Abstract
Neuropeptides provide functional flexibility to microcircuits, their inputs and effectors by modulating pre- and postsynaptic properties and intrinsic currents. Recent studies have relied less on applied neuropeptide and more on their neural release. In rhythmically active microcircuits (central pattern generators, CPGs), recent studies show that neuropeptide modulation can activate particular activity patterns by organizing specific circuit motifs. Neuropeptides can also modify microcircuit output indirectly, by modulating circuit inputs. Recently elucidated consequences of neuropeptide modulation include changes in motor patterns and behavior, stabilization of rhythmic motor patterns and changes in CPG sensitivity to sensory input. One aspect of neuropeptide modulation that remains enigmatic is the presence of multiple peptide family members in the same nervous system and even the same neurons.
Introduction
Neuropeptides are pervasive intercellular signaling molecules that function as neurotransmitters, via paracrine actions, and/or as circulating hormones. With few exceptions [1,2], neuropeptides act via G protein-coupled receptors (GPCRs) and thereby cause relatively long-lasting changes in intrinsic and/or synaptic properties [3,4]. There is a growing literature documenting the ability of neuropeptides to modulate microcircuit output, either directly [4–11, 12••,13••,16•,18••,19••,20••,22••, 23••, 24] or by influencing inputs to these circuits [25–27,28••,29] (Fig. 1a). Neuropeptides thus contribute substantially to the multifunctional nature of microcircuits, enabling the generation of distinct output patterns when influenced by different neuromodulators [10,11,30–32]. Here we focus on recent work providing new insights into neuropeptide modulation of microcircuit output, particularly with respect to sensory (olfaction, proprioception) and rhythmic motor systems.
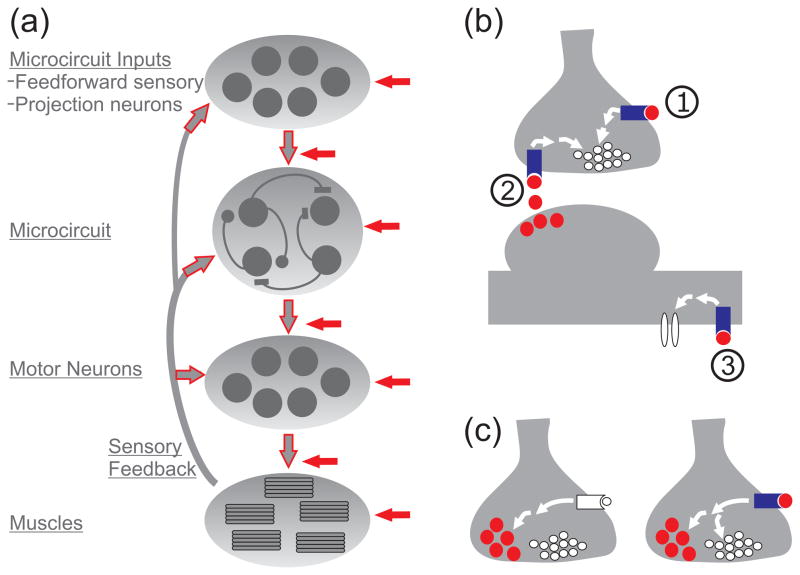
Figure 1.
Schematic illustrations showing that peptidergic modulation occurs at multiple sites. (a) Neuropeptides modulate the cellular and synaptic properties of neurons at every processing stage of microcircuits, including their feedforward (e.g. exteroceptors and projection neurons) and feedback (e.g. muscle sensory neurons) inputs, their effectors (e.g. motor neurons and muscles) and the microcircuit itself. These modulatory actions collectively alter the behavioral output of these systems. Red-filled arrows indicate sites of peptide modulation. Red outlined arrows indicate neurons that use neuropeptide transmitters. (b) Neuropeptides act presynaptically to alter transmitter release, commonly via second messenger systems (represented by white arrows) that are activated by neuropeptide binding to GPCRs. These neuropeptides (red circles) can be delivered from external inputs to a presynaptic site (site 1) or as retrograde messengers from the postsynaptic neuron (site 2). In addition to modulation of synaptic properties, neuropeptides can act via GPCRs to alter intrinsic currents (site 3). (c) Left, Metabotropic receptor activation can selectively regulate neuropeptide (red circles) release from a presynaptic terminal [39••]. Right, In addition, peptide binding to presynaptic GPCRs can regulate peptide release (and possibly small molecule transmitter release as well; white circles) [18••].
Presynaptic peptidergic actions
Many neuropeptide actions are shared with those mediated by metabotropic receptors for small molecule transmitters. For example, they all commonly act via GPCRs, enabling changes in the cellular and synaptic properties of target neurons. One shared site of action is the presynaptic terminal, where metabotropic actions are well-established to regulate neurotransmitter release [33] (Fig. 1b,c). It is important to note that transmitter release can also be regulated by postsynaptic activation of retrograde messengers, some of which are neuropeptides [34,35•] (Fig. 1b).
With respect to microcircuit operation, previous studies have primarily focused on presynaptic modulation of transmitter release by small molecule transmitters [29,36–38, 39••,40], with fewer studies establishing a comparable action by neuropeptides [5]. However, several recent papers document the ability of neuropeptides to presynaptically modulate neurotransmitter release in the microcircuit context [29,35•,41, 42•,43], particularly in the olfactory system.
Peptidergic presynaptic modulation plays multiple roles in the first-stage microcircuit in the olfactory system of both invertebrates (antennal lobe) and vertebrates (olfactory bulb) [18••,23••,29,44••,45,46,47••]. There is both up- and downregulation of synaptic transmission from olfactory receptor neurons (ORNs) onto 2nd order neurons as well as at later stages of processing within these olfactory microcircuits. These recent examples of peptidergic presynaptic modulation influence olfactory cue recognition and olfactory guided behaviors such as feeding, and identifying known conspecifics [18••,23••,29,44••,45,46,47••]. Yet more complexity will undoubtedly be identified in these circuits, as presaged by the recent finding that the fly antennal lobe contains additional peptides [48].
Modulation of neuropeptide release
Presynaptic modulation can in turn regulate peptidergic transmission (Fig. 1c). For example, presynaptic inhibition of neuropeptide release in a C. elegans ORN is pivotal to food searching and odor adaptation behaviors [18••]. This regulation, mediated by a (peptidergic) feedback synapse, limits the duration of ORN peptide release and thereby limits the local search for food and promotes odor adaptation. Whether this feedback inhibition also inhibits cotransmitter (glutamate) release was not determined, although one indirect measure of transmitter release (amplitude and duration of intracellular Ca2+ transients) suggests that glutamate release is also compromised.
