Abstract
Background
Poor reading efficiency is the most persistent problem for adults with developmental dyslexia. Previous research has demonstrated a relationship between left posterior temporal cortex (pTC) function and reading ability, regardless of dyslexia status.
Objective/Hypothesis
In this study, we tested whether enhancing left lateralization of pTC using transcranial direct current stimulation (tDCS) improves reading efficiency in adults without dyslexia.
Method
Twenty-five right-handed adults with no history of learning disorder participated. Real and sham “Left lateralizing” tDCS were applied to the pTC in separate sessions. Standardized word and nonword reading tests were given immediately after stimulation.
Results
Modeling of the induced electrical field confirmed that tDCS was likely to increase left pTC excitability and reduce right pTC excitability as intended. Relative to sham, real tDCS induced improvements in word reading efficiency in below average readers.
Conclusions
Enhancing left lateralization of the pTC using tDCS improves word reading efficiency in below-average readers. This demonstrates that left lateralization of the pTC plays a role in reading ability, and provides stimulation parameters that could be used for a trial of tDCS in adults with developmental dyslexia. Such short-term gains could amplify the effect of appropriate reading interventions when performed in conjunction with them.
Keywords: dyslexia, alexia, language, reading fluency
Developmental dyslexia affects 5-17% of children.1 Intense instructional programs are effective in improving reading accuracy in children with dyslexia, but in many cases reading efficiency (i.e., speed of accurate reading) lags behind, remaining impaired into adulthood.2 New methods for improving reading efficiency could be useful to augment traditional remediation strategies in adults.
Left posterior temporal cortex (pTC), both inferior and superior, appears to be critical for reading competence. Lesions to pTC cause acquired alexias,3 and functional neuroimaging demonstrates reduced left pTC activity in dyslexics.4-6 Nondyslexic children show increasing left lateralization of inferior pTC activity as they learn to read,7,8 and left lateralization of superior pTC activity increases in dyslexic children after successful reading remediation.9 A neuroimaging case report of a hyperlexic boy demonstrated that he activated the left superior pTC to a greater extent than controls during reading.10 Based on these findings, we hypothesized that enhancing left lateralization of pTC function would improve reading efficiency, especially in slower readers.
Transcranial direct current stimulation (tDCS) provides a safe noninvasive method of inducing local changes in cortical excitability by applying low levels of direct electrical current through the scalp.11 This subtle modulation of network properties has shown promising beneficial effects on naming in aphasic patients12,13 and novel word learning in healthy controls.14 TDCS is an ideal method of external neuromodulation for dyslexia because it is safe, portable, silent, and well tolerated, allowing it to be paired with traditional behavioral interventions to improve reading. Furthermore, electrical field modeling suggests that tDCS may affect a large area of the cortex,15 which is disadvantageous for fine dissection of brain-behavior relationships, but has the potential to be clinically advantageous in dyslexia where hypoactivation occurs in both superior and inferior left pTC.6
In this study, we investigated the relationship between left lateralization of pTC function and reading efficiency in adults without a prior diagnosis of dyslexia with the use of tDCS. We used an electrode montage designed to enhance left lateralization of the pTC, based on electrical field modeling, and measured both reading efficiency and untimed reading accuracy after real or sham tDCS in a within-subjects design. Enhancement of left lateralization in the pTC might improve reading efficiency by easing access to lexical, semantic, or phonologic representations, but should not impact untimed reading accuracy, which would require explicit reading instruction for improvement. The effect should be greatest in individuals with poor reading efficiency, as they are expected to underengage the left pTC at baseline.
Materials and Methods
Participants
Twenty-five right-handed native English-speakers (15 female, ages 20-50, mean = 26.7) participated. Subjects had at least 12 years of education, no history of neurologic disorder, psychiatric disorder, significant head trauma, hearing loss, or personal or family history of learning disorder (including dyslexia). Subjects denied the presence of metal in the head, implanted electrical devices, or history of seizure. The University of Pennsylvania Medical Center Institutional Review Board approved all procedures. Written informed consent was obtained.
