% higher than incentives at the other two sites. The amount of monetary incentives that can be provided to participants varies by clinical center based on the guidelines of their respective institutional review boards. Financial incentives are effective in recruiting individuals to participate in clinical trials [37]. The motivation for participation in a HIV vaccine trial varied by gender, race and ethnicity [38]. Among men, 86% of whom were white, only 12% were motivated by financial reimbursement [38]. Among women, 71% of whom were minorities, 43% cited financial incentives as a reason for participation [38]. Two randomized trials have demonstrated the positive effect of financial incentives in retaining participants on an intervention [39, 40]. Among intravenous drug users who participated in a hepatitis vaccine B study which required 3 vaccination doses over 6 months, 69% of those who received monetary incentives completed the study in comparison to 23% among those who did not [40]. In a study conducted in STD clinics, participants randomized to receive monetary incentives were more likely to enroll and complete multiple sessions of HIV risk reduction counseling than participants randomized to receive non-monetary incentives of equivalent value [40].
There are a number of limitations to this study. Our study population was primarily African-American men and may not be representative of a more diverse population. The participating clinical centers were located in the southeastern United States which may differ from STD centers in a wider geographic area. Since this was a secondary data analysis of data collected to meet the clinical trial’s objectives, detailed information regarding income level was not available to allow an assessment of income’s association with retention rate.
Optimally, all study participants complete all follow-up visits for a clinical trial. The study population for this study was drawn from STD clinics and their retention rate reflects the challenges inherent with conducting studies with participants at these clinics. Options for enhancing retention rates include enhancing participant awareness of the potential long-term health consequences of their NGU, implementing aggressive strategies for contacting participants to encourage attendance at study visits. These strategies might include the use of text messaging or social media to communicate with study participants.
Conclusions
The retention rate for this study suggests that strategies are needed for improving the proportion of study participants that complete a clinical trial among predominantly African-American heterosexual young men with a sexually transmitted disease. This study demonstrated that educational level and clinical center are associated with retention rate in a population of minority men treated at STD clinics that serve low-income populations. Phone calls to clinical trial participants from clinic staff were used to encourage participants to return to clinic for study visits. Strategies for improving retention may include communicating with participants using text messaging or social media. Further research is needed to further define factors that enhance or deter retention on clinical trials and the potential use of novel means of communicating with study participants to encourage their continued participation in clinical trials.
Acknowledgements
Our thanks to the site investigators: Ann Rompalo (Baltimore, Maryland), Arlene Seña (Durham, North Carolina), and Stephanie Taylor (New Orleans, Louisiana). Support for this research was provided by the Division of Microbiology and Infectious Diseases (DMID) of the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institute of Health (NIH) DMID contract number N01AI40073C, through the collaboration of the Sexually Transmitted Infections Clinical Trials Group (STI CTG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jeannette Y. Lee, University of Arkansas for Medical Sciences, 4301 West Markham, #781, Little Rock, Arkansas 72205, jylee@uams.edu
Shelly Y. Lensing, University of Arkansas for Medical Sciences, 4301 West Markham, #781, Little Rock, Arkansas 72205, sylensing@uams.edu
Jane R. Schwebke, University of Alabama at Birmingham, ZRB 239, 1530 3RD Avenue South, Birmingham, Alabama 35294-0007, schwebke@uab.edu
References