Presynaptic modulation can also selectively inhibit peptidergic cotransmission (Fig. 1c). In the isolated crab stomatogastric nervous system (STNS), an identified muscle stretch-sensitive sensory neuron (GPR neuron) inhibits the axon terminals of a multi-transmitter projection neuron (MCN1), weakening MCN1 peptidergic modulation without altering the strength of its GABAergic transmission [39••]. MCN1 activity drives the gastric mill (chewing) rhythm, in vitro and in vivo [49,50]. The selective regulation of peptidergic cotransmission enables the sensory input to have a phase-specific influence on the gastric mill rhythm [39••]. The intracellular mechanism underlying this event is not known, but likely involves an aspect of the neuropeptide release process that is not shared with that for small molecule transmitter release [51–53].
Interestingly, Shakiryanova et al. [54•] recently established that synaptic neuropeptide release can be evoked from a Drosophila motor neuron in the absence of extracellular Ca2+ by octopamine (the arthropod equivalent of norepinephrine) application. Octopamine evokes this release via a parallel activation of cAMP-activated protein kinase and Ca2+ release from intracellular stores. Whether octopamine also enables extracellular Ca2+-independent release of the small molecule cotransmitter (glutamate) was not determined.
Modulating microcircuit organization and dynamics
Several novel insights into the circuit consequences of peptidergic modulation were recently elucidated in two well-defined CPG systems that generate rhythmic motor patterns underlying feeding-related movements in the mollusc Aplysia californica [55] and the STNS of crabs and lobsters [10,14]. The studies highlighted below focus on the acute actions of peptidergic modulation, but it is noteworthy that neuropeptides can also have longer-term actions such as facilitating recovery of CPG function after loss of all modulatory input by decentralization [16•,56].
Several recent studies in Aplysia reveal the organizational ability of single neuropeptides to select the set of participating CPG neurons and/or their relative influence on circuit output [19••,20••,57••]. For instance a single neurally-released peptide (SCP) selects which circuit neurons are activated by an input pathway, via parallel coordinated actions within the feeding CPG [57••]. Specifically, stimulating axons in the esophageal nerve (EN) configures the feeding network into the egestion state, by exciting egestion-specific neurons (modulatory neuron B65, CPG neuron B20) and inhibiting an ingestion-specific CPG neuron (B40). This egestion state results largely from the EN axons releasing SCP, whose parallel and serial modulatory actions on these neurons enable the activation of the egestion-specific neurons and inhibition of ingestion-specific interneurons [57••] (Fig. 2a).
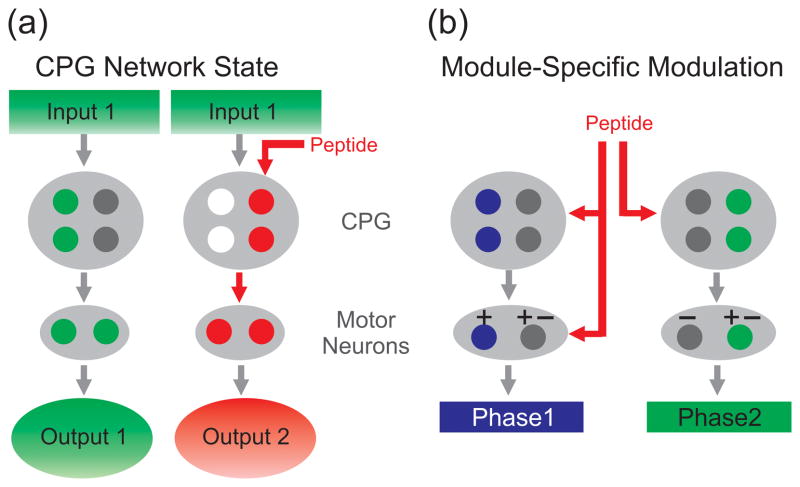
Figure 2.
Schematic illustrations representing peptidergic modulation of microcircuit output. (a) Peptidergic modulation of CPG neurons can select the set of active neurons and thereby establish a network state that determines the output elicited by a parallel input [57••]. Left, For example, Input 1 activates specific CPG neurons (green circles) to drive a particular motor neuron firing pattern (green) and elicit Output 1. Right, In contrast, in the presence of a particular neuropeptide, the same Input 1 now elicits a distinct output (Output 2) by activating different CPG neurons (red circles) while inhibiting the formerly active CPG neurons (white circles). To further ensure selection of Output 2, the peptide activates feedforward loops by enhancing excitatory and inhibitory synaptic actions from an “upstream” CPG neuron onto its “downstream” CPG targets (not shown; see [57••]). Symbols: Colored (red, green) circles, active neurons; grey circles, inactive neurons; white circles, inhibited by peptide input. (b) Peptidergic modulation can be module-specific [19••]. The left motor neuron receives alternating excitation (Phase 1) and inhibition (Phase 2) from the CPG during a two-phase rhythmic motor pattern. The activity of this motor neuron is directly modulated by a peptidergic input (red). The right motor neuron receives concurrent excitation and inhibition from the CPG during both phases of the rhythm. This motor neuron is indirectly modulated by the peptidergic modulation of CPG neurons. Symbols: +, excitation; −, inhibition; colored (blue, green) circles, active neurons; grey circles, inactive neurons.
Another type of peptide-mediated circuit reorganization in the Aplysia feeding system is based on the long-lasting actions of two co-released peptides (Fig. 2b) [19••]. The ability of neurally-released peptides to trigger long-lasting circuit effects is not new [27,58–62]. What is new is that there are module-specific effects of the neuropeptides released by the projection neuron CBI-2 [19••]. Specifically, CBI-2-released peptides (CP2: cerebral peptide 2; FCAP: feeding-circuit activating peptide) directly enhance the activity of a protraction motor neuron (B48) and indirectly, via modulation of the ingestion CPG, inhibit the activity of a retractor motor neuron (B8) (e.g. motor neuron 1 vs. motor neuron 2; Fig. 2b). The authors propose the novel organizational principle that this peptidergic modulation is module-specific to optimize control of each neuron based on their pattern of synaptic input. In this case, B48 receives alternating excitation (during protraction) and inhibition (during retraction), so its direct modulation can separately influence its excitability during each phase (Fig. 2b). In contrast, B8 receives concurrent excitation and inhibition during both phases, and during different feeding patterns it tends to be active during either protraction or retraction (Fig. 2b). A phase specific B8 activity pattern is less likely to be supported by direct modulation of B8, as such modulation would likely influence its excitability during both phases. Both modes of synaptic regulation (concurrent vs alternating excitation and inhibition) are established in many different motor systems, but it remains to be determined whether direct vs. indirect modulation is module-specific as in the Aplysia ingestion motor program.