Procedures
Subjects participated in two sessions on different days in which real or sham tDCS was administered using a Magstim Eldith device via two 5 × 5-cm saline-soaked pads. In both sessions, the anode was centered over the left pTC (midway between T7 and TP7) and the cathode was centered over the right pTC (between T8 and TP8). Because the anode facilitates neuronal activity and the cathode inhibits it, this montage is expected to drive processing to the left hemisphere, enhancing left lateralization. During real tDCS sessions, 1.5 mA of current was applied for 20 minutes, with 10 seconds ramp up and down. During sham sessions, current was ramped up to provide initial sensations (e.g., tingling) associated with tDCS and then extinguished over 10 seconds. These parameters are within safety limits established in prior studies on humans and animals.16-19
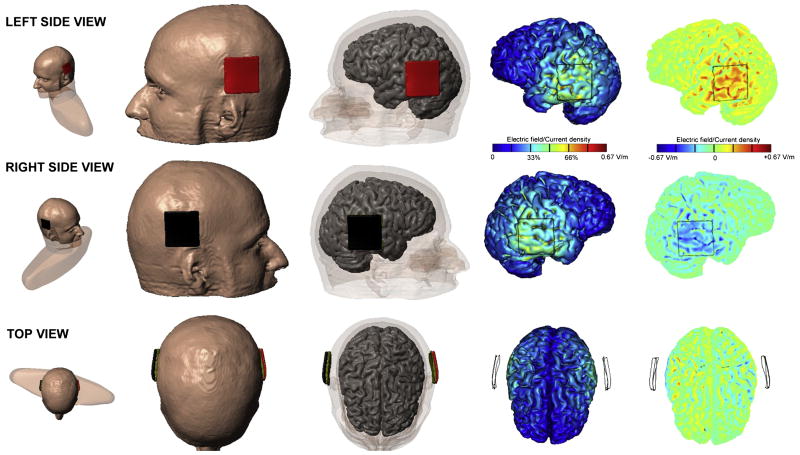
Induced electrical fields were modeled in a single individual to assess the likely area of effect (Figure 1). The goal of the model was to verify the lateralized (bipolar) dominant mode of polarization under each respective electrode, namely, dominantly inward current under the anode leading to somatic depolarization/increased excitability, and dominantly outward current under the cathode leading to somatic hyperpolarization/decreased excitability.20-22 A head model was created from a high resolution magnetic resonance imaging (MRI) of an adult male, and segmented into gray matter, white matter, cerebrospinal fluid (CSF), skull, scalp, eye region, muscle, and air compartments (Custom Segmentation, Soterix Medical, New York, NY). The finite element mesh generated from the segmentation masks was exported to COMSOL Multiphysics 3.5a (Burlington, MA) for computation of electric fields (EF).15 To complete the model, a synthetic region was added to replace tissue clipped by the MRI acquisition volume. The following isotropic electrical conductivities (in S/m) were assigned: gray matter: 0.276; white matter: 0.126; CSF: 1.65; skull: 0.01; scalp: 0.465; eye region: 0.4; muscle: 0.334; air: 1e-15; synthetic region: 0.17; sponge: 1.4; electrode: 5.8e7. The Laplace equation was solved and induced cortical EF maps were determined.15
Figure 1.
Modeling of electrical fields induced by tDCS.
Placement of the electrode pads are shown in the first three columns. The anode is shown in red, and the cathode in black. The fourth column shows the magnitude of the induced electrical field, which is maximal directly under the electrodes. The rightmost column shows the directional fields normal to the cortical surface, demonstrating mainly inward current in left pTC, and outward in right pTC. This pattern of directional current is expected to increase left pTC excitability, and decrease right pTC excitability, thus enhancing left lateralization of the pTC. Individual variation in gyral anatomy may result in differences in the precise location of the effect across subjects, but the model serves to confirm the expected lateralizing effect of this montage on the pTC in general.
Tasks
During each session, subjects performed either a phoneme perception task (n = 15) or a color perception task (n = 10) for the last 15 minutes of tDCS to maintain attention and arousal. After tDCS, they performed a computerized version of the Woodcock Reading Mastery Test-Revised-Normative Update (WRMT)23 Word ID and Word Attack subtests (untimed tests of word and nonword reading accuracy respectively), followed by the primary outcome measure, the Test of Word Reading Efficiency (TOWRE),24 in which subjects read aloud a list of either real words (Sight Reading Efficiency subtest) or nonwords (Phonetic Decoding Efficiency subtest) as quickly as possible. Two equivalent forms of the WRMT and TOWRE were given, one at each session. Psychometric data establishing equivalency of forms are available in the manuals of the tests.23,24 Three subjects were excluded because they scored at ceiling on the TOWRE at both sessions. The remaining 22 subjects (13 female; Mean Edinburgh Handedness = 91.4, range = 60-100) were counterbalanced for order of sessions, order of TOWRE and WRMT subtests, and TOWRE form administered at real versus sham sessions. Subjects completed a questionnaire at the end of each session asking them to rate the severity of a variety of symptoms during and after tDCS (1-5 scale, 26 questions total, data available for 21/22 subjects).
