Abstract
Affinity chromatography is a separation technique that has become increasingly important in work with biological samples and pharmaceutical agents. This method is based on the use of a biologically-related agent as a stationary phase to selectively retain analytes or to study biological interactions. This review discusses the basic principles behind affinity chromatography and examines recent developments that have occurred in the use of this method for biomedical and pharmaceutical analysis. Techniques based on traditional affinity supports are discussed, but an emphasis is placed on methods in which affinity columns are used as part of HPLC systems or in combination with other analytical methods. General formats for affinity chromatography that are considered include step elution schemes, weak affinity chromatography, affinity extraction and affinity depletion. Specific separation techniques that are examined include lectin affinity chromatography, boronate affinity chromatography, immunoaffinity chromatography, and immobilized metal ion affinity chromatography. Approaches for the study of biological interactions by affinity chromatography are also presented, such as the measurement of equilibrium constants, rate constants, or competition and displacement effects. In addition, related developments in the use of immobilized enzyme reactors, molecularly imprinted polymers, dye ligands and aptamers are briefly considered.
Keywords: Affinity chromatography, Lectin affinity chromatography, Boronate affinity chromatography, Immunoaffinity chromatography, Immobilized metal ion affinity chromatography
INTRODUCTION
Liquid chromatography and high performance liquid chromatography have been extensively used for decades in clinical and pharmaceutical laboratories. Examples of liquid chromatographic methods that are commonly used in these fields include reversed-phase, ion-exchange, size-exclusion and normal-phase chromatography [1,2]. However, another liquid chromatographic technique that has become increasingly important in work with biological samples and pharmaceutical agents is affinity chromatography [3]. This review will discuss the basic principles behind affinity chromatography and will examine recent developments that have occurred in the use of this method for biomedical and pharmaceutical analysis.
Overview of Affinity Chromatography
Affinity chromatography can be defined as a type of liquid chromatography that uses a biologically-related agent, or “affinity ligand”, as a stationary phase to selectively retain analytes or to study biological interactions [4–6]. The affinity ligand can consist of a wide variety of binding agents, ranging from a protein or enzyme to an antibody, an antigen, a sequence of DNA or RNA, a biomimetic dye, an enzyme substrate or inhibitor, or a low mass compound (e.g., a drug or hormone). The affinity ligand is immobilized within a column and used to selectively bind a given target or group of targets within a sample. Because of the highly selective nature of many affinity ligands, the result is a column that can be used to isolate, measure, or study specific targets even they are present in complex biological samples [4–8].
The immobilized ligand is an important factor that determines the success of an affinity chromatographic method. Most affinity ligands that are used in this technique are obtained from a biological source, such as antibodies, enzymes, transport proteins, and carbohydrate-binding proteins [3–8]. However, affinity chromatography can also use synthetic ligands such as metal chelates, boronates and biomimetic dyes [3–5,7]. The type of ligand that is employed in this method can be used to divide affinity chromatography into several categories. This review will discuss developments in many of these categories, including lectin affinity chromatography, boronate affinity chromatography, immunoaffinity chromatography, and immobilized metal ion affinity chromatography [3,5,7].
Another critical factor in the design and use of an affinity column is the type of support to which the ligand is immobilized. Many methods that use affinity chromatography for only sample pretreatment or target isolation employ a carbohydrate support such as agarose or cellulose. This type of material can be easily modified for ligand attachment, can be used with a wide range of elution conditions, and has low non-specific binding for many biological compounds. However, the limited mechanical stability and relatively low efficiency of many carbohydrate-based materials means that they tend to work best for off-line methods and for columns that will be operated at only low pressures and flow rates [5,9,10].
An alternative to traditional carbohydrate-based supports is to place the affinity ligand onto supports that consist of HPLC media like silica particles or, more recently, monolithic materials [5,9–13]. This alternative approach is known as high performance affinity chromatography (HPAC), or high performance liquid affinity chromatography (HPLAC). The use of affinity ligands with monolithic supports is also referred to as affinity monolith chromatography (see example in Figure 1) [12–17]. The more rigid and efficient particles or synthetic polymers that are employed in HPAC are often capable of withstanding much higher flow rates and pressures than traditional carbohydrate-based supports and possess better mass transfer properties than these other materials. The result is that HPAC columns can be used on-line with other HPLC columns or analytical methods while providing an increase in speed, precision, and ease of automation versus traditional affinity supports [5,9–13]. This review will discuss recent examples in which both traditional and high performance affinity columns have been used in biomedical and pharmaceutical analysis. However, the emphasis will be placed on methods in which affinity columns are used as part of HPLC systems or in combination with other analytical methods (e.g., mass spectrometry).
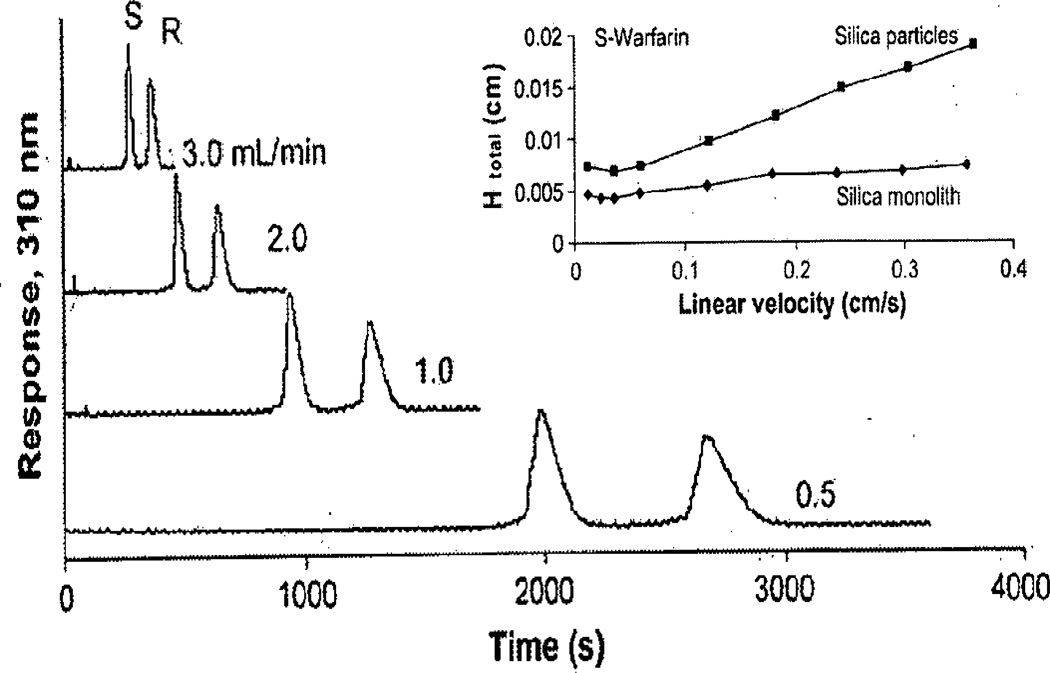
Figure 1.
Separation of R- and S-warfarin on a 10 cm×4.6 mm I.D. silica monolith column containing immobilized α1-acid glycoprotein. These separations were obtained at room temperature using pH 7.0, 0.067 M phosphate buffer as the mobile phase. The inset compares the total plate height (Htotal) that was measured for S-warfarin on the AGP silica monolith column to the total plate height that was determined for the same target and affinity ligand when using 7 ìm silica particles as the support. Adapted with permission from Ref. [17].
General Formats for Affinity Chromatography
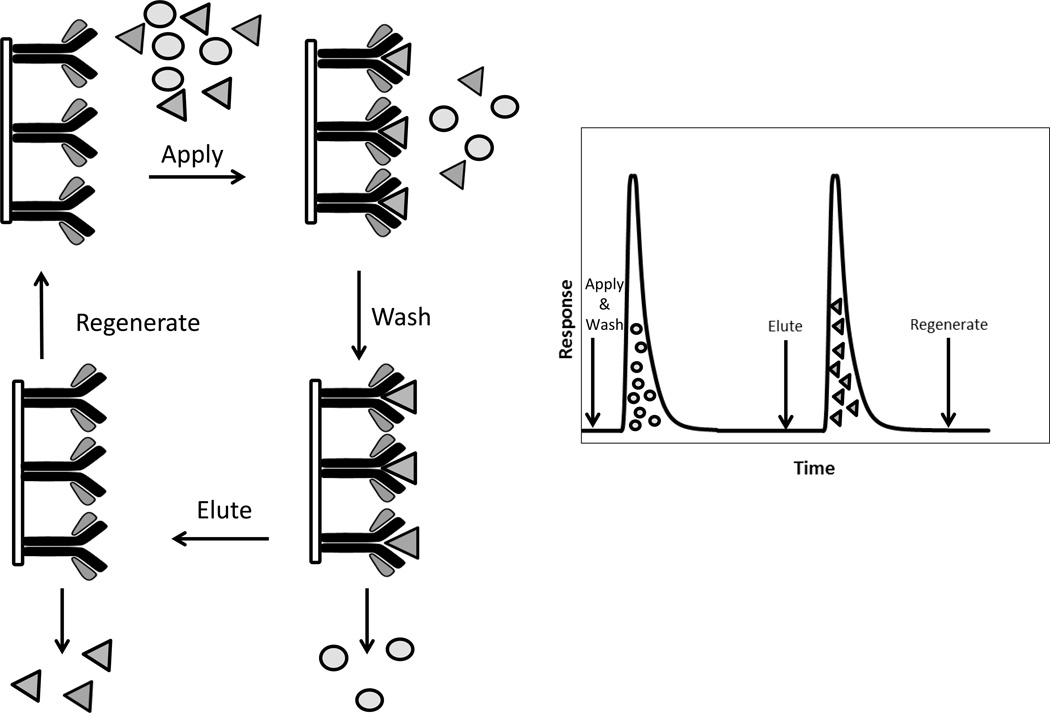
The most common scheme for performing a separation in traditional affinity chromatography and HPAC is shown in Figure 2 [5]. First, a sample is injected onto the affinity column under conditions that allow strong binding by the target or analyte of interest with the immobilized ligand. These application conditions typically involve the use of an aqueous buffer that has a pH and ionic strength that mimic the native environment of the ligand and its target. Compounds in the sample that have little or no interaction with the ligand will elute from the column during this step, giving a non-retained peak. The next step utilizes an elution buffer to dissociate the target from the affinity ligand. This elution step often requires changing the mobile phase composition to promote target elution, as can be accomplished by altering the pH or by adding a competing agent to displace the target from the column. During this elution step, the released target can be collected for later analysis or use. If an HPLC support is used in the affinity column, it may also be possible during this step to monitor the eluting target directly by an on-line method. Both the on-line and off-line approaches can be combined with detection methods such as absorbance, fluorescence or mass spectrometry [18]. After the target has been eluted, the column can then be regenerated prior to the next sample application by passing through the original application buffer.
Figure 2.
The on/off elution format for affinity chromatography and a general chromatogram for this format.
The format that is shown in Figure 2 for target capture and elution is sometimes known as the “on/off” or step elution mode of affinity chromatography [5,19]. This approach has been widely employed for compound isolation and sample pretreatment in biomedical and pharmaceutical analysis because of its simplicity, flexibility, selectivity, and ease of use [5–8,18]. This format can also be utilized with many types of affinity ligands, such as lectins, boronates, and antibodies [3]. In addition, it is relatively easy to automate this format when using affinity columns that are appropriate for work in HPAC or as part of HPLC systems; it is even possible to use this mode in some cases for the direct detection of analytes [18]. Direct detection in the step elution mode is commonly performed using on-line absorbance or fluorescence detectors, but detection based on mass spectrometry or a post column reactor is also possible [18–22].
Methods that employ isocratic elution can be developed in affinity chromatography if the retained target has weak or moderate affinity for the immobilized ligand. This situation allows a single mobile phase to be used as both the sample application and elution buffer. The result is an approach known as weak affinity chromatography (WAC) or dynamic affinity chromatography [19,23–27]. WAC has been used in several pharmaceutical and biomedical applications. One common example is the use of isocratic elution for chiral separations based on affinity columns that contain immobilized enzymes such as trypsin, α-chymotrypsin and cellobiohydrolase I or serum proteins such as human serum albumin (HSA), bovine serum albumin (BSA) and α1-acid glycoprotein (AGP) [19,28,29]. An example of such a separation is provided in Figure 1. In addition, WAC has been explored as a tool for drug screening assays. For instance, this method has been used with trypsin and thrombin columns plus HPLC and mass spectrometry for the fragment-based drug discovery/design screening of amidines, or arginine mimetic ligands [30]. This screening approach has also been utilized in the evaluation of ligands or inhibitors of cholera toxin [31,32]. The combined use of affinity microcolumns with HSA is another option that has been recently explored as a means for the high-throughput screening of drug-protein interactions [33,34].
Another format that is often utilized for affinity chromatography in biomedical analysis is affinity extraction. In this approach, a specific analyte or group of analytes is removed or extracted from the sample by a column that contains an immobilized affinity ligand. The retained targets are then eluted and passed to a second method for analysis. Antibodies are often placed in columns for use in affinity extraction, giving a method known as an immunoextraction [18,35]. However, other ligands (e.g., boronates or lectins) can also be used in this format [36,37]. When it is part of an HPAC system, affinity extraction can be utilized with some ligands to remove targets from samples in less than a few seconds (see Figure 3). This feature has been used to measure the free fractions of drugs and hormones in serum or drug/protein mixtures with columns that contain immobilized antibodies or HSA as the affinity ligand [38–41].