Distinct CPG outputs can also result from changes upstream to the CPG. In the crab STNS, two different extrinsic inputs (VCNs, POC neurons) trigger long-lasting but distinct gastric mill (chewing) rhythms by coactivating the same projection neurons (MCN1, CPN2) to drive the gastric mill CPG in the stomatogastric ganglion (STG) [27,63,64•]. The persistent influence of the POC neurons on these projection neurons results from its release of the tachykinin-related peptide CabTRP Ia [27]. The core rhythm generator underlying both gastric mill rhythms is composed of the same neurons, supporting the hypothesis that the distinct rhythms result entirely from differences in upstream modulation of the projection neurons [64•,65].
In the Aplysia feeding system, the same neuropeptide (ATRP: allatotropin-related peptide), via its release from an identified projection neuron (CBI-4) [66] and a motor neuron, provides feedforward compensation (Fig. 3a). This configuration results from ATRP coordinately altering the ingestion CPG (e.g. reducing protraction duration) and causing a compensatory strengthening of the associated protraction motor neurons (increasing their firing rate) and protraction muscles (strengthening contractions and increasing relaxation rate) [20••]. The ATRP actions on the protraction muscles result from its corelease, with the neuropeptide myomodulin, from protraction motor neurons [20••]. Without the compensatory strengthening at the motor neuron and muscle levels, a shorter protraction duration would weaken the protraction muscle movements (Fig. 3a). Thus, ATRP released coordinately at central and peripheral sites stabilizes the strength of the ingestive behavior during cycle frequency changes that would otherwise compromise the behavior.
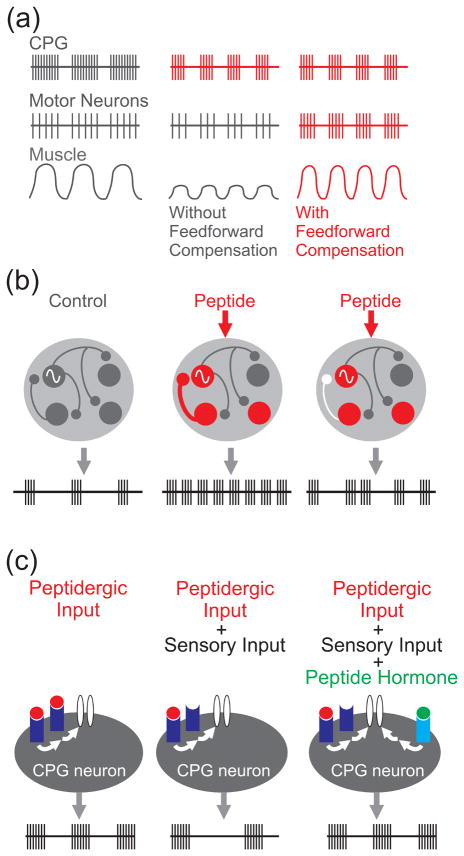
Figure 3.
Schematic illustrations of the functional consequences of neuropeptide modulation. (a) Left, Middle: Peptidergic modulation (red) solely to the CPG increases the rhythm frequency, shortening the duration of CPG neuron activity during each cycle. This in turn causes fewer motor neuron action potentials per cycle and weaker muscle contractions. Right, The addition of peripheral modulation by the same peptide (released by a motor neuron) provides feedforward compensation. In this latter case, there is a faster motor neuron firing frequency and a strengthened muscle response to motor neuron input, maintaining behaviorally-appropriate muscle contractions despite the shorter duration of this phase of the motor pattern [20••]. (b) Peptidergic modulation of a CPG feedback synapse is necessary for reducing the variability in the cycle duration during rhythmic motor activity [70••,71]. Left, Middle: Peptidergic modulation (red) increases the frequency of rhythmic output relative to control. Right, When the peptide-enhanced feedback synapse is selectively suppressed (white synapse), the peptide modulated intrinsic properties of circuit neurons maintain the faster rhythm, but the cycle duration is more variable. Sine wave indicates endogenously oscillatory pacemaker neuron. (c) Peptidergic modulation gates out sensory feedback [39••]. Left, A modulatory input uses peptide transmitter (red circle) binding to its receptor (blue) to activate an intrinsic current in a CPG neuron. This modulation helps generate a particular rhythmic motor pattern in the isolated nervous system. Middle, The inclusion of sensory feedback decreases the release of this peptide via presynaptic inhibition, slowing activation of the intrinsic current and thereby slowing the rhythm. Right, Modulation by a peptide hormone (green circle) gates out the influence of the sensory input, stabilizing the motor pattern and decreasing its sensitivity to perturbation. This is accomplished by the actions of the peptide hormone converging onto the same intrinsic current activated by the peptidergic input in the circuit neuron, albeit via a different receptor (Iight blue) [13••,28••].
Feedforward compensation also underlies other coordinated movements, such as those mediating anticipatory postural adjustments, but the detailed cellular mechanisms underlying these latter events remain to be determined [67]. There are still few systems where network neurons are identified at multiple functional levels (e.g. projection, microcircuit, motor and sensory neurons), including their cotransmitter complements and synaptic actions. It therefore remains to be determined whether the coordinated actions of a single neuropeptide at multiple network levels, as in the Aplysia feeding system, is happenstance or is itself a new organizing principle. One additional system in which serial peptidergic actions are known to occur is the crab STNS, where the aforementioned POC neurons and the projection neuron MCN1 both release CabTRP Ia to trigger (POC neurons) and drive (MCN1) the gastric mill rhythm [27,59,68,69]. However, it is not known if there is a functional relationship between these separate CabTRP Ia sources analogous to those involving ATRP in Aplysia.
Two recent studies in the crab STNS also show that peptide modulation can directly modify CPG dynamics without changing the set of participating neurons or substantially changing the associated motor pattern. Instead, these peptide actions either stabilize the motor pattern [70••] or alter the CPG sensitivity to a parallel perturbation [13••,28••].