Statistical analysis
All statistical tests were performed in SPSS 16 for Mac, with a two-tailed alpha of .05. Dependent variables were standardized scores on each reading measure (mean = 100, SD = 15). The effect of tDCS on each reading measure was assessed using paired t tests comparing performance after real tDCS to sham. Significant effects were further evaluated using repeated-measures analysis of variance (ANOVA) with tDCS as a within-subject factor (real versus sham) and task (phoneme versus color perception), ability (above average versus below average score at sham session, that is, greater or less than 100), and session order (real versus sham first) as between-subject factors. Differences in cumulative symptom ratings between sessions were entered as a covariate.
Results
Tolerability and blinding of tDCS
All subjects completed the protocol and no significant adverse events were reported. On the post-tDCS symptom questionnaire, median ratings were 1 (none or very mild) for both sessions on all items assessing symptoms occurring after tDCS. Ratings for symptoms during tDCS were 1 on all questions, except difficulty concentrating, itching, and tingling, which were 2 at both sessions. Subjects were not directly asked to guess the session at which they received real stimulation, so we assessed the likelihood of unblinding based on cumulative symptom ratings (the sum of all 26 ratings). Eleven subjects rated severity of symptoms as higher during real tDCS, nine subjects rated severity as higher during sham stimulation, and one subject rated the symptoms equally at both sessions. There was no significant difference in the cumulative symptom scores between sessions (median real tDCS cumulative score = 33 (standard deviation [SD] = 6.65), sham = 31 (SD = 5.17); Wilcoxon signed rank Z = 1.41; P = 0.16).
Effect of tDCS on reading
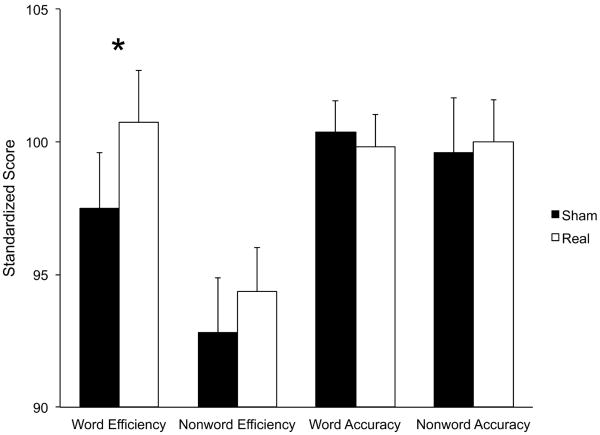
Only word reading efficiency demonstrated a significant effect of tDCS (TOWRE Sight Word Efficiency subtest T(21) = 2.26, P = 0.034; Figure 2, Table 1). Sixteen of 22 subjects scored higher after real tDCS than sham (binomial P = 0.026). The main effect of tDCS on word reading efficiency was no longer significant in the repeated-measures ANOVA including task, ability, session order, and symptoms as between-subjects factors (F(1,18) = 1.29, P = 0.27). There were no interactions between tDCS and task (F(1,15) = 1.61, P = 0.22), session order (F(1,15) = 0.087, P = 0.77), or symptoms (F(1,15) = 0.072, P = 0.79). However, there was a significant interaction of tDCS with reading ability (F(1,15) = 9.82, P = 0.007). There were no second-order interactions between reading ability and task (F(1,18) = 1.65, P = 0.22) or session order (F(1,18) = 1.00, P = 0.33).
Figure 2.
Effect of tDCS on standardized reading scores.
Standardized scores have a population mean of 100 and SD of 15. Word Efficiency = TOWRE Sight Efficiency Test. Nonword Efficiency = TOWRE Phonetic Decoding Efficiency Test. Word Accuracy = WJRMT Word ID. Nonword Accuracy = WJRMT Word Attack. *P = 0.034.