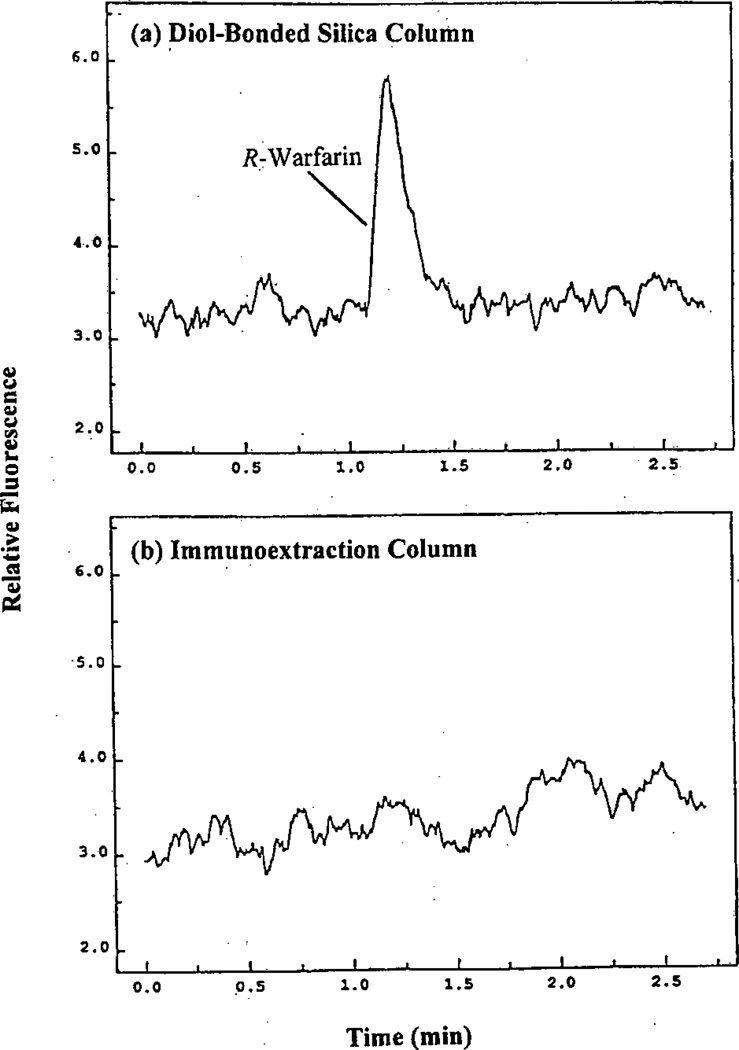
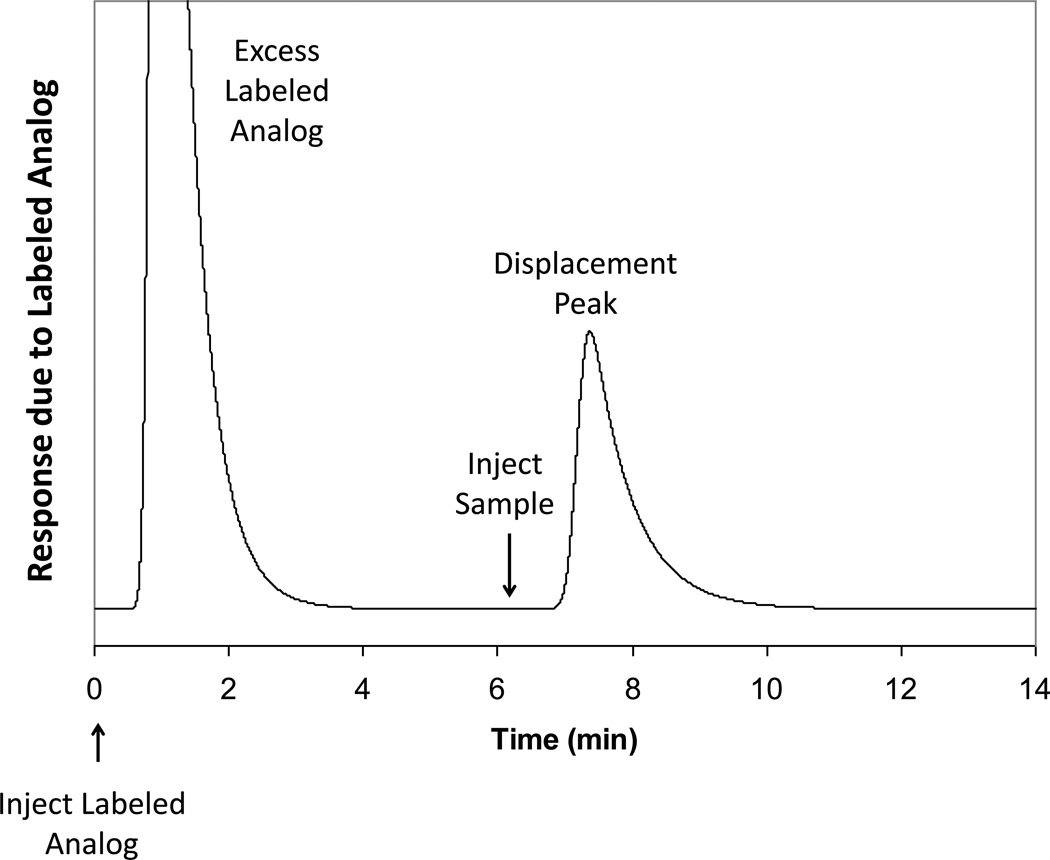
Figure 3.
(a) Injection of R-warfarin onto an inert control column and (b) affinity extraction of R-warfarin by an immunoaffinity microcolumn of the same size but that contained anti-warfarin antibodies. The contact time between the injected sample and the immunoaffinity layer in (b) was only 60 ms. Adapted with permission from Ref. [38].
A closely-related method to affinity extraction is affinity depletion. In this technique an affinity column is used to remove abundant compounds from a complex sample prior to analysis of the non-retained sample components by a second method. This format also frequently uses an antibody as the affinity ligand [35]. A typical application of this method is in proteomics, in which antibody columns have been used to remove highly abundant proteins such as HSA and immunoglobulin G (IgG) from serum prior to the analysis of the lower abundance proteins in such a sample [37,42].
Lectin Affinity Chromatography
One particular technique that has seen growing interest over the last few years is lectin affinity chromatography. This is a type of affinity chromatography in which an immobilized lectin is used as the stationary phase. Lectins are non-immune system proteins that have the ability to recognize and bind certain types of carbohydrate residues [43,44]. These carbohydrate-binding proteins are found in plants and animals, as well as in microorganisms [44–48]. Due to their ability to bind to carbohydrate residues, lectins have been widely used to isolate and identify glycoproteins, glycopeptides, glycolipids and oligosaccharides [43–45]. The most common types of lectins that are used in affinity chromatography are concanavalin A (Con A), wheat germ agglutinin (WGA), and jacalin [8,43,49]. Con A specifically binds to targets that contain α-D-mannose or α-D-glucose residues. WGA binds to D-N-acetylglucosamine residues, and jacalin binds to galactose or mannose residues [43–45,49].
Lectins have often been used for sample pretreatment and target purification in applications where agarose is employed as the support [8,43]. For example, in one recent study galactrox (i.e., a lectin that binds to galactose residues and that is found in snake venom from Botrox atrox) was purified using a lactosyl affinity column that made use of an agarose support [50]. Small columns containing Sambucus nigra agglutinin (SNA) on agarose have been used for glycoprotein enrichment and to capture sialylated glycopeptides prior to glycosylation site mapping by mass spectrometry [51].
Other reports have examined the use of lectin supports along with other HPLC methods and/or mass spectrometry. In one study, glycoproteins were isolated from human serum using lectin affinity columns, followed by tryptic digestion of these glycoproteins and sequential capture of their acidic peptides by using a strong anion-exchange column and a copper-immobilized metal ion affinity column [52]. Other reports have placed several lectins (i.e., Con A, WGA and jacalin) in a single agarose column for use in a technique called multilectin affinity chromatography (M-LAC). For instance, one study used immobilized antibodies and an affinity depletion method to remove highly abundant proteins such as HSA and IgG from serum samples, followed by passage of the non-retained fraction of this support through an M-LAC column to separate glycoproteins from the other remaining proteins in the sample. These protein fractions were later digested and analyzed by liquid chromatography/mass spectrometry (LC-MS) to locate potential biomarkers for a particular disease [53].
Additional supports have been used with lectin columns for biomedical analysis. As an example, M-LAC columns have been prepared using a coated polystyrene–divinylbenzene support. This lectin column was then used along with an antibody column as part of an automated HPLC platform [37]. The same affinity methodology has been combined with isoelectric focusing and a digital proteome chip for proteomic studies. In each case, LC-MS was used to detect changes in the various protein fractions that were obtained from healthy individuals versus those with a given disease state (see scheme in Figure 4) [54,55].
Figure 4.
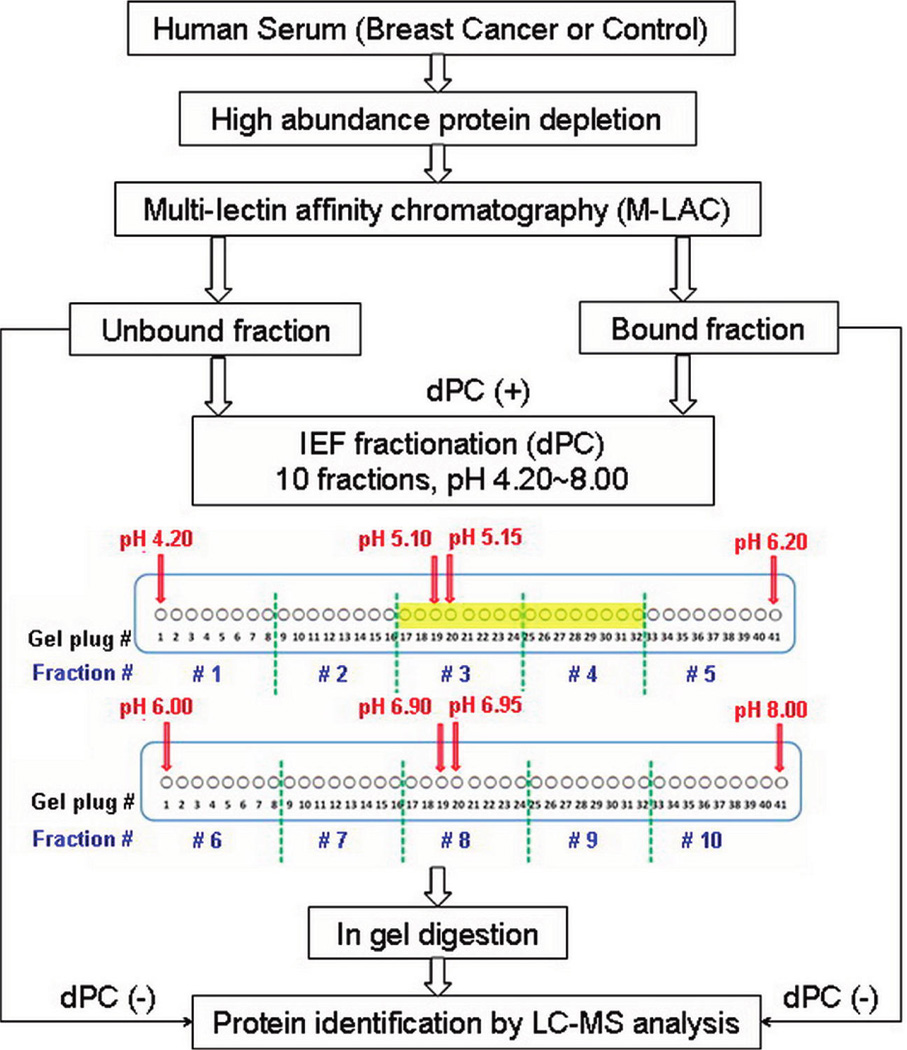
Protocol used with multiple lectin affinity chromatography for the study of glycoproteins in serum. Four techniques were used in this separation: immunodepletion, glycoprotein fractionation, isoelectric focusing (IEF) fractionation using a digital proteome chip (dPC), and reversed-phase liquid chromatography in conjunction with mass spectrometry (LC-MS). Reproduced with permission from Ref. [55].
Sets of lectin columns have been employed for the isolation and purification of glycoproteins and glycoconjugates in an approach known as serial lectin affinity chromatography (S-LAC). This method has been used with small agarose columns containing Con A, WGA or SNA and a hydrophilic interaction column to extract and enrich glycoproteins and glycopeptides prior to their analysis by matrix-assisted laser desorption/ionization mass spectrometry (MALDI MS) and liquid chromatography/tandem mass spectrometry (LC-MS/MS) [56].
Boronate Affinity Chromatography
Boronate affinity chromatography is a separation method that uses a boronate as the affinity ligand [57]. At a basic pH, most boronate derivatives will bind to targets that contain cis-diol groups, such as are present in many carbohydrate-containing compounds. Boronate columns have been used for several decades in clinical laboratories for the quantitation of glycated hemoglobin as an assessment of long-term diabetes management [58–61]. Methods employing this technique have used both agarose and supports that can be employed in HPAC methods [59–66]. Similar methods have been utilized to look at other types of glycoproteins, such as glycated albumin and some apolipoproteins [67,68].
Concerning the analysis of glycated hemoglobin, hemoglobin A1c (HbA1c) is the major component of glycated hemoglobin found in human blood [69,70]. A recent study evaluated the influence of different hemoglobin variants on the HbA1c values that are measured when using ion-exchange HPLC or boronate affinity-based HPLC. Of particular interest was the influence of hemoglobin variants Hb G-Taichung, Hb E, and Hb H on the measurement of HbA1c [71]. It was found that HbA1c measurements may be affected by the presence of Hb E and Hb H but not Hb G-Taichung. Boronate chromatography has also been combined with other analytical methods for HbA1c measurements. One report utilized a boronate affinity column and a cation-exchange column that were coupled with isotope dilution analysis and inductively-coupled plasma mass spectrometry for the quantitation of HbA1c in diabetic patients [36].