The peptide proctolin modulates the strength and short-term dynamics of the sole (inhibitory) CPG feedback synapse onto the pyloric pacemaker neurons in the crab STG [70••] (Fig. 3b). Further, although proctolin directly excites most pyloric circuit neurons [49], its presynaptic modulation of this feedback synapse alone is necessary and sufficient for the proctolin-mediated stabilization of the pyloric rhythm [70••,71]. This stability-enhancing feature occurred without a concomitant alteration of the pyloric motor pattern. Modulation of this same synapse by another neuropeptide (RPCH) was previously proposed to also stabilize the pyloric rhythm [72].
The Zhao et al. [70••] study used bath-applied proctolin, but their results likely reflect the influence of neurally-released proctolin insofar as the other, aforementioned proctolin influences on the pyloric CPG are equivalent to those mediated by the proctolinergic projection neuron MPN [58]. Interestingly, this equivalence occurs despite the fact that MPN contains cotransmitters, a priori suggesting that its influence on the pyloric CPG would result from the collective actions of these cotransmitters [49,59,73].
Peptide modulation can also modify circuit dynamics by influencing intrinsic currents, as shown in the crab gastric mill CPG [10,14] (Fig. 3c). The gastric mill rhythm is a two-phase motor pattern (protraction, retraction) whose core CPG includes reciprocal inhibition between the protractor neuron LG and retractor neuron Int1 [74,75]. During gastric mill rhythms driven by the projection neuron MCN1, the peptide hormone CCAP selectively albeit modestly increases the protraction phase duration [76]. This CCAP action results from its activating a single ionic current (IMI) in the LG neuron, which is the same current activated in LG by the MCN1-released peptide CabTRP Ia (Fig. 3c) [13••].
Surprisingly, the dynamics of the MCN1- and CCAP-activated IMI in LG are distinct during the gastric mill rhythm, because only MCN1-activated IMI is weakened by feedback inhibition during each protraction phase [13••,77]. Consequently, during protraction the declining influence of MCN1-activated IMI is buoyed for a time by the small but maintained influence of CCAP-activated IMI [13••]. Additionally, the unchanged retraction phase duration results not from a lack of CCAP influence but from CCAP-activated IMI in LG preventing an increase in retraction duration [13••]. Without access to these mechanisms, inaccurate conclusions would likely have been drawn (e.g. the unchanged retraction duration resulted from CCAP being ineffective during this phase). Such inaccurate conclusions in turn would have compromised the subsequent insights attained with respect to the state-dependent influence of this CCAP action (see below).
Whereas the CCAP-mediated changes in the IMI dynamics in LG only modestly alter the gastric mill rhythm in the isolated STNS, these altered dynamics gate out the influence of the aforementioned GPR sensory neuron [28••] (Fig. 3c). Using a dynamic clamp version of CCAP-activated IMI to either add or subtract (in the presence of CCAP) IMI in LG, DeLong and Nusbaum [28••] showed that the CCAP influence on LG is necessary and sufficient to eliminate this sensory feedback action. Thus, the CCAP modulation of an intrinsic current in a CPG neuron mediates the state-dependence of sensory regulation of circuit output.
Context-dependent modulation was also recently established for substance P in the mammalian respiratory CPG [12••]. Specifically, substance P excitation of the respiratory rhythm in the medullary pre-Bötzinger complex is modest during co-activation of noradrenergic and/or serotonergic input pathways, but its influence is pivotal when these parallel inputs are silent. Although the points of convergence in these separate actions remain to be determined, it is likely that the substance P influence becomes critical during sleep when the parallel aminergic pathways are weakly active or silent [12••].
Neuropeptide families
Neuropeptides differ from other metabotropic-acting transmitters in that they commonly are members of extended families, wherein family members often exhibit only small differences in their amino acid sequences [73,78–82]. Additionally, multiple peptide family members are often found in the same nervous system, and at least sometimes likely colocalize to the same neurons [83–85,86•]. In the STNS, separate application of different peptide family members have indistinguishable actions on the pyloric and gastric mill rhythms [73,87,88]. Interestingly, the influence of two different RFamide peptide families (FRFamides, FMRFamide) in Aplysia have distinct (but complementary) actions on the ingestion motor pattern [86•]. When the focus narrows to a single muscle target of the FRFamides, some family members have quantitatively equivalent actions while the dose-response curve for others is shifted [86•]. These observations raise questions, and hypotheses, regarding the purpose of colocalizing peptide family members.
Recent work in insects provides some insight by revealing that a single neuropeptide-activated GPCR can (a) differentially activate separate intracellular signaling systems when it is bound by different peptide family members [89,90] or (b) respond to only some family members [91•]. In the stable fly Stomxys calcitrans, an insect tachykinin (TK) receptor (STKR) has different relative influences on two separate signaling pathways when it is challenged with insect TK peptides containing an alanine vs. glycine residue near their C-terminal core motif [89]. In contrast, there is no such dichotomy for the Drosophila ortholog of STKR, called DTKR [90]. However, the other Drosophila tachykinin receptor (NKD) shares the STKR selectivity and responds only to the one Drosophila TK, of the six identified DTKs, with the alanine residue in its C-terminal core motif [91•].
Another degree of freedom available to peptide families results from the presence of different GPCRs in a single cell that can bind the same neuropeptide [42•]. The presence of multiple receptors binding the same neuropeptide in a given nervous system, and particularly in a single neuron, provides the possibility that different peptide family members could have different affinities for each receptor type and thereby alter cellular physiology in distinct ways.
The above possibilities, however, are not sufficient to explain why different peptide family members have comparable actions on CPGs. It may be that some differences in amino acid sequences among peptide family members serve no distinct function and are simply “tolerated” by the system. Alternatively, it may be that different family members have overlapping but distinct actions, but the method of analysis (bath application) does not mimic the peptide actions when they are neurally-released and thus the differences between their actions are masked. For example, bath application might overwhelm the extracellular peptidases that normally limit peptide actions, and a given peptidase might not be equally effective at cleaving all peptide family members [68,92–94]. The persistent presence of the applied peptide during bath application, even at an appropriate concentration, might also enable the occurrence of relatively low affinity peptide-receptor binding events that do not occur during the more transient presence resulting from neural release.
Conclusions and Future Directions
Studies of peptidergic actions are providing new insights regarding the flexibility intrinsic to microcircuits. It is heartening that many of these new insights result from the neural release of peptides instead of their bath application. Bath application is a valuable tool for studying peptide modulation and in some situations is an appropriate and effective mimic of neural release, but this is not always the case (e.g. peptides are often coreleased with other transmitters). The consequences of cotransmission for microcircuits are established in only a few contexts, and promises to be a significant growth industry in the coming years. It is also likely that the number of degrees of freedom provided to microcircuits by peptidergic modulation will continue to grow, particularly as more effective tools become available to understand the influence of coreleased members of the same peptide family and the ability of peptide receptors to distinguish among them.