Table 1. Effect of tDCS on standardized reading scores.
| Test | Mean score after sham tDCS (SD) |
Mean score after real tDCS (SD) |
P value |
|---|---|---|---|
| TOWRE-sight reading | 97.5 (9.8) | 100.7 (9.2) | 0.034 |
| TOWRE-phonetic decoding | 92.8 (9.6) | 94.4 (7.8) | 0.327 |
| WRMT-word ID | 100.4 (5.6) | 99.8 (5.6) | 0.533 |
| WRMT-word attack | 99.6 (9.7) | 100 (7.5) | 0.825 |
Scores have a population mean of 100 and SD of 15.
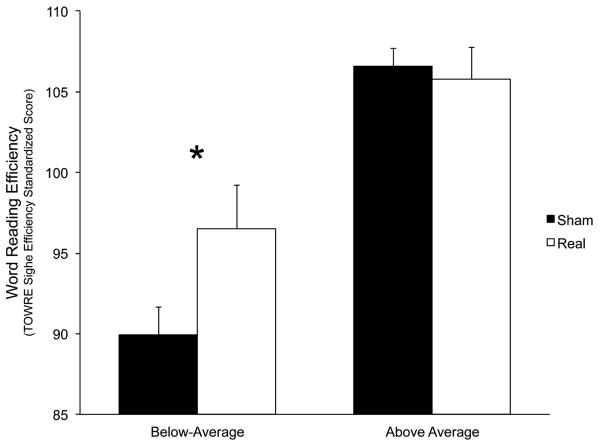
All 12 below-average subjects scored higher on the TOWRE Sight Word Efficiency subtest after real tDCS than sham (mean difference = 6.58 points, T(11) = 4.24, P = 0.001; binomial P(12/12) = 0.0002, Figure 3). This group was unbalanced on session order (eight received tDCS in the second session) and TOWRE form assignment (eight received form A during the real tDCS session). To ensure that these imbalances did not impact the results, we removed every combination of subjects that rebalanced the group for these factors and rechecked the paired t test for each rebalanced group (n = 8 for each). The effect of tDCS was significant in every case (range of P = 0.0019 to 0.04; binomial P(8/8) = 0.0038).There was no effect of tDCS in above-average subjects (mean difference = −0.8, T(9) = 0.42, P = 0.68).
Figure 3.
Word reading efficiency results by reading ability.
Standardized scores have a population mean of 100 and SD of 15.
Discussion
These results demonstrate that enhancing left lateralization of pTC using tDCS can induce short-term improvement in word reading efficiency in below-average readers. This confirms the relationship between pTC lateralization and reading ability, and provides a possible avenue for development of the first neuromodulatory intervention for dyslexia based on decades of evidence for the biologic basis of the disorder.1 Because this study was designed as a clinical proof of principle, rather than a fine-grained cognitive neuroscience experiment, we focus below on avenues of research that might help to optimize the potential applications of tDCS for dyslexia.
This protocol provides stimulation parameters that could be used for a double-blind trial of tDCS in adults with persistent reading difficulties because of developmental dyslexia. Still, several factors should be considered in designing clinical investigations of tDCS for dyslexia. Subject blinding was not directly assessed here, and unblinding of subjects could have biased the outcome, although the results of the symptom questionnaire mitigate this concern considerably. Testing the electrode montage used in this study against other active stimulation configurations that induce identical sensations would be useful to limit unblinding to the degree possible. Clinical investigations should also determine whether improvement in reading efficiency is sustained after repeated sessions, as has been demonstrated previously in a variety of conditions (e.g., aphasia,12,13 hemiparesis,25,26 and depression27,28). Even short-term enhancement of reading efficiency may be valuable though. When administered concurrently with appropriate reading instruction programs, short-term changes induced by tDCS could amplify the effectiveness of these programs, as been shown previously for novel word learning.14 Ultimately, tDCS may be useful in primary remediation of children with dyslexia who are predicted to fail standard educational approaches,29 but substantially more evidence of its safety in children is needed before this strategy is pursued.
To optimize the effect of stimulation on successful remediation, the specific anatomic source of the effect should be delineated. The “lateralizing” electrode montage used in this study is specialized in that the EF model predicts a focal directional effect under the electrodes, whereas other standard montages may have anatomically broader and less directional effects.15 However, we cannot determine whether reading efficiency improved in this study because of alteration of the balance between left and right pTC processing, or strictly because of facilitation of the left or inhibition of the right. This is an important issue to address because the right hemisphere cathodal stimulation might negatively impact cognitive processes not examined in this experiment. If the right pTC cathode is not essential to the efficacy of the technique, unilateral stimulation would be preferable to reduce this concern. Extending the behavioral battery used, and investigating electrode montages that impact only one hemisphere will address these questions. Because individual variation in gyral anatomy may result in differences in the precise location of maximal current flow across subjects, targeting enhancement or inhibition more precisely using MRI-guided TMS will help to further delineate the locus of the effect within the pTC of each hemisphere and may help to optimize electrode placement for clinical applications of tDCS in dyslexia.