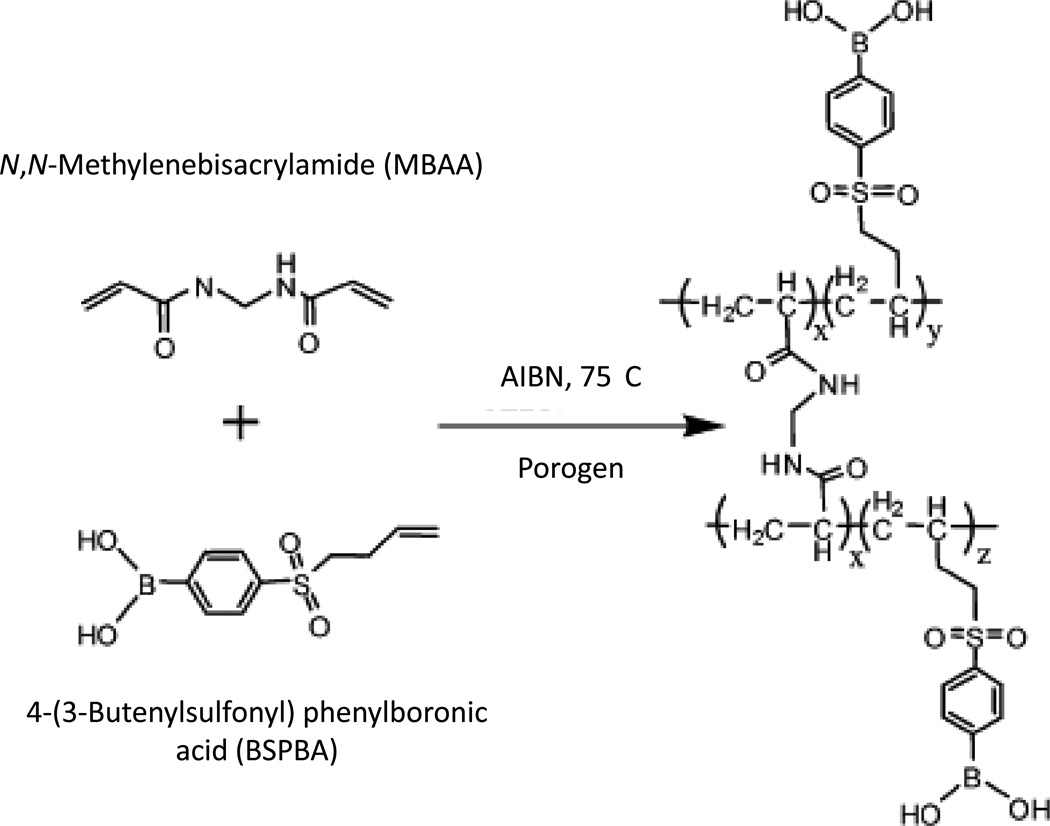
Several recent studies have considered new ways of preparing boronate affinity columns for work with biological samples. A capillary boronate affinity monolith was prepared at a neutral pH to capture glycoproteins like horseradish peroxidase and lactoferrin [72]. The synthesis of this monolith was achieved by the in situ free radical polymerization of 4-vinylphenylboronic acid (VPBA) in the presence of N,N’-methylenebisacrylamide (MBAA) as a hydrophilic cross-linking agent. By substituting VPBA with 4-(3-butenylsulfonyl) phenylboronic acid (BSPBA), as shown in Figure 5, it was possible to obtain strong retention of the glycoconjugates at a neutral pH [73]. Another study used “click chemistry” to create a new boronate ligand. In this work, 3-(prop-2-ynyloxycarbonylamino) phenylboronic acid was coupled to azide-activated agarose using a Cu(I)-catalyzed 1,3-dipolar cycloaddition reaction to create a material that could be used for the separation of glycoproteins such as ovalbumin and RNase B [74].
Figure 5.
Synthesis of a monolith containing a boronate, as based on in situ free radical polymerization using 4-(3-butenylsulfonyl) phenylboronic acid and N,N-methylenebisacrylamide. The azobisisobutyronitrile (AIBN) was used to initiate polymerization. The porogen was a mixture of two solvents that was used to promote the formation of pores in the monolith during its formation. Adapted with permission from Ref. [73].
A number of hybrid affinity methods using boronates have been described for the analysis of biomedical samples. One report combined the use of boronates and lectins in a single column to separate glycoproteins. This technique, referred to as boronic acid-lectin affinity chromatography, was created by combining an agarose support that contained immobilized Con A and WGA with an agarose support that contained immobilized m-aminophenyl boronic acid. This mixed-bed column was then used to separate a set of glycoproteins that included RNase B, glutathione peroxidase, and myoglobin [75]. Another report used a silica support and immobilized m-aminophenyl boronic acid along with ion-pair chromatography and flow injection analysis to simultaneously determine several catecholamines in urine samples [76].
Immunoaffinity Chromatography
The use of affinity columns that contain immobilized antibodies or related agents is a technique known as immunoaffinity chromatography [18,77]. Many immunoaffinity methods have been developed in the past for the isolation and purification of hormones, enzymes, peptides, viruses, and other biologically-relevant substances [77,78]. Like other affinity ligands, antibodies can be used on traditional affinity supports like agarose or attached to supports that can be used in HPAC, such as silica or monolithic materials [11–13,18,77]. The combination of immunoaffinity chromatography and these latter supports produces a method known as high performance immunoaffinity chromatography (HPIAC) [18,77].
There are several elution formats that can be utilized in immunoaffinity chromatography and HPIAC. The most common approach involves the step elution mode that was illustrated in Figure 2 [18,77]. Elution of the retained target in this case is often accomplished by using a change in the pH of the mobile phase, although the addition of a chaotropic agent or organic modifier can also be employed [77]. Step elution is frequently used during the isolation of target compounds by immunoaffinity columns prior to the analysis of these targets by another method [18,35,37,42,77]. In addition, this approach can be used for the direct detection of targets if they are present at sufficient levels in the sample. Analytes that have been measured by this approach have included acetylcholinesterase, benzidine, HSA, IgG, insulin, and transferrin [35,77]. Detection in this situation may be carried out by using many of the methods that were discussed earlier (e.g., absorbance, fluorescence, and mass spectrometry). Other detection schemes that have been employed are pulsed amperometry and radiometric detection [18,35,77].
Another format that can be used with immunoaffinity chromatography and HPIAC is a flow-based immunoassay, or “chromatographic immunoassay” [79,80]. These methods can be classified as either competitive or non-competitive immunoassays. Competitive binding immunoassays typically involve competition between the target analyte and a labeled analyte analog for a limited number of binding sites in an antibody column. Either the retained or non-retained fraction of the labeled analog is then detected, such as through the use of fluorescence, absorbance, chemiluminescence, or electrochemical formats [18,80]. The resulting signal provides an indirect measure of the amount of target that was in the original sample [79,80].
There are several ways to perform a competitive binding immunoassay by immunoaffinity chromatography. Three possible approaches are the simultaneous injection mode, the sequential injection mode, and a displacement immunoassay [35,79–82]. The simultaneous injection method involves application of the labeled analog and the target at the same time onto a column that contains a limited amount of immobilized antibodies for these agents [82]. The sequential injection format involves application of the target followed by injection of the labeled analog [81,82]. The displacement format (see Figure 6) uses the adsorption of an excess of the labeled analog onto a column that contains the immobilized antibody; a sample of the target is later applied to the column and displaces some of the labeled analog [79,80]. Simultaneous injection immunoassays have been utilized in the analysis of adrenocorticotropic hormone, caffeine, cortisol, digoxin, gentamicin, HSA, IgG, methotrexate, testosterone, and theophylline [35,80]. Sequential injection immunoassays have been used to measure α-amylase, digoxin, HSA, and IgG [35]. Displacement immunoassays have been employed for the analysis of benzoylecgonine, cocaine, cortisol, phenytoin, and thyroxine [18,35,39–40,79,80].
Figure 6.
An example of a displacement immunoassay. Adapted with permission from Ref. [40].
There are two common types of non-competitive immunoassays: the one-site immunometric assay and the two-site immunometric assay [79,80]. A one-site immunometric assay is performed by incubating the sample with a known excess of labeled antibodies that bind to the target. This mixture is then injected onto a column that contains an immobilized analyte analog. The immobilized analog binds to any antibodies that are not already bound to the target, while antibodies that are bound to the target elute as a non-retained fraction. Either the amount of non-retained or retained antibodies can then be measured to determine the amount of the target that was in the original sample [83]. This type of immunoassay has been used alone or as part of post column detection schemes for analytes such as digoxin, α-fetoprotein, granulocyte colony-stimulating factor, interleukin-10, and thyroxine [35,83].
A two-site immunometric assay, or sandwich immunoassay, utilizes two different antibodies that can bind simultaneously to the same target. In this assay, one of the antibodies is immobilized to a support and used to extract the target, while the other antibody contains a label that is used for detection. After non-retained components and excess labeled antibodies have been washed from the column, the retained target and labeled antibodies are also eluted. The signal that is generated by the labeled antibodies is used as a direct measure of the amount of target in the sample [80]. Examples of analytes that have been measured by this approach are human chorionic gonadotropin, HSA, IgG, interleukin-5, parathyroid hormone, and thyroid-stimulating hormone [35,80].
Many reports have used immunoaffinity chromatography in combination with other techniques for the analysis of complex biological samples. Examples of these methods that have already been discussed are immunoextraction [18,35,38–41] and immunodepletion [37,42]. Off-line immunoextraction has been coupled with HPLC, gas chromatography (GC) and other methods for the determination of such analytes as α1-anti-trypsin, BSA, chloramphenicol, cortisol, clenbuterol and phenytoin, among others [18,35,77]. On-line immunoextraction methods have also been reported for a wide range of drugs, proteins and other compounds of biomedical interest [18,35]. These on-line methods have been created by combining immunoaffinity columns with GC, mass spectrometry, size-exclusion chromatography, ion-exchange chromatography, and capillary electrophoresis. However, most of these on-line methods have involved immunoextraction and reversed-phase HPLC. This combination reflects the popularity of reversed-phase HPLC in biological separations and the relative ease with which an immunoaffinity column can be coupled with a reversed-phase column [18,35,77].
Immobilized Metal Ion Affinity Chromatography
Immobilized metal ion affinity chromatography (IMAC) is another type of affinity chromatography that has seen growing use in biomedical analysis. IMAC makes use of the specific interactions that can occur between immobilized metal ions and targets such as amino acids, peptides, proteins, and nucleic acids [84–87]. The metal ions are immobilized within a column through the use of chelating agents like iminodiacetic acid, nitrilotriacetic acid, carboxymethylated-aspartic acid, and L-glutamic acid. Metal ions that are often chelated to these groups include Ni2+, Zn2+, Cu2+ and Fe3+ [88–90]. IMAC was originally developed for the isolation and separation of metal- and histidine-containing proteins [87]. During this type of analysis, the sample is passed through an IMAC column, and targets that can bind to the immobilized metal ions will be retained. These targets are later eluted through the addition of a competing agent (e.g., imidazole) and/or by changing the pH [87–90].
IMAC has become a powerful tool for analyzing membrane proteins, histidine-tagged proteins, and phosphorylated proteins [89,91–93]. In recent work, IMAC has also played a role in sample pretreatment for the detection of drugs. Examples of drugs that have been examined through the use of IMAC are tetracyclines, quinolones, macrolides, β-lactams, and aminoglycosides [94,95].
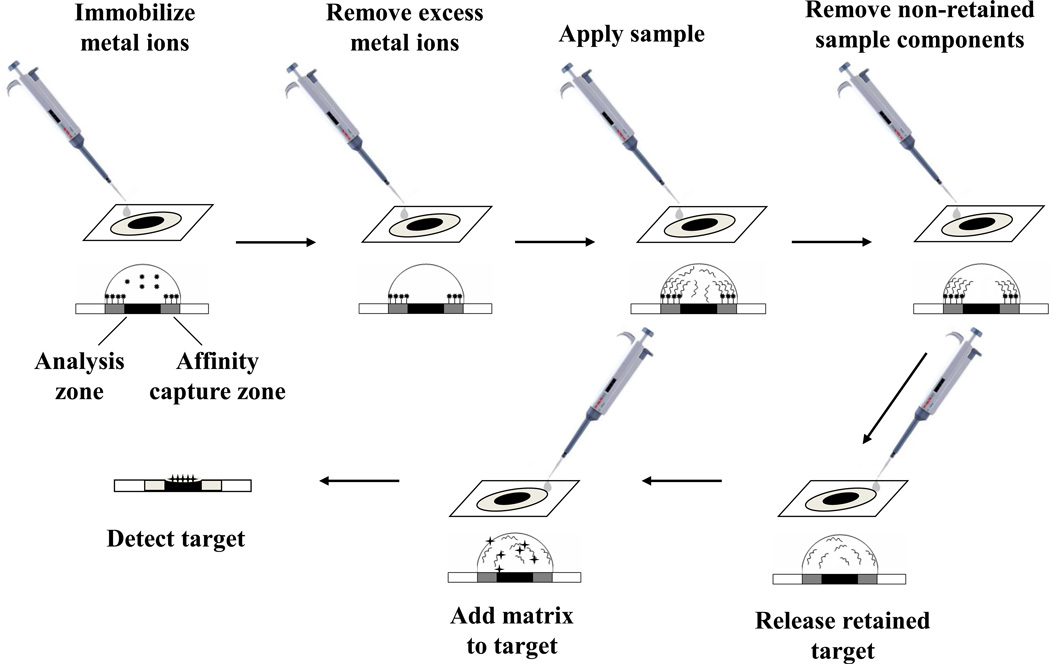
Another growing application for IMAC has been in the detection of biomarkers for disease diagnosis. This type of work has often involved the use of IMAC with mass spectrometry in surface-enhanced laser desorption/ionization (SELDI) [96–98]. An example of this approach is shown in Figure 7. The first step in this method is the immobilization of the desired metal ions onto a surface such as a chip. After excess metal ions have been washed away, the sample is applied and the target is allowed to bind to the immobilized metal ions while non-retained substances are washed away. An elution buffer is next added to release the retained target from the immobilized metal ions so the target can enter an analysis zone. An appropriate matrix is then placed on this surface to aid in the ionization and detection of the target by mass spectrometry [99]. This technique has been used for detecting biomarkers in various samples, including serum, urine, and tissues [96–98].
Figure 7.
Scheme for the purification and mass spectrometric analysis of a target by using surface-enhanced laser desorption/ionization (SELDI) with a surface that contains immobilized metal ions. Based on information provided in Ref. [99].
The refinement of IMAC for protein purification is another active area of research. Although there have been many studies aimed at optimizing supports and ligands for IMAC, there are still relatively few reports in which IMAC has been used for large-scale protein purification [89,100–102]. One problem that still needs to be addressed for such applications is the potential for leakage of metal ions from IMAC columns. This issue is of concern because the presence of metal ions in the collected fractions may interfere with the purity, shelf-life and apparent activity of a protein that is isolated by IMAC [103–105]. When IMAC is used to isolate recombinant histidine-tagged proteins, other issues that must be considered include the need for eventual removal of the histidine tag from the recombinant protein and the possible co-elution of this target with other proteins from the sample [89,105,106]. Many of the same issues will need to be addressed as applications for IMAC expand in the areas of biomedical and pharmaceutical analysis. This will probably require the development of new chelating ligands for IMAC, as well as the use of focusing methods to improve the selectivity of IMAC during the isolation of proteins from biological samples.