Highlights.
Neuropeptides contribute to the extensive functional flexibility of microcircuits
Modulation of synaptic and intrinsic circuit properties alters circuit output
Circuit output is also altered indirectly via modulation of circuit inputs
Circuit modules can be differentially modulated by the same neuropeptide
Functional consequences include stabilization at circuit and effector levels
Acknowledgments
The work in the Nusbaum lab is supported by NIH grant NS29436. The work in the Blitz lab is supported by NSF grant IOS-1153417.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Lingueglia E, Deval E, Lazdunski M. FMRFamide-gated sodium channel and ASIC channels: a new class of ionotropic receptors for FMRFamide and related peptides. Peptides. 2006;27:1138–1152. doi: 10.1016/j.peptides.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 2.Dürrnagel S, Kuhn A, Tsiairis CD, Williamson M, Kalbacher H, Grimmelikhuijzen CJP, Holstein TW, Gründer S. Three homologous subunits form a high affinity peptide-gated ion channel in Hydra. J Biol Chem. 2010;285:11958–11965. doi: 10.1074/jbc.M109.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salio C, Lossi L, Ferrini F, Merighi A. Neuropeptides as synaptic transmitters. Cell Tissue Res. 2006;326:583–598. doi: 10.1007/s00441-006-0268-3. [DOI] [PubMed] [Google Scholar]
- 4.Nässel DR. Neuropeptide signaling near and far: how localized and timed is the action of neuropeptides in brain circuits? Invert Neurosci. 2009;9:57–75. doi: 10.1007/s10158-009-0090-1. [DOI] [PubMed] [Google Scholar]
- 5.Parker D, Grillner S. Cellular and synaptic modulation underlying substance P-mediated plasticity of the lamprey locomotor network. J Neurosci. 1998;18:8095–8110. doi: 10.1523/JNEUROSCI.18-19-08095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svensson E, Grillner S, Parker D. Gating and braking of short- and long-term modulatory effects by interactions between colocalized neuromodulators. J Neurosci. 2001;21:5984–5992. doi: 10.1523/JNEUROSCI.21-16-05984.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nusbaum MP. Regulating peptidergic modulation of rhythmically active neural circuits. Brain Behav Evol. 2002;60:378–87. doi: 10.1159/000067791. [DOI] [PubMed] [Google Scholar]
- 8.Fort TJ, Brezina V, Miller MW. Regulation of the crab heartbeat by FMRFamide-like peptides: multiple interacting effects on center and periphery. J Neurophysiol. 2007;98:2887–2902. doi: 10.1152/jn.00558.2007. [DOI] [PubMed] [Google Scholar]
- 9.Jing J, Vilim FS, Horn CC, Alexeeva V, Hatcher NG, Sasaki K, Yashina I, Zhurov Y, Kupfermann I, Sweedler JV, et al. From hunger to satiety: reconfiguration of a feeding network by Aplysia neuropeptide Y. J Neurosci. 2007;27:3490–3502. doi: 10.1523/JNEUROSCI.0334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- 11.Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol. 2008;164:96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Doi A, Ramirez J-M. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci. 2010;30:8251–8262. doi: 10.1523/JNEUROSCI.5361-09.2010. In vivo recordings in anesthetized mice, and in vitro recordings from ventral medullary brain slices through the Pre-Bötzinger complex, show that modulation of respiration by endogenously released substance P, via activation of neurokinin-1 (NK-1) receptors, is blunted or absent during coactivation of receptors for other modulatory pathways [e.g. serotonin (5-HT2) or noradrenergic (α1 NE) receptors]. In contrast, during relatively slow respiratory rhythms, such as when these parallel inputs are weakly active or silent (e.g. during sleep), NK-1 receptor activation is critical to maintain breathing. This state-dependent modulation by substance P highlights the importance of studying modulatory actions under multiple conditions. It remains to be determined whether the blunted influence of substance P truly represents no influence (e.g. its actions are occluded by 5-HT2 and/or α1 NE receptor activation) or if instead it produces a latent alteration in network dynamics that becomes evident under another condition (a different, parallel input) not yet examined (see [28••]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.DeLong ND, Kirby MS, Blitz DM, Nusbaum MP. Parallel regulation of a modulator-activated current via distinct dynamics underlies comodulation of motor circuit output. J Neurosci. 2009;29:12355–12367. doi: 10.1523/JNEUROSCI.3079-09.2009. Voltage- and dynamic clamp manipulations of a pivotal gastric mill CPG neuron (LG neuron) in the isolated crab stomatogastric ganglion (STG) show that the same current (IMI: modulator-activated, voltage-dependent inward current) in LG is activated by a neurally-release peptide (CabTRP Ia release by the projection neuron MCN1) and a circulating peptide hormone (CCAP). However, the dynamics of MCN1- and CCAP-activated IMI in LG are distinct during the MCN1-driven gastric mill rhythm. Specifically, during this rhythm only the MCN1-activated current is phasically weakened, by feedback inhibition of the MCN1 axon terminals. These distinct dynamics enable CCAP-activated IMI to (a) selectively, but only modestly (~10–15%) prolong the gastric mill protractor phase by slowing the overall decay rate of IMI during protraction and (b) compensate for a decreased amount of MCN1-activated IMI during retraction to prevent a prolongation of this phase. These altered dynamics have little impact on the basic motor pattern, but see [28••] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein W. Modulation of stomatogastric rhythms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195:989–1009. doi: 10.1007/s00359-009-0483-y. [DOI] [PubMed] [Google Scholar]
- 15.Thörn Pérez C, Hill RH, El Manira A, Grillner S. Endocannabinoids mediate tachykinin-induced effects in the lamprey locomotor network. J Neurophysiol. 2009;102:1358–1365. doi: 10.1152/jn.00294.2009. [DOI] [PubMed] [Google Scholar]