The activation state of networks during brain stimulation impacts the physiologic effect of stimulation,30 and tDCS in particular has a purely modulatory effect on neuronal populations likely making its effect dependent on the activity state of the neurons impacted by the EF.31 Thus, the effect of tDCS on reading efficiency could potentially be optimized by pairing stimulation with behaviors that engage the specific cognitive processes, and hence the neuronal populations, maximally impacted by stimulation. We elected to use standardized reading measures in this study because these scores would provide a metric of the tDCS effect that is meaningful to clinicians and educators. The fact that tDCS induced changes in these gross measures reinforces the possibility of practical applications for dyslexia, but using these measures limited our ability to control for word regularity, concreteness, or other variables that might help to clarify the specific cognitive operations impacted by tDCS. However, the specific effect of tDCS in improving word but not nonword reading efficiency suggests improved access to lexical or semantic representations necessary for word, but not nonword reading.3,32 In this study, subjects performed tasks during tDCS that do not require engagement of lexical or semantic systems and the effect of tDCS did not interact with the specific task performed. For the purpose of optimizing clinical efficacy, pairing stimulation with behaviors that require lexical or semantic access should be investigated.
Conversely, activity-state dependent neuromodulatory effects of tDCS might potentially be exploited clinically. Selectively engaging neuronal populations involved in specific reading subprocesses (e.g., grapheme-to-phoneme conversion) during stimulation might sensitize them to the effect of tDCS. Thus, by pairing stimulation with specific approaches to reading remediation, the effect of tDCS might be tuned to improve a specific individual's area of weakness. Clinical investigations of tDCS for dyslexia should thus pair stimulation with different instructional remediation strategies to evaluate these interactions. Optimizing the effect of tDCS for reading remediation in dyslexia will depend on further basic and clinical investigations into the physiologic and cognitive effects of stimulation in different behavioral contexts.
Acknowledgments
This work was supported by the International Dyslexia Association (General Grant to P.E.T.), the American Academy of Neurology Foundation (Clinical Research Training Fellowship to P.E.T.), the National Institutes of Health (NIH) (no. S06 GM008168 NS054783 to M.B.).
Dr. Turkeltaub is funded by the American Academy of Neurology Foundation (Clinical Research Training Fellowship) and the International Dyslexia Association (General Grant 2009). Dr. Hamilton is funded by an NIH grant (K01NS060995), and receives research support from the Harold Amos Foundation. Dr. Datta is cofounder of Soterix Medical.
The City University of New York has patent applications in Dr. Datta's name on brain stimulation. Dr. Bikson is funded by the National Institutes of Health (NIH) (no. S06 GM008168 NS054783), the Andy Grove Foundation, and PSC CUNY. The City University of New York has patent applications in Dr. Bikson's name on brain stimulation. Dr. Bikson is cofounder of Soterix Medical. Dr. Coslett is funded by the NIH (R01MH076227, RO1DC005672, 24HD050836, RO1DC000191).