Analytical Affinity Chromatography
Besides its application for isolating and measuring specific targets, affinity chromatography can also be used to study interactions in biological systems. When affinity chromatography is utilized for this purpose, the method is referred to as analytical affinity chromatography, quantitative affinity chromatography or biointeraction affinity chromatography [107–110]. This approach has been used in many recent studies to examine drug binding with agents that include serum proteins, enzymes, and receptors, as well as the interactions of biomolecules with lectins, aptamers, and antibodies [107–114]. Information that can be obtained by this approach includes the overall extent of binding, the equilibrium constants for an interaction, the number and types of sites involved in the binding process, and the rate constants for the interaction [107–110,113].
One method that is often used to conduct these measurements is zonal elution. In this type of experiment, a small plug of a drug or analyte is injected onto an affinity column that contains the immobilized binding agent of interest. The retention factor or peak profile for the analyte is then analyzed under various conditions to obtain quantitative or qualitative information for a biological interaction [108,110]. There are many different applications for zonal elution. For instance, retention factors can be used directly as a measure of the overall affinity of solute-ligand binding [108]. This application has been used in HPAC for the high throughput analysis of drug interactions with HSA [33,34,115]. The measurement of retention factors as the mobile phase or column conditions are changed (e.g., a variation in pH, ionic strength, solvent polarity or temperature) can also provide clues as to the forces and mechanisms that underlie a drug-protein interaction [108,110]. Such an approach has been used in numerous studies to examine the chiral selectivity of proteins such as HSA, AGP, trypsin, α-chymotrypsin and cellobiohydrolase I [19,28,29,108,110,114,116]. A comparison of retention factors that are measured by zonal elution for a set of structurally similar compounds can further be used to create quantitative structure-retention relationships that describe the interaction of these compounds with a given protein or binding agent, as has been used to examine drug binding sites on HSA and AGP [108,114,117–120].
Another common use of zonal elution experiments is in competition and displacement studies. In this type of experiment, injections of a target or probe are carried out in the presence of a fixed concentration of a competing agent in the mobile phase [108]. Analysis of how the retention factor of the target changes with respect to the concentration of the competing agent can then be used to identify the type of competition that is occurring between these two solutes. In some cases, the same data can be further used to determine the association equilibrium constants and number of sites that are involved in the interaction [108,110].
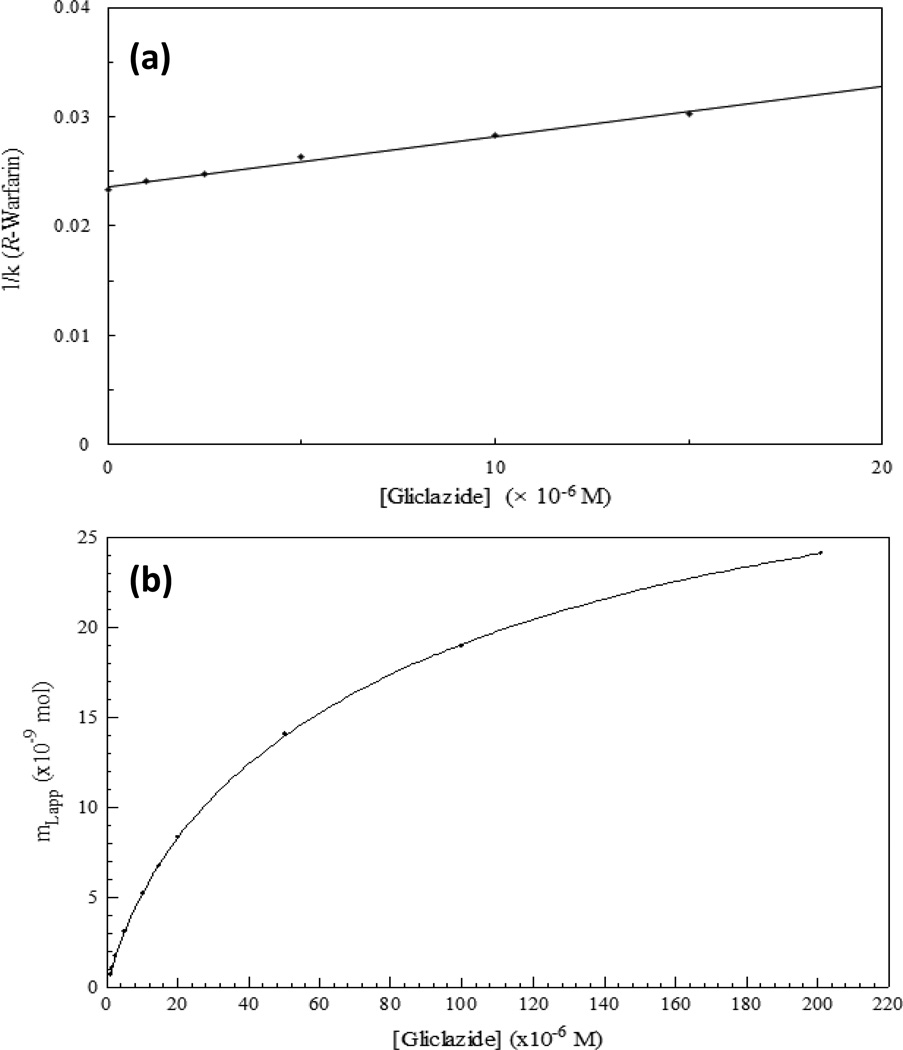
Figure 8(a) provides an example of a competition study for an HSA column in which R-warfarin was an injected probe for Sudlow site I of HSA and gliclazide was employed as a competing agent [121]. As the concentration of gliclazide in the mobile phase was increased during this experiment, the retention factor for R-warfarin shifted to smaller values. When a plot of 1/k versus gliclazide concentration was made, the result was a linear relationship, which is representative of direct competition by R-warfarin and gliclazide at a single common region on HSA. It was then possible to use the slope and intercept of this plot to determine the equilibrium constant for gliclazide at the common binding region on HSA [121]. Comparable experiments and plots have been used to examine systems with other types of interactions [108,110].
Figure 8.
Examples of (a) a zonal elution competition study with R-warfarin and gliclazide as a competing agent on an HSA column and (b) a frontal analysis experiment for gliclazide applied to an HSA column, with data being fitted to a two-site binding model. Terms: k, retention factor; mL,app, moles of applied target that are needed to reach the mean position of the breakthrough curve at a given concentration of the target. Adapted with permission from Ref. [121].
Similar experiments to those in Figure 8(a) have been employed in numerous studies. Examples include work that has examined the displacement of D/L-thyronine and D/L-tryptophan from HSA by bilirubin or caprylate [122]; the competition of R/S-warfarin with oxazepam and lorazepam on HSA [123]; and the effects of fatty acids on the binding of HSA with various drugs (e.g., warfarin, phenylbutazone, tolbutamide, acetohexamide, and gliclazide) [124,125]. This method has also been utilized to compare various coumarin and indole derivatives as possible probes for Sudlow sites I or II of HSA [126,127]. Recent work has used a modified version of this method to provide quantitative information on the allosteric interactions that take place on HSA between ibuprofen and benzodiazepines, L-tryptophan and phenytoin, or warfarin and tamoxifen [128–130]. Competition studies using site-specific probes have further been employed to investigate the changes that occur in solute interactions with HSA that has been modified at specific binding regions through synthetic methods [131,132] or natural processes (e.g., glycation) [121,133–135].
A second approach that can be used for binding studies in affinity chromatography is frontal analysis, or frontal affinity chromatography (FAC) [107–111,136]. In this technique, a known concentration of a target is continuously applied to an affinity column that contains an immobilized binding agent. As the immobilized binding agent becomes saturated with the target, a breakthrough curve will form. The mean position or shape of the breakthrough curve is then analyzed. For instance, the mean position of this curve can be fit to various binding models to determine the type of interaction that is taking place in the column, as is illustrated in Figure 8(b) [121]. From the best-fit parameters, it is possible to determine the equilibrium constants and number of binding sites that are present between the target and immobilized binding agent [108]. Information on the interaction kinetics can also be obtained from the shape of the breakthrough curve [112].
Frontal analysis has been used to investigate the binding of HSA to R/S-warfarin [137] or D/L-tryptophan [138,139], to compare the binding of monomeric versus dimeric HSA for various drugs [140], to examine the effects of chemical modification on the binding of site-specific probes with HSA [132], and to examine the multi-site binding of thyroxine with HSA [119,141]. This method has been employed more recently to examine the overall binding of drugs such as acetohexamide, carbamazepine, imipramine, lidocaine, phenytoin, tolbutamide and verapamil with HSA [142–147], as well as the interactions of various coumarin and indole derivatives with HSA [126,127]. Similar experiments have been used to investigate the binding of propranolol, carbamazepine, and lidocaine with AGP [116,145,148] and the interactions of propranolol and verapamil with high-density lipoprotein [149]. Additional work has been conducted in the development and evaluation of methods for the detection of multi-site binding by frontal analysis [141,150].
Frontal analysis can also be utilized to look at how changing the mobile phase or temperature will affect the affinity and number of binding sites that are involved in a solute-ligand interaction. As an example, this approach has been used to examine the changes in binding by D/L-tryptophan to HSA as a function of pH [138]. The effect of temperature on biological interactions has been frequently studied by this method, as illustrated by reports that have investigated the interactions of R/S-warfarin, D/L-tryptophan with HSA [137,138], the binding of propranolol and carbamazepine with AGP [116,148], and the interactions of propranolol with high-density lipoprotein [149]. This approach has further been utilized to determine the overall changes in drug or solute interactions that can occur during the modification of HSA at its binding regions [121,132,133–135].
Another format in which frontal analysis can be employed is as a tool for competitive binding experiments, particularly when used with mass spectrometry. This combination of methods is known as frontal affinity chromatography-mass spectrometry (FAC-MS) [110,136,151,152]. FAC-MS has been explored in numerous reports as a tool for screening mixtures of compounds for binding to a given immobilized ligand [136,151–159]. Binding agents that have been investigated by this technique include lectins [155,159], enzymes [136,152,157] and galactosaminoglycans [154].
Another application of analytical affinity chromatography is as a tool for studying the kinetics of a biological interaction [108,160]. There are several approaches for this type of work. One such approach is the plate height method, in which band-broadening is measured for a target on a column that contains the desired affinity ligand and on a control column that contains no ligand. The measured plate heights for the analyte are then plotted as a function of flow rate or linear velocity to find the plate height contribution due to stationary phase mass transfer, which is related to the dissociation rate constant between the target and affinity ligand [160]. Two examples of this approach have been its use to examine the interaction kinetics of R/S-warfarin and D/L-tryptophan with HSA [161,162].
Peak profiling is a variation on the plate height method in which retention times and peak widths are measured for both a target and a non-retained solute on an affinity column [160]. These values are then compared and used to determine the rate constant for target dissociation from the affinity ligand. During this process, the results for the non-retained solute are used to correct for band-broadening processes other than the target-ligand interaction. This method has been used to estimate the dissociation rate constants for L-tryptophan, carbamazepine, imipramine and phenytoin metabolites from HSA [163–166]. Peak profiling methods based on the use of either single flow rates or multiple flow rates have been described [163,164], and techniques have been developed for the use of this technique with solutes that have multiple interactions with a column (e.g., binding to both the affinity ligand and to the support) [165]. In addition, this method has been combined with chiral separations to simultaneously examine the interaction kinetics of HSA with the enantiomers of drug metabolites [166].
Another approach that has recently been used to examine the kinetics of biological interactions by analytical affinity chromatography is the peak decay method. This technique is carried out by first injecting a pulse of a target onto a small affinity column. The mobile phase is then quickly passed through to prevent re-association of the target as it is released from the affinity ligand. Under the appropriate conditions, the result is a decay curve that can be used to measure the dissociation rate constant for the target from the ligand [160]. This method has been used in recent studies to estimate the dissociation rate constants for various drugs from HSA [167,168] and has been explored as a high-throughput method for such measurements using affinity microcolumns and/or affinity monoliths [168].
Curve fitting has been used in both zonal elution and frontal analysis methods to obtain information on the kinetics of biological interactions [160]. For instance, frontal analysis profiles were recently used to examine the association kinetics of target interactions with antibodies in immunoaffinity columns [112,169]. The same approach has been used to examine binding of some aptamers with their targets [169]. Zonal elution studies carried out under non-linear elution conditions have also been employed in kinetic studies with affinity columns. An example is the use of this approach in several reports to examine the interactions of various inhibitors with immobilized nicotinic acetylcholine receptor membrane columns [170–175].
Other Biomedical Applications of Affinity Chromatography
There are a number of areas related to affinity chromatography that have also been of great interest in pharmaceutical and biomedical analysis. One such area is in the use of affinity columns for enzyme isolation [43,176]. This approach may use affinity ligands that are substrates, inhibitors, cofactors, or proteins that are associated with the biochemical pathways of the target enzyme [176]. Some examples are the use of immobilized flavin mononucleotides for the purification of flavin adenine dinucleotide synthetase [177] and the use of inhibitors to purify proprotein convertase furine [178]. Other reports have sought to develop and use new types of immobilized enzyme reactors (IMERs) for screening potential enzyme inhibitors, research in drug metabolism, or the digestion of proteins for peptide mapping [179–182]. Part of this work has involved the use of improved chromatographic supports for IMERs, such as monolithic media [12,13].
Synthetic dyes are another group of ligands that can be employed in affinity chromatography, creating a method know as dye-ligand affinity chromatography [183]. For instance, columns containing triazine dyes are commonly used to purify albumin and other blood proteins, along with various enzymes and proteins of interest as pharmaceutical agents [183–185]. Dye-ligand columns have also been employed to remove prion proteins, human immunodeficiency virus-1, and hepatitis B viral particles from biological fluids [186–188]. The use of computational methods or combinatorial chemistry to screen dyes and other ligands for a particular target is known as biomimetic affinity chromatography; this approach has been of great recent interest as a tool for the purification of protein-based pharmaceuticals [183,189].