- 16•.Zhang Y, Khorkova O, Rodriguez R, Golowasch J. Activity and neuromodulatory input contribute to the recovery of rhythmic output after decentralization in a central pattern generator. J Neurophysiol. 2009;101:372–386. doi: 10.1152/jn.01290.2007. In the isolated STNS, complete removal of descending neuromodulatory input to the STG (i.e. decentralization) initially terminates the spontaneously active pyloric rhythm. However, the pyloric CPG eventually recovers its ability to spontaneously generate the pyloric rhythm, despite the continued absence of modulatory input. In this follow-up study, the authors find that several types of manipulations prior to decentralization, including bath application of the neuropeptide proctolin, significantly reduce the latency to onset of the recovery process. Interestingly, these long-term proctolin actions appear to be distinct from its acute modulatory influence on the pyloric rhythm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Mabrouk F, Tryba AK. Substance P modulation of TRPC3/7 channels improves respiratory rhythm regularity and ICAN-dependent pacemaker activity. Eur J Neurosci. 2010;31:1219–1232. doi: 10.1111/j.1460-9568.2010.07156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Chalasani SH, Kato S, Albrecht DR, Nakagawa T, Abbott LF, Bargmann CI. Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci. 2010;13:615–621. doi: 10.1038/nn.2526. In a C. elegans sensorimotor circuit that drives two food searching-related turning behaviors, the authors use neuron-specific genetic manipulations, Ca2+ imaging and behavioral analysis to identify a peptidergic feedback synapse that regulates the duration of these behaviors at an early processing stage. Specifically, the AWC olfactory receptor neuron responds to food-related olfactory stimuli and initiates food searching behaviors using (at least partly) divergent cotransmitter (glutamate, NLP-1 peptide) actions on its target neurons. Overall, its glutamatergic signaling is necessary for turning behaviors while its peptidergic signaling limits the duration of these behaviors. One of its peptide targets (AIA interneuron) is shown to have an inhibitory peptidergic (INS peptide) feedback action on the AWC neuron that is responsible for limiting the duration of the turning behaviors. At the cellular level, this inhibition limits the duration of the odor-mediated increase in intracellular Ca2+ concentration in AWC, which is likely directly responsible for limiting the duration of NLP-1 (and presumably glutamate) release and, hence, the duration of the turning behaviors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Friedman AK, Weiss KR. Repetition priming of motoneuronal activity in a small motor network: intercellular and intracellular signaling. J Neurosci. 2010;30:8906–8919. doi: 10.1523/JNEUROSCI.1287-10.2010. Repetitively stimulating the peptidergic CBI-2 projection neuron enables it to have long-lasting modulatory actions on the Aplysia ingestion CPG. This repetition priming effect directly or indirectly regulates the activity of particular motor neurons based on whether their excitatory and inhibitory synaptic inputs from the ingestion CPG are alternating (direct modulation: e.g. B48 motor neuron) or concurrent (indirect modulation: e.g. B8 motor neuron). In line with these distinct synaptic input patterns, B48 is a dedicated protraction neuron while B8 can be active during protraction or retraction, depending on the balance of concurrent excitation and inhibition as dictated by how each modulatory input (e.g. CBI-2) configures the CPG. The authors hypothesize that these direct vs. indirect modulatory pathways for regulating the motor neurons is an organizational feature that optimizes phase-specific actions on the basis of whether a given neuron is driven by alternating vs. concurrent excitation and inhibition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Jing J, Sweedler JV, Cropper EC, Alexeeva V, Park J-H, Romanova EV, Xie F, Dembrow NC, Ludwar BC, Weiss KR, et al. Feedforward compensation mediated by the central and peripheral actions of a single neuropeptide discovered using representational difference analysis. J Neurosci. 2010;30:16545–16558. doi: 10.1523/JNEUROSCI.4264-10.2010. A novel neuropeptide (ATRP) is identified in the Aplysia CNS and localized to specific neurons, including the projection neuron CBI-4 and protraction motor neurons. ATRP speeds up the ingestion motor pattern by reducing protraction duration, an action documented previously for CBI-4 stimulation. Simply reducing protraction duration, however, would compromise the ingestion behavior by weakening the protraction movement, due to the relatively slow contraction rate of the protractor muscles. However, CBI-4 and ATRP also increase protraction motor neuron firing rate, which enables these motor neurons to maintain muscle contraction strength by increased release of its cotransmitters (ACh, which directly causes muscle contractions; ATRP which strengthens contraction; myomodulin peptide, which increases relaxation rate). These parallel central and peripheral ATRP actions provide a feedforward compensation of contraction strength that enables the motor pattern to cycle faster while stabilizing its behavioral effectiveness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai T, Abe H, Akazome Y, Oka Y. Neuromodulatory effect of GnRH on the synaptic transmission of the olfactory bulbar neural circuit in goldfish, Carassius auratus. J Neurophysiol. 2010;104:3540–3550. doi: 10.1152/jn.00639.2010. [DOI] [PubMed] [Google Scholar]
- 22••.Lepousez G, Mouret A, Loudes C, Epelbaum J, Viollet C. Somatostatin contributes to in vivo gamma oscillation modulation and odor discrimination in the olfactory bulb. J Neurosci. 2010;30:870–875. doi: 10.1523/JNEUROSCI.4958-09.2010. In vivo pharmacological manipulations, knock-out mice, field potential recordings and behavioral experiments are used to show that the peptide somatostatin (SST) is an intrinsic modulator in the rodent olfactory bulb. SST is localized to a subset of GABAergic interneurons in the external plexiform layer, and SST-2 type receptors are present on mitral cell dendrites. SST is also shown to regulate odor discrimination performance, likely by enhancing odor-activated, mitral cell-mediated gamma oscillation amplitude. The latter effect appeared to result from SST weakening the strength of the mitral cell excitation of granule cells at their dendro-dendritic synapses, which in turn weakens the subsequent granule cell inhibition of the mitral cell. The results support the hypothesis that SST regulates odor-mediated gamma oscillation amplitude and, hence, odor discrimination by its weakening mitral cell transmitter release (or possibly down-regulation of mitral cell GABA receptors) at the dendro-dendritic synapses between mitral- and granule cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Tobin VA, Hashimoto H, Wacker DW, Takayanagi Y, Langnaese K, Caquineau C, Noack J, Landgraf R, Onaka T, Leng G, et al. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature. 