References
- 1.Gabrieli JD. Dyslexia: a new synergy between education and cognitive neuroscience. Science. 2009;325(5938):280–283. doi: 10.1126/science.1171999. [DOI] [PubMed] [Google Scholar]
- 2.Lyon GR, Moats LC. Critical conceptual and methodological considerations in reading intervention research. J Learn Disabil. 1997;30(6):578–588. doi: 10.1177/002221949703000601. [DOI] [PubMed] [Google Scholar]
- 3.Coslett HB. Acquired dyslexia. Semin Neurol. 2000;20(4):419–426. doi: 10.1055/s-2000-13174. [DOI] [PubMed] [Google Scholar]
- 4.Paulesu E, Demonet JF, Fazio F, et al. Dyslexia: cultural diversity and biological unity. Science. 2001;291(5511):2165–2167. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- 5.Temple E, Deutsch GK, Poldrack RA, et al. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proc Natl Acad Sci U S A. 2003;100(5):2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Ann NY Acad Sci. 2008;1145:237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- 7.Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6(7):767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- 8.Simos PG, Breier JI, Fletcher JM, Foorman BR, Mouzaki A, Papanicolaou AC. Age-related changes in regional brain activation during phonological decoding and printed word recognition. Dev Neuropsychol. 2001;19(2):191–210. doi: 10.1207/S15326942DN1902_4. [DOI] [PubMed] [Google Scholar]
- 9.Simos PG, Fletcher JM, Bergman E, et al. Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology. 2002;58(8):1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- 10.Turkeltaub PE, Flowers DL, Verbalis A, Miranda M, Gareau L, Eden GF. The neural basis of hyperlexic reading: an FMRI case study. Neuron. 2004;41(1):11–25. doi: 10.1016/s0896-6273(03)00803-1. [DOI] [PubMed] [Google Scholar]
- 11.Zaghi S, Acar M, Hultgren B, Boggio PS, Fregni F. Noninvasive brain stimulation with low-intensity electrical currents: putative mechanisms of action for direct and alternating current stimulation. Neuroscientist. 2010;16(3):285–307. doi: 10.1177/1073858409336227. [DOI] [PubMed] [Google Scholar]
- 12.Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41(6):1229–1236. doi: 10.1161/STROKEAHA.109.576785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiori V, Coccia M, Marinelli CV, et al. Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. J Cogn Neurosci. 2010 Oct 14; doi: 10.1162/jocn.2010.21579. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Floel A, Rosser N, Michka O, Knecht S, Breitenstein C. Noninvasive brain stimulation improves language learning. J Cognitive Neurosci. 2008;20(8):1415–1422. doi: 10.1162/jocn.2008.20098. [DOI] [PubMed] [Google Scholar]
- 15.Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2(4):201–207. 7e1. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiology. 2003;114(11):2220–2222. doi: 10.1016/s1388-2457(03)00235-9. author reply 2-3. [DOI] [PubMed] [Google Scholar]
- 17.Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64(5):872–875. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- 18.Liebetanz D, Koch R, Mayenfels S, Konig F, Paulus W, Nitsche MA. Safety limits of cathodal transcranial direct current stimulation in rats. Clin Neurophysiology. 2009;120(6):1161–1167. doi: 10.1016/j.clinph.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Bikson M, Datta A, Elwassif M. Establishing safety limits for transcranial direct current stimulation. Clin Neurophysiology. 2009;120(6):1033–1034. doi: 10.1016/j.clinph.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2009;2(4):215–228. 28 e1–3. doi: 10.1016/j.brs.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radman T, Su Y, An JH, Parra LC, Bikson M. Spike timing amplifies the effect of electric fields on neurons: implications for endogenous field effects. J Neurosci. 2007;27(11):3030–3036. doi: 10.1523/JNEUROSCI.0095-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bikson M, Inoue M, Akiyama H, et al. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol. 2004;557(Pt 1):175–190. doi: 10.1113/jphysiol.2003.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodcock RW. Woodcock reading mastery test—revised/normative update. Circle Pines, MN: American Guidance Service; 1998. [Google Scholar]
- 24.Torgesen JK, Wagner RK, Rashotte CA. Test of word reading efficiency (TOWRE) Austin, TX: Pro-Ed; 1999. [Google Scholar]
- 25.Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25(2):123–129. [PubMed] [Google Scholar]
- 26.Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75(24):2176–2184. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrucci R, Bortolomasi M, Vergari M, et al. Transcranial direct current stimulation in severe, drug-resistant major depression. J Affective Disorders. 2009;118(1-3):215–219. doi: 10.1016/j.jad.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Boggio PS, Rigonatti SP, Ribeiro RB, et al. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychopharmacol. 2008;11(2):249–254. doi: 10.1017/S1461145707007833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoeft F, McCandliss BD, Black JM, et al. Neural systems predicting long-term outcome in dyslexia. Proc Natl Acad Sci U S A. 2011;108(1):361–366. doi: 10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science. 2007;317(5846):1918–1921. doi: 10.1126/science.1146426. [DOI] [PubMed] [Google Scholar]
- 31.Reato D, Rahman A, Bikson M, Parra LC. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J Neurosci. 2010;30(45):15067–15079. doi: 10.1523/JNEUROSCI.2059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coltheart M. Disorders of reading and their implications for models of normal reading. Visible Language. 1981;15:245–286. [Google Scholar]