- 1.Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52(2):163–170. doi: 10.1093/cid/ciq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peipert JF. Clinical practice. Genital chlamydial infections. N Engl J Med. 2003;349(25):2424–2430. doi: 10.1056/NEJMcp030542. [DOI] [PubMed] [Google Scholar]
- 3.Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium compared to chlamydia, gonorrhoea and trichomonas as an aetiological agent of urethritis in men attending STD clinics. Sex Transm Infect. 2009;85(6):438–440. doi: 10.1136/sti.2008.035477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wetmore CM, Manhart LE, Lowens MS, et al. Demographic, behavioral, and clinical characteristics of men with nongonococcal urethritis differ by etiology: a case-comparison study. Sex Transm Dis. 2011;38(3):180–186. doi: 10.1097/OLQ.0b013e3182040de9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. U.S. Department of Health and Human Services. Atlanta, Georgia: 2010. Sexually Transmitted Disease Surveillance 2009. Editor. [Google Scholar]
- 6.Newman LM, Warner L, Weinstock HS. Predicting subsequent infection in patients attending sexually transmitted disease clinics. Sex Transm Dis. 2006;33(12):737–742. doi: 10.1097/01.olq.0000218865.37084.f6. [DOI] [PubMed] [Google Scholar]
- 7.Gifford AL, Cunningham WE, Heslin KC, et al. Participation in research and access to experimental treatments by HIV-infected patients. N Engl J Med. 2002;346(18):1373–1382. doi: 10.1056/NEJMsa011565. [DOI] [PubMed] [Google Scholar]
- 8.Colon-Otero G, Smallridge RC, Solberg LA, Jr., et al. Disparities in participation in cancer clinical trials in the United States : a symptom of a healthcare system in crisis. Cancer. 2008;112(3):447–454. doi: 10.1002/cncr.23201. [DOI] [PubMed] [Google Scholar]
- 9.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 10.Stewart JH, Bertoni AG, Staten JL, et al. Participation in surgical oncology clinical trials: gender-, race/ethnicity-, and age-based disparities. Ann Surg Oncol. 2007;14(12):3328–3334. doi: 10.1245/s10434-007-9500-y. [DOI] [PubMed] [Google Scholar]
- 11.Lara PN, Jr., Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23(36):9282–9289. doi: 10.1200/JCO.2005.02.6245. [DOI] [PubMed] [Google Scholar]
- 12.Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 13.Brown M, Moyer A. Predictors of awareness of clinical trials and feelings about the use of medical information for research in a nationally representative US sample. Ethn Health. 2010;15(3):223–236. doi: 10.1080/13557851003624281. [DOI] [PubMed] [Google Scholar]
- 14.Carey MP, Senn TE, Vanable PA, et al. Do STD clinic patients who consent to sexual health research differ from those who decline? Findings from a randomized controlled trial with implications for the generalization of research results. Sex Transm Dis. 2008;35(1):73–77. doi: 10.1097/olq.0b013e318148b4ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gwadz MV, Colon P, Ritchie AS, et al. Increasing and supporting the participation of persons of color living with HIV/AIDS in AIDS clinical trials. Curr HIV/AIDS Rep. 2010;7(4):194–200. doi: 10.1007/s11904-010-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman PA, Duan N, Roberts KJ, et al. HIV vaccine trial participation among ethnic minority communities: barriers, motivators, and implications for recruitment. J Acquir Immune Defic Syndr. 2006;41(2):210–217. doi: 10.1097/01.qai.0000179454.93443.60. [DOI] [PubMed] [Google Scholar]
- 17.Katz RV, Kegeles SS, Kressin NR, et al. The Tuskegee Legacy Project: willingness of minorities to participate in biomedical research. J Health Care Poor Underserved. 2006;17(4):698–715. doi: 10.1353/hpu.2006.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou B, Krill LS, Horton BB, et al. Disparities in human papillomavirus vaccine completion among vaccine initiators. Obstet Gynecol. 2011;118(1):14–20. doi: 10.1097/AOG.0b013e318220ebf3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeVita DA, White MC, Zhao X, et al. Determinants of subject visit participation in a prospective cohort study of HTLV infection. BMC Med Res Methodol. 2009;9:19. doi: 10.1186/1471-2288-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magruder KM, Bichun O, Miller S, et al. Retention of under-represented minorities in drug abuse treatment studies. Clin Trials. 2009;6(3):252–260. doi: 10.1177/1740774509105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akl EA, Briel M, You JJ, et al. LOST to follow-up Information in Trials (LOST-IT): a protocol on the potential impact. Trials. 2009;10:40. doi: 10.1186/1745-6215-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamasuna R, Takahashi S, Kiyota H, et al. Effect of gatifloxacin against Mycoplasma genitalium-related urethritis: an open clinical trial. Sex Transm Infect. 2011;87(5):389–390. doi: 10.1136/sti.2010.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stamm WE, Batteiger BE, McCormack WM, et al. A randomized, double-blind study comparing single-dose rifalazil with single-dose azithromycin for the empirical treatment of nongonococcal urethritis in men. Sex Transm Dis. 2007;34(8):545–552. doi: 10.1097/01.olq.0000253348.44308.8c. [DOI] [PubMed] [Google Scholar]