2010;464:413–417. doi: 10.1038/nature08826. Social recognition memory in rats is regulated by vasopressin (VP) peptide modulation via V1a receptors in the olfactory bulb (OB). V1a receptors localize to many cell types within the OB, including periglomerular and mitral cells. The VP-containing neurons influencing social recognition memory are local interneurons in the external plexiform layer, similar to the SST-containing neurons in [22••], but unlike these latter neurons the VP neurons do not colocalize with GABA. Pharmacological and molecular (e.g. siRNA) manipulations establish that VP facilitates social recognition memory but not other, unrelated behaviors. In vivo recordings in anesthetized rats show that VP inhibits the mitral cell response to odors, although it remains to be determined whether this action is direct and/or upstream of the mitral cell. VP therefore acts to filter social cue-specific olfactory excitation of mitral cells, enabling recognition of recently visited conspecifics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagenaar DA, Hamilton MS, Huang T, Kristan WB, French KA. A hormone-activated central pattern generator for courtship. Curr Biol. 2010;20:487–495. doi: 10.1016/j.cub.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blitz DM, Nusbaum MP. Distinct functions for cotransmitters mediating motor pattern selection. J Neurosci. 1999;19:6774–6783. doi: 10.1523/JNEUROSCI.19-16-06774.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brocard F, Bardy C, Dubuc R. Modulatory effect of substance P to the brain stem locomotor command in lampreys. J Neurophysiol. 2005;93:2127–2141. doi: 10.1152/jn.00401.2004. [DOI] [PubMed] [Google Scholar]
- 27.Blitz DM, White RS, Saideman SR, Cook A, Christie AE, Nadim F, Nusbaum MP. A newly identified extrinsic input triggers a distinct gastric mill rhythm via activation of modulatory projection neurons. J Exp Biol. 2008;211:1000–1011. doi: 10.1242/jeb.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.DeLong ND, Nusbaum MP. Hormonal modulation of sensorimotor integration. J Neurosci. 2010;30:2418–2427. doi: 10.1523/JNEUROSCI.5533-09.2010. Computational modeling and dynamic clamp manipulations that test model predictions in the isolated crab STG show that the peptide hormone CCAP has a state-dependent action on the MCN1-driven gastric mill rhythm. Specifically, it causes only a modest change in the basic rhythm [76], but it gates out the rhythm-altering influence of an identified sensory feedback pathway (GPR neurons). The sensory feedback acts via metabotropic inhibition of the axon terminals of MCN1 to selectively reduce MCN1 peptide cotransmission [39••]. Surprisingly, the CCAP gating action does not involve modulation of GPR, its synapses onto MCN1 or the MCN1 axon terminals. Instead, it acts downstream, on a CPG neuron (LG neuron) where it activates the same voltage-dependent current (IMI) as MCN1-released peptide (CabTRP Ia) and thereby compensates for the GPR-mediated reduction in peptide release by MCN1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang JW. Presynaptic modulation of early olfactory processing in Drosophila. Dev Neurobiol. 2012;72:87–99. doi: 10.1002/dneu.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- 31.Dickinson PS. Neuromodulation of central pattern generators in invertebrates and vertebrates. Curr Opin Neurobiol. 2006;16:604–614. doi: 10.1016/j.conb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Harris-Warrick RM. Neuromodulation and flexibility in central pattern generator networks. Curr Opin Neurobiol. 2011;21:685–692. doi: 10.1016/j.conb.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Jong APH, Verhage M. Presynaptic signal transduction pathways that modulate synaptic transmission. Curr Opin Neurobiol. 2009;19:245–253. doi: 10.1016/j.conb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–170. doi: 10.1016/j.neuron.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Iremonger KJ, Kuzmiski JB, Baimoukhametova DV, Bains JS. Dual regulation of anterograde and retrograde transmission by endocannabinoids. J Neurosci. 2011;31:12011–12020. doi: 10.1523/JNEUROSCI.2925-11.2011. An elaborate local circuit is revealed involving the dendrites of vasopressin (VP) neurons and their glutamatergic inputs in the paraventricular nucleus of the rat hypothalamus. In response to coincident pre- and postsynaptic firing, the VP neurons exhibit dendritic release of endocannabinoids (eCBs) which feedback to inhibit glutamate release by the presynaptic neuron. This eCB action prevents the occurrence of long-term depression (LTD) of this glutamatergic synapse. When eCBs are not released, sufficient glutamate is released to activate postsynaptic mGluRs which in turn trigger dendritic release of the opioid peptide dynorphin from the same dendrites that release eCBs. Dynorphin then feeds back to the presynaptic terminal to mediate LTD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapman RJ, Issberner JP, Sillar KT. Group I mGluRs increase locomotor network excitability in Xenopus tadpoles via presynaptic inhibition of glycinergic neurotransmission. Eur J Neurosci. 2008;28:903–913. doi: 10.1111/j.1460-9568.2008.06391.x. [DOI] [PubMed] [Google Scholar]
- 37.El Manira A, Kyriakatos A, Nanou E, Mahmood R. Endocannabinoid signaling in the spinal locomotor circuitry. Brain Res Rev. 2008;57:29–36. doi: 10.1016/j.brainresrev.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 38.Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.DeLong ND, Beenhakker MP, Nusbaum MP. Presynaptic inhibition selectively weakens peptidergic cotransmission in a small motor system. J Neurophysiol. 2009;102:3492–3504. doi: 10.1152/jn.00833.2009. Electrophysiological (including dynamic clamp) manipulations and computational modeling in the isolated crab STG show that the GPR muscle stretch-sensitive sensory neuron causes a 5HT-mediated, slow inhibition in the axon terminals of the projection neuron MCN1 that selectively inhibits peptidergic cotransmission from MCN1. This sensory feedback action slows MCN1-gastric mill rhythm by selectively prolonging the retraction phase. GPR acts by slowing the rate of MCN1 peptide release, without altering its GABAergic cotransmission. Modeling and experimental inhibition of both cotransmitter actions are shown to instead shorten the gastric mill protraction phase, resulting in a faster gastric mill rhythm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaudry Q, Kristan WB. Behavioral choice by presynaptic inhibition of tactile sensory terminals. Nat Neurosci. 2009;12:1450–1457. doi: 10.1038/nn.2400. [DOI] [PubMed] [Google Scholar]