Molecularly imprinted polymers (MIPs) make up another group of alternative ligands and supports that have been undergoing development for use in affinity methods [190,191]. Binding sites in MIPs are created by using functional monomers that can form a complex with a template such as the target analyte. After the monomers have been cross-linked, the template is removed and leaves behind a pocket that will recognize and retain the target. Most applications for MIPs up to the present have been in the area of affinity extractions [190,191], including their recent use for on-line methods [192,193].
Another relatively new class of ligand is the aptamer. Aptamers are made up of nucleic acid sequences that can take on three-dimensional structures that will bind a given target [183]. Aptamers are generated by screening the binding of the target with members of a large, random library of single-stranded DNA or RNA sequences. This method is carried out through a process known as SELEX, or the “systematic evolution of ligands by exponential enrichment” [183]. Aptamer columns have been utilized to purify recombinant proteins [194], as well as for chemical analysis [195–200]. Some aptamers have been used as part of microfluidic systems [197], while others have been immobilized within monolithic supports [198,199].
Conclusion
As has been shown in this review, affinity chromatography is a versatile technique that has become a valuable separation tool for biomedical and pharmaceutical analysis. This method can be carried out with traditional carbohydrate supports or with HPLC supports, such as silica and monolithic materials. This method can be used with step elution or isocratic elution and as part of affinity extraction or affinity depletion schemes. It can also be carried out with a wide range of ligands, such as lectins, boronates, antibodies, immobilized metal ions, molecularly imprinted polymers, dye ligands, and aptamers. These affinity methods can either be used alone or in combination with other techniques, such as reversed-phase HPLC or mass spectrometry. In addition, affinity chromatography can be used to study biological interactions and the competition or displacement of solutes on proteins or other binding agents. Based on the many recent applications of this method, it is expected that affinity chromatography will continue to grow in importance and popularity as a tool for the separation and analysis of biological and pharmaceutical agents in complex samples.
HIGHLIGHTS.
Affinity chromatography is a separation technique that has become increasingly important in work with biological samples and pharmaceutical agents.
This method can be used for alone or in combination with other methods, as well as for affinity extraction, affinity depletion, or biointeraction studies.
Affinity methods that have been of recent interest include lectin affinity chromatography, boronate affinity chromatography, immunoaffinity chromatography, and immobilized metal ion affinity chromatography.
Acknowledgements
This research was supported, in part, by the National Institutes of Health under grants R01 DK069629 and R01 GM044931 and by the NSF/EPSCoR program under grant EPS-1004094.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Aboul-Enein HY, editor. Separation Techniques in Clinical Chemistry. New York: Marcel Dekker; 2003. [Google Scholar]
- 2.Corradini D, Phillips TM, editors. Handbook of HPLC. 2nd ed. Boca Raton: CRC Press; 2011. [Google Scholar]
- 3.Hage DS, editor. Handbook of Affinity Chromatography. Boca Raton, FL: CRC Press; 2005. [Google Scholar]
- 4.Hage DS, Ruhn PF. An introduction to affinity chromatography. In: Hage DS, editor. Handbook of Affinity Chromatography. Boca Raton, FL: CRC Press; 2005. pp. 3–13. [Google Scholar]
- 5.Walters RR. Affinity chromatography. Anal. Chem. 1985;57:1099A–1114A. doi: 10.1021/ac00288a001. [DOI] [PubMed] [Google Scholar]
- 6.Cuatrecasas P, Wilchek M, Anfinsen CB. Selective enzyme purification by affinity chromatography. Proc. Natl. Acad. Sci. USA. 1968;68:636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turkova J. Affinity Chromatography. Amsterdam: Elsevier; 1978. [Google Scholar]
- 8.Hermanson GT, Mallia AK, Smith PK. Immobilized Affinity Ligand Techniques. New York: Academic Press; 1992. [Google Scholar]
- 9.Larsson PO. High-performance liquid affinity chromatography. Methods Enzymol. 1987;104:212–223. doi: 10.1016/s0076-6879(84)04091-x. [DOI] [PubMed] [Google Scholar]
- 10.Gustavsson PE, Larsson PO. Support materials for affinity chromatography. In: Hage DS, editor. Handbook of Affinity Chromatography. Boca Raton, FL: CRC Press; 2005. pp. 15–33. [Google Scholar]
- 11.Schiel JE, Mallik R, Soman S, Joseph KS, Hage DS. Applications of silica supports in affinity chromatography. J. Sep. Sci. 2006;29:719–737. doi: 10.1002/jssc.200500501. [DOI] [PubMed] [Google Scholar]
- 12.Mallik R, Hage DS. Affinity monolith chromatography. J. Sep. Sci. 2006;29:1686–1704. doi: 10.1002/jssc.200600152. [DOI] [PubMed] [Google Scholar]
- 13.Yoo MJ, Hage DS. Affinity monolith chromatography: principles and recent developments, Chap. 1. In: Wang P, editor. Monolithic Chromatography and Its Modern Applications. UK: ILM Publications; 2010. [Google Scholar]
- 14.Uzun L, Yavuz H, Say R, Ersoez A, Denizli A. Poly(ethylene dimethacrylate-glycidyl methacrylate) monolith as a stationary phase in dye-affinity chromatography. Ind. Eng. Chem. Res. 2004;43:6507–6513. [Google Scholar]
- 15.Jiang T, Mallik R, Hage DS. Affinity monoliths for ultrafast immunoextraction. Anal. Chem. 2005;77:2362–2372. doi: 10.1021/ac0483668. [DOI] [PubMed] [Google Scholar]
- 16.Okanda FM, El Rassi Z. Affinity monolithic capillary columns for glycomics/proteomics: 1. Polymethacrylate monoliths with immobilized lectins for glycoprotein separation by affinity capillary electrochromatography and affinity nano-liquid chromatography in either a single column or columns coupled in series. Electrophoresis. 2006;27:1020–1030. doi: 10.1002/elps.200500766. [DOI] [PubMed] [Google Scholar]
- 17.Mallik R, Xuan H, Hage DS. Development of an affinity silica monolith containing α1-acid glycoprotein for chiral separations. J. Chromatogr. A. 2007;1149:294–304. doi: 10.1016/j.chroma.2007.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hage DS. A survey of recent advances in analytical applications of immunoaffinity chromatography. J. Chromatogr. 1998;715:3–28. doi: 10.1016/s0378-4347(97)00621-x. [DOI] [PubMed] [Google Scholar]
- 19.Hage DS, Xuan H, Nelson MA. Application and elution in affinity chromatography. In: Hage DS, editor. Handbook of Affinity Chromatography. 2nd edition. Boca Raton, FL: CRC Press; 2005. pp. 79–97. [Google Scholar]
- 20.Kool J, Giera M, Irth H, Niessen WMA. Advances in mass spectrometry-based postcolumn bioaffinity profiling of mixtures. Anal. Bional. Chem. 2011;399:2655–2668. doi: 10.1007/s00216-010-4406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nuwaysir LM, Stults JT. Electrospray ionization mass spectrometry of phosphopeptides isolated by on-line immobilized metal-ion affinity chromatography. J. Am. Soc. Mass Spectrom. 1993;4:662–669. doi: 10.1016/1044-0305(93)85031-R. [DOI] [PubMed] [Google Scholar]
- 22.de Frutos M M, Regnier FE. Tandem chromatographic-immunological analyses. Anal. Chem. 1993;65:17A–25A. doi: 10.1021/ac00049a001. [DOI] [PubMed] [Google Scholar]
- 23.Ohlson S, Lundblad A, Zopf D. Novel approach to affinity chromatography using “weak” monoclonal antibodies. Anal. Biochem. 1988;169:204–208. doi: 10.1016/0003-2697(88)90275-8. [DOI] [PubMed] [Google Scholar]
- 24.Wikstroem M, Ohlson S. Computer simulation of weak affinity chromatography. J. Chromatogr. 1992;597:83–92. [Google Scholar]
- 25.Ohlson S, Bergstroem M, Pahlsson P, Lundblad A. Use of monoclonal antibodies for weak affinity chromatography. J. Chromatogr. A. 1997;758:199–208. doi: 10.1016/s0021-9673(96)00733-9. [DOI] [PubMed] [Google Scholar]
- 26.Strandh M, Andersson HS, Ohlson S. Weak affinity chromatography. Methods Mol. Biol. 2000;147:7–23. doi: 10.1007/978-1-60327-261-2_2. [DOI] [PubMed] [Google Scholar]
- 27.Vosseller K, Trinidad JC, Chalkley RJ, Specht CG, Thalhammer A, Lynn AJ, et al. O-Linked N-acetylglucosamine proteomics of postsynaptic density preparations using lectin weak affinity chromatography and mass spectrometry. Mol. Cell Proteomics. 2006;5:923–934. doi: 10.1074/mcp.T500040-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Patel S, Wainer IW, Lough JW. Affinity-based chiral stationary phases. In: Hage DS, editor. Handbook of Affinity Chromatography. 2nd ed. Boca Raton: CRC Press; 2006. pp. 571–592. [Google Scholar]
- 29.Allenmark S. Chromatographic Enantioseparation: Methods and Applications. 2nd ed. New York: Ellis Horwood; 1991. [Google Scholar]
- 30.Duong-Thi M-D, Meiby E, Bergstroem M, Fex T, Isaksson R, Ohlson S. Weak affinity chromatography as a new approach for fragment screening in drug discovery. Anal. Biochem. 2011;414:138–146. doi: 10.1016/j.ab.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Cheshev P, Morelli L, Marchesi M, Podlipnik C, Bergstrom M, Bernadi A. Synthesis and affinity evaluation of a small library of bidentate cholera toxin ligands: towards nonhydrolyzable ganglioside mimics. Chemistry. 2010;16:1951–1967. doi: 10.1002/chem.200902469. [DOI] [PubMed] [Google Scholar]
- 32.Bergstrom M, Liu S, Kiick K, Ohlson S. Cholera toxin inhibitors studied with high-performance liquid affinity chromatography: a robust method to evaluate receptor-ligand interactions. Chem. Biol. Drug Des. 2009;73:132–141. doi: 10.1111/j.1747-0285.2008.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo MJ, Hage DS. Evaluation of silica monoliths in affinity microcolumns for high-throughput analysis of drug-protein interactions. J. Sep. Sci. 2009;32:2776–2785. doi: 10.1002/jssc.200900346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoo MJ, Schiel JS, Hage DS. Evaluation of affinity microcolumns containing human serum albumin for rapid analysis of drug-protein binding. J. Chromatogr. B. 2010;878:1707–1713. doi: 10.1016/j.jchromb.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moser AC, Hage DS. Immunoaffinity chromatography. Bioanalysis. 2010;2:769–790. doi: 10.4155/bio.10.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.del Castillo E, Montes-Batyón M, Anón E, Sanz’Medel A. Quantitative targeted biomarker assay for glycated haemoglobin by multidimensional LC using mass spectrometric detection. J. Proteomics. 2011;74:35–43. doi: 10.1016/j.jprot.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Kullolli M, Hancock WS, Hincapie M. Automated platform for fractionation of human plasma glycoproteome in clinical proteomics. Anal. Chem. 2010;82:115–120. doi: 10.1021/ac9013308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke W, Roy Choudhuri A, Hage DS. Analysis of free drug fractions by ultra-fast immunoaffinity chromatography. Anal. Chem. 2001;73:2157–2164. doi: 10.1021/ac0009752. [DOI] [PubMed] [Google Scholar]
- 39.Clarke W, Schiel JE, Moser A, Hage DS. Analysis of free hormone fractions by an ultrafast immunoextraction/displacement immunoassay: studies using free thyroxine as a model system. Anal. Chem. 2005;77:1859–1866. doi: 10.1021/ac040127x. [DOI] [PubMed] [Google Scholar]
- 40.Ohnmacht CM, Schiel JE, Hage DS. Analysis of free drug fractions using near-infrared fluorescent labels and an ultrafast immunoextraction/displacement assay. Anal. Chem. 2006;78:7547–7556. doi: 10.1021/ac061215f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mallik R, Yoo MJ, Briscoe CJ, Hage DS. Analysis of drug-protein binding by ultrafast affinity chromatography using immobilized human serum albumin. J. Chromatogr. A. 2010;1217:2796–2803. doi: 10.1016/j.chroma.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cellar NA, Karnoup AS, Albers DR, Langhorst ML, Young SA. Immunodepletion of high abundance proteins coupled on-line with reversed-phase liquid chromatography: A two-dimensional LC sample enrichment and fractionation technique for mammalian proteomics. J. Chromatogr. B. 2009;877:79–85. doi: 10.1016/j.jchromb.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 43.Hage DS, Bian M, Burks R, Karle E, Ohnmacht C, Wa C. Bioaffinity chromatography. In: Hage DS, editor. Handbook of Affinity Chromatography. 2nd ed. Boca Raton, FL: CRC Press; 2005. pp. 107–135. [Google Scholar]
- 44.Liener IE, Sharon N, Goldstein IJ IJ, editors. The Lectins: Properties, Functions and Applications in Biology and Medicine. Waltham, MS: Academic Press; 1986. [Google Scholar]
- 45.Yamamoto K, Tsuji T, Osawa T. Affinity chromatography of oligosaccharides and glycopeptides with immobilized lectins. In: Walker J, editor. The Protein Protocols Handbook. Totowa, NJ: Humana Press; 2002. pp. 917–931. [Google Scholar]
- 46.Hauri HP, Appenzeller C, Kuhn F, Nufer O. Lectins and traffic in the secretory pathway. FEBS Lett. 2000;476:32–37. doi: 10.1016/s0014-5793(00)01665-3. [DOI] [PubMed] [Google Scholar]