- 24.Mena LA, Mroczkowski TF, Nsuami M, et al. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48(12):1649–1654. doi: 10.1086/599033. [DOI] [PubMed] [Google Scholar]
- 25.Golden MR, Whittington WL, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352(7):676–685. doi: 10.1056/NEJMoa041681. [DOI] [PubMed] [Google Scholar]
- 26.Thurman AR, Holden AE, Shain RN, et al. Preventing recurrent sexually transmitted diseases in minority adolescents: a randomized controlled trial. Obstet Gynecol. 2008;111(6):1417–1425. doi: 10.1097/AOG.0b013e318177143a. [DOI] [PubMed] [Google Scholar]
- 27.Feldblum PJ, Halpern V, Lie CC, et al. What predicts non-retention in microbicide trials? Contemp Clin Trials. 2011;32(4):512–516. doi: 10.1016/j.cct.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Wendler D, Krohmal B, Emanuel EJ, et al. Why patients continue to participate in clinical research. Arch Intern Med. 2008;168(12):1294–1299. doi: 10.1001/archinte.168.12.1294. [DOI] [PubMed] [Google Scholar]
- 29.Hessol NA, Weber KM, Holman S, et al. Retention and attendance of women enrolled in a large prospective study of HIV-1 in the United States. J Womens Health (Larchmt) 2009;18(10):1627–1637. doi: 10.1089/jwh.2008.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Bruyn G, Hudgens MG, Sullivan PS, et al. Participant retention in clinical trials of candidate HIV vaccines. J Acquir Immune Defic Syndr. 2005;39(4):499–501. doi: 10.1097/01.qai.0000148532.12329.df. [DOI] [PubMed] [Google Scholar]
- 31.Fortune T, Wright E, Juzang I, et al. Recruitment, enrollment and retention of young black men for HIV prevention research: experiences from The 411 for Safe Text project. Contemp Clin Trials. 2010;31(2):151–156. doi: 10.1016/j.cct.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howe CJ, Cole SR, Napravnik S, et al. Enrollment, retention, and visit attendance in the University of North Carolina Center for AIDS Research HIV clinical cohort, 2001–2007. AIDS Res Hum Retroviruses. 2010;26(8):875–881. doi: 10.1089/aid.2009.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osann K, Wenzel L, Dogan A, et al. Recruitment and retention results for a population-based cervical cancer biobehavioral clinical trial. Gynecol Oncol. 2011;121(3):558–564. doi: 10.1016/j.ygyno.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdool Karim SS, Richardson BA, Ramjee G, et al. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS. 25(7):957–966. doi: 10.1097/QAD.0b013e32834541d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leichliter JS, Chesson HW, Sternberg M, et al. The concentration of sexual behaviours in the USA: a closer examination of subpopulations. Sex Transm Infect. 2010;86(Suppl 3):iii45–iii51. doi: 10.1136/sti.2010.042283. [DOI] [PubMed] [Google Scholar]
- 37.Caldwell PH, Hamilton S, Tan A, et al. Strategies for increasing recruitment to randomised controlled trials: systematic review. PLoS Med. 2010;7(11):1–16. doi: 10.1371/journal.pmed.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colfax G, Buchbinder S, Vamshidar G, et al. Motivations for participating in an HIV vaccine efficacy trial. J Acquir Immune Defic Syndr. 2005;39(3):359–364. doi: 10.1097/01.qai.0000152039.88422.ec. [DOI] [PubMed] [Google Scholar]
- 39.Seal KH, Kral AH, Lorvick J, et al. A randomized controlled trial of monetary incentives vs. outreach to enhance adherence to the hepatitis B vaccine series among injection drug users. Drug Alcohol Depend. 2003;71(2):127–131. doi: 10.1016/s0376-8716(03)00074-7. [DOI] [PubMed] [Google Scholar]
- 40.Kamb ML, Rhodes F, Hoxworth T, et al. What about money? Effect of small monetary incentives on enrollment, retention, and motivation to change behaviour in an HIV/STD prevention counselling intervention. The Project RESPECT Study Group. Sex Transm Infect. 1998;74(4):253–255. doi: 10.1136/sti.74.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]