- 47.Sing RS, Bhari R, Kaur HP. Current trends of lectins from microfungi. Crit. Rev. Biotechnol. 2011;31:193–210. doi: 10.3109/07388551.2010.505911. [DOI] [PubMed] [Google Scholar]
- 48.Alexandre KB, Gray ES, Lambson BE, Moore PL, Choge IA, Mlisana K, Abdool K, Salim S, McMahon J, O'Keefe B, Chikwamba R, Morris L. Mannose-rich glycosylation patterns on HIV-1 subtype C gp120 and sensitivity to the lectins Griffithsin, Cyanovirin-N and Scytovirin. Virology. 2010;402:187–196. doi: 10.1016/j.virol.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bourne Y, Astoul CH, Zamboni V, Peumans WJ, Menu-Bouaouiche L, Van Damme EJM, Barre A, Rouge P. Structural basis for the unusual carbohydrate-binding specificity of jacalin towards galactose and mannose. J. Biochem. 2002;364:173–180. doi: 10.1042/bj3640173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Paula E, de Melo R, Sartim MA, Callejon DR, Paiva HH, Antonucci GA, Rosa JC, Oliveira AC, Franco JJ, Candiani E, Dias-Baruffi M, Vilela S. Isolation, functional, and partial biochemical characterization of galactrox, an acidic lectin from Bothrops atrox snake venom. Acta Biochim. Biophys. Sin. 2011;43:181–192. doi: 10.1093/abbs/gmr003. [DOI] [PubMed] [Google Scholar]
- 51.Vivekananda S, Nickens Z, Shah P, Sinnathamby G, Semmes OJ, Philip R. Investigation of sialylation aberration in N-linked glycopeptides by lectin and tandem labeling (LTL) quantitative proteomics. Anal. Chem. 2010;82:9201–9210. doi: 10.1021/ac101486d. [DOI] [PubMed] [Google Scholar]
- 52.Qiu R, Zhang X, Regnier FE. A method for the identification of glycoproteins from human serum by a combination of lectin affinity chromatography along with anion exchange and Cu-IMAC selection of tryptic peptides. J. Chromatogr. B. 2007;845:143–150. doi: 10.1016/j.jchromb.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Plavina T, Wakshull E, Hancock WS, Hincapie M. Combination of abundant protein depletion and multi-lectin affinity chromatography (MLAC) for plasma protein biomarker discovery. J. Prot. Res. 2007;6:662–671. doi: 10.1021/pr060413k. [DOI] [PubMed] [Google Scholar]
- 54.Zeng Z, Hincapie M, Haab BB, Hanash S, Pitteri SJ, Kluck S, Hogan JM, Kennedy J, Hancock WS. The development of an integrated platform to identify breast cancer glycoproteome changes in human serum. J. Chromatogr. A. 2010;1217:3307–3315. doi: 10.1016/j.chroma.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng Z, Hincapie M, Pitteri SJ, Hanash S, Schalkwijk J, Hogan JM, Wang H, Hancock WS. A proteomics platform combining depletion, multi-lectin affinity chromatography (M-LAC), and isoelectric focusing to study the breast cancer proteome. Anal. Chem. 2011;83:4845–4854. doi: 10.1021/ac2002802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calvano CD, Zambonin CG, Jensen ON. Assessment of lectin and HILIC based enrichment protocols for characterization of serum glycoproteins by mass spectrometry. J. Proteomics. 2008;71:304–317. doi: 10.1016/j.jprot.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 57.Liu XC, Scouten W. Boronate affinity chromatography. In: Hage DS, editor. Handbook of Affinity Chromatography. Boca Raton, FL: CRC Press; 2005. pp. 243–261. [Google Scholar]
- 58.Mayer TK, Freedman ZR. Protein glycosylation in diabetes mellitus: a review of laboratory measurements and of their clinical utility. Clin. Chim. Acta. 1983;127:147–184. doi: 10.1016/s0009-8981(83)80002-3. [DOI] [PubMed] [Google Scholar]
- 59.Bouriotis V, Stott J, Galloway A, Bellingham AJ, Dean PDG. Measurement of glycosylated haemoglobins using affinity chromatography. Diabetologia. 1981;21:579–580. doi: 10.1007/BF00281553. [DOI] [PubMed] [Google Scholar]
- 60.Middle FA, Bannister A, Bellingham AJ, Dean PDG. Separation of glycosylated haemoglobins using immobilized phenylboronic acids. Biochem. J. 1983;209:771–779. doi: 10.1042/bj2090771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mallia AK, Hermanson GT, Krohn RI, Fujimoto EK, Smith PK. Preparation and use of a boronic acid affinity support for the separation and quantitation of glycosylated hemoglobins. Anal. Lett. 1981;14:649–661. [Google Scholar]
- 62.Hjerten S, Li JP. High-performance liquid chromatography of proteins on deformed non-porous agarose beads: fast boronate affinity chromatography of haemoglobin at neutral pH. J. Chromatogr. 1990;500:543–553. doi: 10.1016/s0021-9673(00)96091-6. [DOI] [PubMed] [Google Scholar]
- 63.Gould BJ, Hall PM, Cook JGH. Measurement of glycosylated haemoglobins using an affinity chromatography method. Clin. Chim. Acta. 1982;125:41–48. doi: 10.1016/0009-8981(82)90043-2. [DOI] [PubMed] [Google Scholar]
- 64.Singhal RP, DeSilva SSM. Boronate affinity chromatography. Adv. Chromatogr. 1992;31:293–335. [PubMed] [Google Scholar]
- 65.Kitagawa N, Treat-Clemens LG. Chromatographic study of immobilized boronate stationary phases. Anal. Sci. 1991;7:195–198. [Google Scholar]
- 66.Stevenson T. Glycosal: the first rapid, point-of-care test for the determination of hemoglobin A1c in patients with diabetes. Diabetes Technol. Ther. 1999;1:425–431. doi: 10.1089/152091599316946. [DOI] [PubMed] [Google Scholar]
- 67.Silver AC, Lamb E, Cattell WR, Dawnay ABSJ. Investigation and validation of the affinity chromatography method for measuring glycated albumin in serum and urine. Clin. Chim. Acta. 1991;202:11–22. doi: 10.1016/0009-8981(91)90251-7. [DOI] [PubMed] [Google Scholar]
- 68.Panteghini M, Bonora R, Pagani F. Determination of glycated apolipoprotein B in serum by a combination of affinity chromatography and immunonephelometry. Ann. Clin. Biochem. 1994;31:544–549. doi: 10.1177/000456329403100603. [DOI] [PubMed] [Google Scholar]
- 69.Peterson KP, Pavlovich JG, Goldstein D, Little R, England J, Peterson CM. What is haemoglobin A1c? An analysis of glycated hemoglobin by electrospay ionization mass spectrometry. Clin. Chem. 1998;44:1951–1958. [PubMed] [Google Scholar]
- 70.Little RR, Roberts WL. A review of variant hemoglobins interfering with hemoglobin A1c measurement. J. Diabetes Science and Technology. 2009;3:446–451. doi: 10.1177/193229680900300307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee S-C, Wang L-S, Tsai S-M, Fang H-Y, Tsai L-Y. Effects of the Hb E, Hb H and Hb G-Taichung variants on HbA1c values by the Bio-Rad variant™ turbo analyzer. Clin. Biochem. 2011;44:1338–1342. doi: 10.1016/j.clinbiochem.2011.08.907. [DOI] [PubMed] [Google Scholar]
- 72.Ren L, Liu Y, Dong M, Liu Z. Synthesis of hydrophilic boronate affinity monolithic capillary for specific capture of glycoproteins by capillary liquid chromatography. J. Chromatogr. A. 2009;1216:8421–8425. doi: 10.1016/j.chroma.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y, Ren L, Liu Z. A unique boronic acid functionalized monolithic capillary for specific capture, separation and immobilization of cis-diol biomolecules. Chem. Commun. 2011;47:5067–5069. doi: 10.1039/c0cc05675h. [DOI] [PubMed] [Google Scholar]
- 74.Suksrichavalita T, Yoshimatsua K, Prachayasittikulb V, Bülowa L, Ye L. “Clickable” affinity ligands for effective separation of glycoproteins. J. Chromatogr. A. 2010;1217:3635–3641. doi: 10.1016/j.chroma.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 75.Monzo A, Bonn G, Guttman A. Boronic acid–lectin affinity chromatography. 1. simultaneous glycoprotein binding with selective or combined elution. Anal. Bioanal. Chem. 2007;389:2097–2102. doi: 10.1007/s00216-007-1627-y. [DOI] [PubMed] [Google Scholar]
- 76.Thomas DH, Taylor JD, Barnaby OS, Hage DS. Determination of free catecholamines in urine by tandem affinity/ion-pair chromatography and flow injection analysis. Clin. Chim. Acta. 2008;398:63–69. doi: 10.1016/j.cca.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hage DS, Phillips TM. Immunoaffinity chromatography. In: Hage DS, editor. Handbook of Affinity Chromatography. 2nd ed. Boca Raton, FL: CRC Press; 2005. pp. 127–172. [Google Scholar]
- 78.Wilchek M, Miron T, Kohn J. Affinity chromatography. Methods Enzymol. 1984;104:3–55. doi: 10.1016/s0076-6879(84)04082-9. [DOI] [PubMed] [Google Scholar]
- 79.Hage DS, Nelson MA. Chromatographic immunoassays. Anal. Chem. 2001;73:198A–205A. [PubMed] [Google Scholar]
- 80.Moser AC, Hage DS. Chromatographic immunoassays, Chapter 29. In: Hage DS, editor. Handbook of Affinity Chromatography. 2nd ed. Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- 81.Hage DS, Thomas DH, Roy Chowdhuri A, Clarke W. Development of a theoretical model for chromatographic-based competitive binding immunoassays with simultaneous injection of sample and label. Anal. Chem. 1999;71:2965–2975. doi: 10.1021/ac990070s. [DOI] [PubMed] [Google Scholar]
- 82.Nelson MA, Reiter WS, Hage DS. Chromatographic competitive binding immunoassays: a comparison of the sequential and simultaneous injection methods. Biomed. Chromatogr. 2003;17:188–200. doi: 10.1002/bmc.241. [DOI] [PubMed] [Google Scholar]
- 83.Oates MR, Clarke W, Zimlich A, II, Hage DS. Optimization and development of an HPLC-based one-site immunometric assay with chemiluminescence detection. Anal. Chim. Acta. 2002;470:37–50. [Google Scholar]
- 84.Todorova D, Vijayalakshmi MA. Immobilized metal-ion affinity chromatography. Chap. 10. In: Hage DS, editor. Handbook of Affinity Chromatography. 2nd ed. Boca Raton: CRC Press; 2005. [Google Scholar]
- 85.Chaga GS. Twenty-five years of immobilized metal ion affinity chromatography: Past, present and future. J. Biochem. Biophys. Methods. 2001;49:313–334. doi: 10.1016/s0165-022x(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 86.Mrabet NT, Vijayalakshmi MA. Immobilized metal-ion affinity chromatography. From phenomenological hallmarks to structure-based molecular insights. In: Vijayalakshmi MA, editor. Biochromatography-Theory and Practice. London: Taylor & Francis; 2002. pp. 272–294. [Google Scholar]
- 87.Porath J, Carlsson J, Olsson I, Belfrage B. Metal chelate affinity chromatography: a new approach to protein fraction. Nature. 1975;258:598–599. doi: 10.1038/258598a0. [DOI] [PubMed] [Google Scholar]
- 88.Li R, Wang Y, Guo LC, Shi M, Wang XG, Chen B, Zheng JB. A novel silica-based metal chelate stationary phase—L-glutamic acid-coppeR(II) . Sep. Sci. Technol. 2011;46:309–314. [Google Scholar]
- 89.Block H, Maertens B, Spriestersbach A, Brinker N, Kubicek J, Fabis R, Labahn J, Schäfer F. Immobilized-metal affinity chromatography (IMAC) : a review. Methods Enzymol. 2009;463:439–473. doi: 10.1016/S0076-6879(09)63027-5. [DOI] [PubMed] [Google Scholar]
- 90.Gutiérrez R, Martín del Valle EM, Galán MA. Immobilized metal-ion affinity chromatography: status and trends. Sep. Purif. Rev. 2007;36:71–111. [Google Scholar]
- 91.Dong XY, Chen LJ, Sun Y. Refolding and purification of histidine-tagged protein by artificial chaperone-assisted metal affinity chromatography. J. Chromatogr. A. 2009;1216:5207–5213. doi: 10.1016/j.chroma.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 92.Wang CZ, Wang LL, Geng XD. Optimization of refolding with simultaneous purification of recombinant human granulocyte colony-stimulating factor from Escherichia coli by immobilized metal ion affinity chromatography. Biochem. Eng. J. 2009;43:197–202. [Google Scholar]
- 93.Ye JY, Zhang XM, Young C, Zhao XL, Hao Q, Cheng L, Jensen ON. Optimized IMAC protocol for phosphopeptide recovery from complex biological samples. J. Proteome Res. 2010;9:3561–3573. doi: 10.1021/pr100075x. [DOI] [PubMed] [Google Scholar]
- 94.Lee YM, Venkataraman K, Hwang S, Han DK, Hla T. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC) Prostaglandins Other Lipid Mediat. 2007;84:154–162. doi: 10.1016/j.prostaglandins.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takeda N, Matsuoka T, Gotoh M. Potentiality of IMAC as sample pretreatment tool in food analysis for veterinary drugs. Chromatographia. 2010;72:127–131. [Google Scholar]
- 96.Felix K, Fakelman F, Hartmann D, Giese NA, Gaida MM, Schnoelzer M, Flad T, Buechler MW, Werner J. Identification of serum proteins involved in pancreatic cancer cachexia. Life Sci. 2011;88:218–225. doi: 10.1016/j.lfs.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 97.Wu C, Wang ZF, Liu LJ, Zhao P, Wang WJ, Yao DK, Shi B, Lu JH, Liao P, Yang YN, Zhu L. Surface enhanced laser desorption/ionization profiling: new diagnostic method of HBV-related hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2009;24:55–62. doi: 10.1111/j.1440-1746.2008.05580.x. [DOI] [PubMed] [Google Scholar]
- 98.Liu ZJ, Wang B, Yan ZY, Song XX, Qian DM, Bai ZQ. Using the SELDI ProteinChip system to detect changes in protein expression in Vero cells after infection. Virol. Sin. 2007;22(1):68–73. [Google Scholar]
- 99.QIAGEN Group. Mass·Spec·Focus IMAC Chip Handbook. third ed. Germany: Hilden; 2007. [Google Scholar]
- 100.Victor MBG, Owen RD. Structural analysis and classification of native proteins from commonly co-purified by immobilised metal affinity chromatography. Biochim. Biophys. Acta. 2006;1760:1304–1313. doi: 10.1016/j.bbagen.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 101.Block H, Kubicek J, Labahn J, Roth U, Schafer F. Production and comprehensive quality control of recombinant human interleukin-1b: a case study for a process development strategy. Protein Expr. Purif. 2008;27:244–254. doi: 10.1016/j.pep.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 102.Lewinson O, Lee AT, Douglas CR. The funnel approach to the precrystallization production of membrane proteins. J. Mol. Biol. 2008;377:62–73. doi: 10.1016/j.jmb.2007.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garberc-Porekar V, Menart V, Jevsevar S, Vidensek A, Stalc A. Histidines in affinity tags and surface clusters for immobilized metal-ion affinity chromatography of trimeric tumor necrosis factor α. J. Chromatogr. A. 1999;852:117–128. doi: 10.1016/s0021-9673(99)00374-x. [DOI] [PubMed] [Google Scholar]
- 104.Wang W. Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int. J. Pharm. 1999;185(2):129–188. doi: 10.1016/s0378-5173(99)00152-0. [DOI] [PubMed] [Google Scholar]
- 105.Garberc-Porekar V, Menart V. Perspectives of immobilized-metal affinity chromatography. J. Biochem. Biophys. Methods. 2001;49:335–360. doi: 10.1016/s0165-022x(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 106.Pedersen J, Lauritzen C, Madsen MT, Dahl SW. Removal of N-terminal polyhistidine tags from recombinant proteins using engineered aminopeptidases. Prot. Express. Purif. 1999;15:389–400. doi: 10.1006/prep.1999.1038. [DOI] [PubMed] [Google Scholar]
- 107.Chaiken IM, editor. Analytical Affinity Chromatography. Boca Raton, FL: CRC Press; 1987. [Google Scholar]
- 108.Hage DS, Chen J. Quantitative affinity chromatography: practical aspects. In: Hage DS, editor. Handbook of Affinity Chromatography. 2nd ed. Boca Raton: CRC Press; 2006. pp. 595–628. [Google Scholar]
- 109.Winzor DJ. Quantitative affinity chromatography: recent theoretical developments. In: Hage DS, editor. Handbook of Affinity Chromatography. 2nd ed. Boca Raton: CRC Press; 2006. pp. 595–628. [Google Scholar]
- 110.Schiel JE, Joseph KS, Hage DS. Biointeraction affinity chromatography, Chapter 4. In: Grinsberg N, Grushka E, editors. Advances in Chromatography. Vol. 48. New York: Taylor & Francis; 2009. [Google Scholar]
- 111.Hage DS, Anguizola JA, Jackson AJ, Matsuda R, Papastavros E, Pfaunmiller E, Tong Z, Vargas-Badilla J, Yoo MJ, Zheng X. Chromatographic analysis of drug interactions in the serum proteome. Anal. Methods. 2011;8:1449–1460. doi: 10.1039/C1AY05068K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nelson MA, Moser AC, Hage DS. Biointeraction analysis by high-performance affinity chromatography: kinetic studies of immobilized antibodies. J. Chromatogr. B. 2010;878:165–171. doi: 10.1016/j.jchromb.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schiel JE, Hage DS. Kinetic studies of biological interactions by affinity chromatography. J. Sep. Sci. 2009;32:1507–1522. doi: 10.1002/jssc.200800685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Patel S, Wainer IW, Lough WJ. Chromatographic studies of molecular recognition and solute binding to enzymes and plasma proteins. In: Hage DS, editor. Handbook of Affinity Chromatography. 2nd ed. Boca Raton: CRC Press; 2006. pp. 663–683. [Google Scholar]
- 115.Kim HS, Wainer IW. Rapid analysis of the interactions between drugs and human serum albumin (HSA) using high-performance affinity chromatography (HPAC) J. Chromatogr. B. 2008;870:22–26. doi: 10.1016/j.jchromb.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xuan H, Hage DS. Evaluation of a hydrazide-linked alpha1-acid glycoprotein chiral stationary phase: separation of R- and S-propranolol. J. Sep. Sci. 2006;29:1412–1422. doi: 10.1002/jssc.200600051. [DOI] [PubMed] [Google Scholar]
- 117.Kalizan R, Noctor TAG, Wainer IW. Stereochemical aspects of benzodiazepine binding to human serum albumin. II. Quantitative relationships between structure and enantioselective retention in high performance liquid affinity chromatography. Mol. Pharmacol. 1992;42:512–517. [PubMed] [Google Scholar]
- 118.Kaliszan R, Nasal A, Turowski M. Quantitative structure-retention relationships in the examination of the topography of the binding site of antihistamine drugs on α1-acid glycoprotein. J. Chromatogr. A. 1996;722:25–32. doi: 10.1016/0021-9673(95)00523-4. [DOI] [PubMed] [Google Scholar]
- 119.Nasal A, Radwanska A, Osmialowski K, Bucinski A, Kaliszan R, Barker GE, Sun P, Hartwick RA. Quantitative relationships between the structure of β-adrenolytic and antihistamine drugs and their retention on an α1-acid glycoprotein HPLC column. Biomed. Chromatogr. 1994;8:125–129. doi: 10.1002/bmc.1130080306. [DOI] [PubMed] [Google Scholar]
- 120.Loun B, Hage DS. Chiral separation mechanisms in protein-based HPLC columns. I. Thermodynamic studies of R- and S-warfarin binding to immobilized human serum albumin. Anal. Chem. 1994;66:3814–3822. doi: 10.1021/ac00093a043. [DOI] [PubMed] [Google Scholar]
- 121.Matsuda R, Anguizola JA, Joseph KS, Hage DS. High-performance affinity chromatography and the analysis of drug interactions with modified proteins: binding of gliclazide with glycated human serum albumin. Anal. Bioanal. Chem. 2011;401:2811–2819. doi: 10.1007/s00216-011-5382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dalgaard L, Hansen JJ, Pedersen JL. Resolution and binding site determination of D,L-thyronine by high-performance liquid chromatography using immobilized albumin as chiral stationary phase. Determination of optical purity of thyroxine in tablets. J. Pharm. Biomed. Anal. 1989;7:361–368. doi: 10.1016/0731-7085(89)80103-7. [DOI] [PubMed] [Google Scholar]
- 123.Domenici E, Bertucci C, Salvadorri P, Wainer IW. Use of human serum albumin-based high-performance affinity chromatography chiral stationary phase for the investigation between warfarin and benzodiazepine binding site. J. Pharm. Sci. 1991;80:164–166. doi: 10.1002/jps.2600800216. [DOI] [PubMed] [Google Scholar]
- 124.Noctor TAG, Wainer IW, Hage DS. Allosteric and competitive displacement of drugs from human serum albumin by octanoic acid, as revealed by high-performance liquid affinity chromatography, on a human serum albumin-based stationary phase. J. Chromatogr. 1992;577:305–315. doi: 10.1016/0378-4347(92)80252-l. [DOI] [PubMed] [Google Scholar]
- 125.Basiaga SBG, Hage DS. Chromatographic studies of changes in binding of sulfonylurea drugs to human serum albumin due to glycation and fatty acids. J. Chromatogr. B. 2010;878:3193–3197. doi: 10.1016/j.jchromb.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Joseph KS, Moser AC, Basiaga S, Schiel JE, Hage DS. Evaluation of alternatives to warfarin as probes for sudlow site I of human serum albumin: characterization by high performance affinity chromatography. J. Chromatogr. A. 2009;1216:3492–3500. doi: 10.1016/j.chroma.2008.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Conrad ML, Moser AC, Hage DS. Evaluation of indole-based probes for studying drug binding to human serum albumin in high-performance affinity separations. J. Sep. Sci. 2009;32:1145–1155. doi: 10.1002/jssc.200800567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen J, Hage DS. Quantitative analysis of allosteric drug-protein binding by biointeraction chromatography. Nature Biotechnol. 2004;22:1445–1448. doi: 10.1038/nbt1022. [DOI] [PubMed] [Google Scholar]
- 129.Chen J, Fitos I, Hage DS. Chromatographic analysis of allosteric effects between ibuprofen and benzodiazepines on human serum albumin. Chirality. 2006;18:24–36. doi: 10.1002/chir.20216. [DOI] [PubMed] [Google Scholar]
- 130.Chen J, Hage DS. Quantitative studies of allosteric effects by biointeraction chromatography: analysis of protein binding to low solubility drugs. Anal. Chem. 2006;78:2672–2683. doi: 10.1021/ac052017b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Noctor TAG, Wainer IW. The in situ acetylation of an immobilized human serum albumin chiral stationary phase for high-performance liquid chromatography in the examination of drug-protein binding phenomenon. Pharm. Res. 1992;9:480–484. doi: 10.1023/a:1015884112039. [DOI] [PubMed] [Google Scholar]
- 132.Chattopadhyay A, Tian T, Kortum L, Hage DS. Development of tryptophan-modified human serum albumin columns for site-specific studies of drug-protein interactions by high-performance affinity chromatography. J. Chromatogr. B. 1998;715:183–190. doi: 10.1016/s0378-4347(98)00140-6. [DOI] [PubMed] [Google Scholar]
- 133.Joseph KS, Anguizola JA, Hage DS. Binding of tolbutamide to glycated human serum albumin. J. Pharm. Biomed. Anal. 2011;54:426–432. doi: 10.1016/j.jpba.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Joseph KS, Anguizola JA, Jackson AJ, Hage DS. Chromatographic analysis of acetohexamide binding to glycated human serum albumin. J. Chromatogr. B. 2010;878:2775–2781. doi: 10.1016/j.jchromb.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Joseph KS, Hage DS. The effects of glycation on the binding of human serum albumin to warfarin and L-tryptophan. J. Pharm. Biomed. Anal. 2010;53:811–818. doi: 10.1016/j.jpba.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schreimer DC. Biosensor alternative: frontal affinity chromatography. Anal. Chem. 2004;76:440–448A. doi: 10.1021/ac041684m. [DOI] [PubMed] [Google Scholar]
- 137.Loun B, Hage DS. Characterization of thyroxine-albumin binding using high-performance affinity chromatography. 1. Interactions at the warfarin and indole sites of albumin. J. Chromatogr. 1992;579:225–235. [PubMed] [Google Scholar]
- 138.Yang J, Hage DS. Role of binding capacity versus binding strength in the separation of chiral compounds on protein-based high-performance liquid chromatographic columns: interactions of D- and L-tryptophan with human serum albumin. J. Chromatogr. B. 1996;725:273–285. doi: 10.1016/0021-9673(95)01009-2. [DOI] [PubMed] [Google Scholar]
- 139.Yang J, Hage DS. Characterization of the binding and chiral separation of D- and L-tryptophan on a high-performance immobilized human serum albumin column. J. Chromatogr. 1993;645:241–250. doi: 10.1016/0021-9673(93)83383-4. [DOI] [PubMed] [Google Scholar]
- 140.Nakano NI, Shimamori Y, Yamaguchi S. Binding capacities of human serum albumin monomer and dimer by continuous frontal affinity chromatography. J. Chromatogr. 1982;237:225–232. doi: 10.1016/s0021-9673(00)83229-x. [DOI] [PubMed] [Google Scholar]
- 141.Tweed SA, Loun B, Hage DS. Effects of ligand heterogeneity in the characterization of affinity columns by frontal analysis. Anal. Chem. 1997;69:4790–4798. doi: 10.1021/ac970565m. [DOI] [PubMed] [Google Scholar]
- 142.Joseph KS, Hage DS. Characterization of the binding of sulfonylurea drugs to HSA by high-performance affinity chromatography. J. Chromatogr. B. 2010;878:1590–1598. doi: 10.1016/j.jchromb.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim HS, Hage DS. Chromatographic analysis of carbamazepine binding to human serum albumin. J. Chromatogr. B. 2005;816:57–66. doi: 10.1016/j.jchromb.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 144.Chen J, Ohnmacht C, Hage DS. Studies of phenytoin binding to human serum albumin by high-performance affinity chromatography. J. Chromatogr. B. 2004;809:137–145. doi: 10.1016/j.jchromb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 145.Soman S, Yoo MJ, Jang YJ, Hage DS. Lidocaine interactions with serum proteins using high-performance affinity chromatography. J. Chromatogr. B. 2010;878:705–708. doi: 10.1016/j.jchromb.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mallik R, Yoo MJ, Chen S, Hage DS. Studies of verapamil binding to human serum albumin by high-performance affinity chromatography. J. Chromatogr. B. 2008;876:69–75. doi: 10.1016/j.jchromb.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yoo MJ, Smith QR, Hage DS. Studies of imipramine binding to human serum albumin by high-performance affinity chromatography. J. Chromatogr. B. 2009;877:1149–1154. doi: 10.1016/j.jchromb.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xuan H, Joseph KS, Wa C, Hage DS. Biointeraction analysis of carbamazepine binding to alpha 1-acid glycoprotein by high-performance affinity chromatography. J. Sep. Sci. 2010;33:2294–2301. doi: 10.1002/jssc.201000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen S, Sobansky MR, Hage DS. Analysis of drug interactions with high-density lipoprotein by high-performance affinity chromatography. Anal. Biochem. 2010;397:107–114. doi: 10.1016/j.ab.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tong Z, Joseph KS, Hage DS. Detection of heterogeneous drug-protein binding by frontal analysis and high-performance affinity chromatography. J. Chromatogr. A. 2011;1218:8915–8924. doi: 10.1016/j.chroma.2011.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chan N, Lewis D, Kelly M, Ng ESM, Schriemer DC. Frontal affinity chromatography-mass spectrometry for ligand discovery and characterization. In: Wanner KT, Hofner G, editors. Mass Spectrometry in Medicinal Chemistry. Germany: Wiley, Weinheim; 2007. pp. 217–246. [Google Scholar]
- 152.Slon-Usakiewicz JJ, Dai JR, Ng W, Foster JE, Deretey E, Toledo-Sherman L, Redden PR, Pasternak A, Reid N. Global kinase screening. Applications of frontal affinity chromatography coupled to mass spectrometry in drug discovery. Anal. Chem. 2005;77:1268–1274. doi: 10.1021/ac048716q. [DOI] [PubMed] [Google Scholar]
- 153.Kovarik P, Hodgson RJ, Covey T, Brook MA, Brennan JD. Capillary-scale frontal affinity chromatography MALDI tandem mass spectrometry using protein-doped monolithic silica columns. Anal. Chem. 2005;77:3340–3350. doi: 10.1021/ac048263p. [DOI] [PubMed] [Google Scholar]
- 154.Iwaki J, Minamisawa T, Tateno H, Kominami J, Suzuki K, Nishi N, Nakamura T, Hirabayashi J. Desulfated galactosaminoglycans are potential ligands for galectins: evidence from frontal affinity chromatography. Biochem. and Biophys. Res. Comm. 2008;373:206–212. doi: 10.1016/j.bbrc.2008.05.190. [DOI] [PubMed] [Google Scholar]
- 155.Tachibana K, Nakamura, Wang H, Iwasaki H, Tachibana K, Maebara K, Cheng L, Hirabayashi J, Narimatsu H. Elucidation of binding specificity of jacalin toward O-glycosylated peptides: quantitative analysis by frontal affinity chromatography. Glycobiology. 2006;16:46–53. doi: 10.1093/glycob/cwj038. [DOI] [PubMed] [Google Scholar]
- 156.Nakamura-Tsuruta S, Uchiyama N, Hirabayashi J. High-throughput analysis of lectin-oligosaccharide interactions by automated frontal affinity chromatography. Methods Enzymol. 2006;415:311–325. doi: 10.1016/S0076-6879(06)15019-3. [DOI] [PubMed] [Google Scholar]
- 157.Ng ESM, Yang F, Kameyama A, Palcic MM, Hindsgaul O, Schriemer DC. High-throughput screening for enzyme inhibitors using frontal affinity chromatography with liquid chromatography and mass spectrometry. Anal. Chem. 2005;77:6125–6133. doi: 10.1021/ac051131r. [DOI] [PubMed] [Google Scholar]
- 158.Arata Y, Ishii N, Tamura M, Nonaka T, Kasai KI. Identification of the amino acid residue in the nematode galectin LEC-1 responsible for Its unique sugar binding property: analysis by combination of site-directed mutagenesis and frontal affinity chromatography. Biol. Pharm. Bull. 2007;30:2012–2017. doi: 10.1248/bpb.30.2012. [DOI] [PubMed] [Google Scholar]
- 159.Itakura Y, Nakamura-Tsuruta S, Kominami J, Sharon N, Kasai K, Hirabayashi J. Systematic comparison of oligosaccharide specificity of Ricinus communis agglutinin I and erythrina lectins: a search by frontal affinity chromatography. J. Biochem. 2007;142:459–469. doi: 10.1093/jb/mvm153. [DOI] [PubMed] [Google Scholar]
- 160.Schiel JE, Hage DS. Kinetic studies of biological interactions by affinity chromatography. J. Sep. Sci. 2009;32:1507–1522. doi: 10.1002/jssc.200800685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Loun B, Hage DS. Chiral separation mechanisms in protein based HPLC columns 2. Kinetic studies of (R) - and (S) -warfarin binding to immobilized human serum albumin. Anal. Chem. 1996;68:1218–1225. doi: 10.1021/ac950827p. [DOI] [PubMed] [Google Scholar]
- 162.Yang J, Hage DS. Effect of mobile phase composition on the binding kinetics of chiral solutes on a protein-based high-performance liquid chromatography column: interactions of D- and L-tryptophan with immobilized human serum albumin. J. Chromatogr. B. 1997;766:15–25. doi: 10.1016/s0021-9673(96)01040-0. [DOI] [PubMed] [Google Scholar]
- 163.Talbert AM, Tranter GE, Holmes E, Francis PL. Determination of drug-plasma protein binding kinetics and equilibria by chromatographic profiling: exemplification of the method using L-tryptophan and albumin. Anal. Chem. 2002;74:446–452. doi: 10.1021/ac010643c. [DOI] [PubMed] [Google Scholar]
- 164.Schiel JE, Ohnmacht CM, Hage DS. Measurement of drug-protein dissociation rates by high-performance affinity chromatography and peak profiling. Anal. Chem. 2009;81:4320–4333. doi: 10.1021/ac9000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tong Z, Schiel JE, Papastavros E, Ohnmacht CM, Smith QR, Hage DS. Kinetic studies of drug-protein interactions by using peak profiling and high-performance affinity chromatography: examination of multi-site interactions of drugs with human serum albumin columns. J. Chromatogr. A. 2011;1218:2065–2071. doi: 10.1016/j.chroma.2010.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tong Z, Hage DS. Characterization of interaction kinetics between chiral solutes and human serum albumin by using high-performance affinity chromatography and peak profiling. J. Chromatogr. A. 2011;1218:6892–6897. doi: 10.1016/j.chroma.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen J, Schiel JE, Hage DS. Noncompetitive peak decay analysis of drug-protein dissociation by high-performance affinity chromatography. J. Sep. Sci. 2009;32:1632–1641. doi: 10.1002/jssc.200900074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yoo MJ, Hage DS. Use of peak decay analysis and affinity microcolumns containing silica monoliths for rapid determination of drug-protein dissociation rates. J. Chromatogr. A. 2011;1218:2072–2078. doi: 10.1016/j.chroma.2010.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pfaunmiller E, Moser AC, Hage DS. Biointeraction analysis of immobilized antibodies and related agents by high-performance immunoaffinity chromatography. Methods. doi: 10.1016/j.ymeth.2011.08.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jozwiak K, Haginaka J, Moaddel R, Wainer I. Displacement and nonlinear chromatographic techniques in the investigation of interaction of noncompetitive inhibitors with an immobilized a3b4 nicotinic acetylcholine receptor liquid chromatographic stationary phase. Anal. Chem. 2002;74:4618–4624. doi: 10.1021/ac0202029. [DOI] [PubMed] [Google Scholar]
- 171.Jozwiak K, Hernandex SC, Kellar KJ, Wainer I. Enantioselective interactions of dextromethorphan and levomethorphan with the a3B4-nicotinic acetylcholine receptor: comparison of chromatographic and functional data. J. Chromatogr. B. 2003;797:373–379. doi: 10.1016/s1570-0232(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 172.Jozwiak K, Ravichandran S, Collins JR, Moaddel R, Wainer I. Interaction of noncompetitive inhibitors with the a3B2 Nicotinic acetylcholine receptor investigated by affinity chromatography and molecular docking. J. Med. Chem. 2007;50:6279–6283. doi: 10.1021/jm070784s. [DOI] [PubMed] [Google Scholar]
- 173.Jozwiak K, Ravichandran S, Collins JR, Wainer I. Interaction of noncompetitive inhibitors with an immobilized a3b4 nicotinic acetylcholine receptor investigated by affinity chromatography. Quantitative-Structure activity relationship analysis and molecular docking. J. Med. Chem. 2004;47:4008–4021. doi: 10.1021/jm0400707. [DOI] [PubMed] [Google Scholar]
- 174.Moaddel R, Jozwiak K, Yamaguchi R, Wainer IW. Direct chromatographic determination of dissociation rate constants of ligand-receptor complexes: assessment of the interaction of noncompetitive inhibitors with an immobilized nicotinic acetylcholine receptor-based Liquid chromatography stationary phase. Anal. Chem. 2005;77:5421–5426. doi: 10.1021/ac0504464. [DOI] [PubMed] [Google Scholar]
- 175.Moaddel R, Wainer I. Conformational mobility of immobilized proteins. J. Pharm. Biomed. Anal. 2007;43:399–406. doi: 10.1016/j.jpba.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 176.Friedberg F, Rhoads AR. Affinity chromatography of enzymes. In: Hage DS, editor. Handbook of Affinity Chromatography. Boca Raton, FL: CRC Press; 2005. pp. 313–346. [Google Scholar]
- 177.Bowers-Komro DM, Yamada Y, McCormick DB. Substrate specificity and variables affecting efficiency of mammalian flavin adenine dinucleotide synthesis. Biochemistry. 1989;28:8439–8844. doi: 10.1021/bi00447a025. [DOI] [PubMed] [Google Scholar]
- 178.Kuester M, Becker GL, Hardes K, Lindberg I, Steinmetzer T, Than ME. Purification of the proprotein convertase furin by affinity chromatography based on PC-specific inhibitors. Biol. Chem. 2011;392:973–981. doi: 10.1515/BC.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bartolini M, Cavrini V, Andrisano V. Characterization of resersible and pseudo-irreversible acetylcholinesterase inihibitors by means of an immobilized enzyme reactor. J. Chromatogr. A. 2007;1144:102–110. doi: 10.1016/j.chroma.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 180.Hodgson RJ, Besanger TR, Brook MA, Brennan JD. Inhibitor screening using immobilized enzyme reactor chromatography/mass spectrometry. Anal. Chem. 2005;77:7512–7519. doi: 10.1021/ac050761q. [DOI] [PubMed] [Google Scholar]
- 181.Kim HS, Wainer IW. The covalent immobilization of microsomal uridine diphospho-glucuronosyltransferase (UDPGT) : initial synthesis and characterization of an UDPGT immobilized enzyme reactor for the on-line study of glucuronidation. J. Chromatogr. B. 2005;823:158–166. doi: 10.1016/j.jchromb.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 182.Mancini F, Naldi M, Cavrini W, Andrisano V. Development and characterization of β-secretase monolithic micro-immobilized enzyme reactor for on-line high-performance liquid chromatography studies. J. Chromatogr. A. 2007;1175:217–226. doi: 10.1016/j.chroma.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 183.Labrou NE, Mazitsos K, Clonis YD. Dye-Ligand and biomimetic affinity chromatography. In: Hage DS, editor. Handbook of Affinity Chromatography. 2nd edition. Boca Raton, FL: CRC Press; 2005. pp. 231–255. [Google Scholar]
- 184.Denizli A, Pikin E. Dye-ligand affinity systems. J. Biochem. Biophys. Methods. 2001;49:391–416. doi: 10.1016/s0165-022x(01)00209-3. [DOI] [PubMed] [Google Scholar]
- 185.Labrou NE. Dye-ligand affinity chromatography for protein separation and purification. In: Walker JM, editor. Methods in Molecular Biology, Affinity Chromatography: Methods and Protocols. Totowa, NJ: Humana Press; 2000. pp. 129–139. [DOI] [PubMed] [Google Scholar]
- 186.Caspi S, Halimi M, Yanai A, Sasson SB, Taraboulos A, Gabizon R. The anti-prion activity of Congo Red. Putative mechanism. J. Biol. Chem. 1998;273:3484–3489. doi: 10.1074/jbc.273.6.3484. [DOI] [PubMed] [Google Scholar]
- 187.Hattori T, Zhang X, Weiss C, Xu Y, Kubo T, Sato Y, Nishikawa S, Sakaida H, Uchiyama T. Triazine dyes inhibit HIV-1 entry by binding to envelope glycoproteins. Microbiol. Immunol. 1997;41:717–724. doi: 10.1111/j.1348-0421.1997.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 188.Brown RA, Combridge BS. Binding of hepatitis virus particles to immobilized Procion Blue-HB and Cibacron Blue 3GA. J. Virol. Methods. 1986;14:267–274. doi: 10.1016/0166-0934(86)90028-5. [DOI] [PubMed] [Google Scholar]
- 189.Lowe CR. Combinatorial approaches to affinity chromatography. Curr. Opin. Chem. Biol. 2001;5:248–256. doi: 10.1016/s1367-5931(00)00199-x. [DOI] [PubMed] [Google Scholar]
- 190.Haginaka J. Monodispersed, molecularly imprinted polymers as affinity-based chromatography media. J. Chromatogr. B. 2008:3–13. doi: 10.1016/j.jchromb.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 191.Haupt K. Molecularly imprinted polymers: artificial receptors for affinity separations. In: Hage DS, editor. Handbook of Affinity Chromatography. 2nd edition. Boca Raton, FL: Taylor & Francis; 2006. pp. 837–856. [Google Scholar]
- 192.Oliveira HM, Segundo MA, Lima JLFC, Miro M, Cerda V. Exploiting automatic on-line renewable molecularly imprinted solid-phase extraction in lab-on-valve format as front end to liquid chromatography: application to the determination of riboflavin in foodstuffs. Anal. Bioanal. Chem. 2010;397:77–86. doi: 10.1007/s00216-010-3522-1. [DOI] [PubMed] [Google Scholar]
- 193.Boonjob W, Yu Y, Miro M, Segundo MA, Wang J, Cerda V. Online hyphenation of multimodal microsolid phase extraction involving renewable molecularly imprinted and reversed-phase sorbents to liquid chromatography for automatic multiresidue assays. Anal. Chem. 2010;82:3052–3060. doi: 10.1021/ac100185s. [DOI] [PubMed] [Google Scholar]
- 194.Romig TS, Bell C, Drolet DW. Aptamer affinity chromatography: combinatorial chemistry applied to protein purification. J. Chromatogr. B. 1999;731:275–284. [PubMed] [Google Scholar]
- 195.German I, Buchanan DD, Kennedy RT. Aptamers as ligands in affinity probe capillary electrophoresis. Anal. Chem. 1998;70:4540–4545. doi: 10.1021/ac980638h. [DOI] [PubMed] [Google Scholar]
- 196.Potyrailo RA, Conrad RC, Ellington AD, Hieftje GM. Adapting selected nucleic acid ligands (aptamers) to biosensors. Anal. Chem. 1998;70:3419–3425. doi: 10.1021/ac9802325. [DOI] [PubMed] [Google Scholar]
- 197.Xu Y, Yang X, Wang E. Review: Aptamers in microfluidic chips. Anal. Chim. Acta. 2010;683:12–20. doi: 10.1016/j.aca.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 198.Zhao Q, Li X-F, Shao Y, Le XC. Aptamer-based affinity chromatographic assays for thrombin. Anal. Chem. 2008;80:7586–7593. doi: 10.1021/ac801206s. [DOI] [PubMed] [Google Scholar]
- 199.Zhao Q, Li X-F, Le XC. Aptamer-modified monolithic capillary chromatography for protein separation and detection. Anal. Chem. 2008;80:3915–3920. doi: 10.1021/ac702567x. [DOI] [PubMed] [Google Scholar]
- 200.Mairal T, Oezalp VC, Sanchez PL, Mir M, Katakis I, O’Sullivan CK. Aptamers: molecular tools for analytical applications. Anal. Bioanal. Chem. 2008;390:989–1007. doi: 10.1007/s00216-007-1346-4. [DOI] [PubMed] [Google Scholar]