- 41.Deng P-Y, Xiao Z, Jha A, Ramonet D, Matsui T, Leitges M, Shin H-S, Porter JE, Geiger JD, Lei S. Cholecystokinin facilitates glutamate release by increasing the number of readily releasable vesicles and releasing probability. J Neurosci. 2010;30:5136–5148. doi: 10.1523/JNEUROSCI.5711-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Klose MK, Dason JS, Atwood HL, Boulianne GL, Mercier AJ. Peptide-induced modulation of synaptic transmission and escape response in Drosophila requires two G-protein-coupled receptors. J Neurosci. 2010;30:14724–14734. doi: 10.1523/JNEUROSCI.3612-10.2010. A member of the FMRFamide peptide family in Drosophila (DPKQDFMRFamide) is shown to act at both the cellular and behavioral levels by coactivating two different GPCRs (Fmrf Receptor, Dromyosuppressin-2 Receptor), both of which are necessary for these actions. At the cellular level, this peptide acts through both GPCRs in the same cell to enhance transmitter release by eliciting Ca2+ release from intracellular stores. It remains to be determined whether the necessity of both GPCRs results from them acting separately or perhaps as heterodimers. It is also not yet known if the 13 identified members of this peptide family in Drosophila each coactivate these two GPCRs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu Z, Pym ECG, Babu K, Vashlishan Murray AB, Kaplan JM. A neuropeptide-mediated stretch response links muscle contraction to changes in neurotransmitter release. Neuron. 2011;71:92–102. doi: 10.1016/j.neuron.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Ignell R, Root CM, Birse RT, Wang JW, Nässel DR, Winther AME. Presynaptic peptidergic modulation of olfactory receptor neurons in Drosophila. Proc Natl Acad Sci USA. 2009;106:13070–13075. doi: 10.1073/pnas.0813004106. In the Drosophila antennal lobe, some local interneurons contain 5 of the 6 identified Drosophila tachykinin peptides (DTK 1–5) while the axon terminals of olfactory receptor neurons (ORNs) contain a DTK receptor (DTKR). The location of the DTKs and DTKR suggest that this peptide acts as a feedback signal to regulate incoming olfactory information. Experimental manipulations (i.e. local DTK application to the antennal lobe, targeted RNAi of DTKR or over-expression of DTKR in ORNs) indicate that DTKs mediate presynaptic inhibition of transmitter release from ORN terminals, acting at least partly by reducing the odorant-activated increase in intracellular Ca2+. These manipulations also alter the fly behavioral response to a particular odorant. Interestingly, the DTK action was only detected behaviorally at high odorant concentrations, suggesting that it acts as a break to overstimulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glauser DA, Goodman MB. Neuropeptides strike back. Nat Neurosci. 2010;13:528–529. doi: 10.1038/nn0510-528. [DOI] [PubMed] [Google Scholar]
- 46.Leinwand SG, Chalasani SH. Olfactory networks: from sensation to perception. Curr Opin Genet Dev. 2011;21:806–811. doi: 10.1016/j.gde.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 47••.Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–144. doi: 10.1016/j.cell.2011.02.008. The neuropeptide sNPF as well as the sNPF receptor (sNPFR1) localize to the axon terminals of some ORNs in the Drosophila antennal lobe. This peptidergic pathway causes presynaptic facilitation of transmitter release from ORNs. This synaptic facilitation is pivotal to promoting feeding behavior during starvation, during which time there is increased expression of sNPFR1. The sNPFR1 levels are regulated by insulin, and insulin levels are low during starvation. In contrast, during satiation, insulin levels are high and sNPFR1 levels are reduced, as is sNPF influence on ORN transmission. Genetic manipulation of sNPFR1 levels result in flies that behave as if they are starved (artificially high sNPFR1 levels) or satiated (artificially low sNPFR1 levels) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlsson MA, Diesner M, Schachtner J, Nässel DR. Multiple neuropeptides in the Drosophila antennal lobe suggest complex modulatory circuits. J Comp Neurol. 2010;518:3359–3380. doi: 10.1002/cne.22405. [DOI] [PubMed] [Google Scholar]
- 49.Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- 50.Hedrich U, Diehl F, Stein W. Gastric and pyloric motor pattern control by a modulatory projection neuron in the intact crab, Cancer pagurus. J Neurophysiol. 2011;105:1671–1680. doi: 10.1152/jn.01105.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghijsen WEJM, Leenders AGM. Differential signaling in presynaptic neurotransmitter release. Cell Mol Life Sci. 2005;62:937–954. doi: 10.1007/s00018-004-4525-0. [DOI] [PubMed] [Google Scholar]
- 52.Hu S, Pawson T, Steven RM. UNC-73/trio RhoGEF-2 activity modulates Caenorhabditis elegans motility through changes in neurotransmitter signaling upstream of the GSA-1/Galphas pathway. Genetics. 2011;189:137–151. doi: 10.1534/genetics.111.131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris G, Mills H, Wragg R, Hapiak V, Castelletto M, Korchnak A, Komuniecki RW. The monoaminergic modulation of sensory-mediated aversive responses in Caenorhabditis elegans requires glutamatergic/peptidergic cotransmission. J Neurosci. 2010;30:7889–7899. doi: 10.1523/JNEUROSCI.0497-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54•.Shakiryanova D, Zettel GM, Gu T, Hewes RS, Levitan ES. Synaptic neuropeptide release induced by octopamine without Ca2+ entry into the nerve terminal. Proc Natl Acad Sci USA. 2011;108:4477–4481. doi: 10.1073/pnas.1017837108. Genetic and pharmacological experiments in Drosophila enable the novel finding that octopamine promotes synaptic peptide release from motor neuron terminals in the absence of extracellular Ca2+. Peptide release was tracked in the peptidergic neuron by selective expression in this neuron of GFP-tagged atrial natriuretic factor. Octopamine mediated this action by its parallel activation of cAMP-activated protein kinase and Ca2+ release from the endoplasmic reticulum, via activation of the IP3 and ryanodine receptors. Whether this octopamine action also evoked release of the small molecule transmitter (glutamate) from the motor neuron terminals was not determined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cropper EC, Evans CG, Hurwitz I, Jing J, Proekt A, Romero A, Rosen SC. Feeding neural networks in the mollusc Aplysia. Neurosignals. 2004;13:70–86. doi: 10.1159/000076159. [DOI] [PubMed] [Google Scholar]
- 56.Khorkova O, Golowasch J. Neuromodulators, not activity, control coordinated expression of ionic currents. J Neurosci. 2007;27:8709–8718. doi: 10.1523/JNEUROSCI.1274-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57••.Wu J-S, Vilim FS, Hatcher NG, Due MR, Sweedler JV, Weiss KR, Jing J. Composite modulatory feedforward loop contributes to the establishment of a network state. J Neurophysiol. 2010;103:2174–2184. doi: 10.1152/jn.01054.2009. The detailed cellular and synaptic mechanisms that establish a particular network state remain elusive in most systems. To address this issue, the authors identify a neuropeptide (SCP) released by axons that project through the esophageal nerve (EN) and establish the egestive state in the Aplysia feeding system. This state is defined by activation of a modulatory CPG neuron (B65) and the CPG neuron B20, plus inactivity in the ingestive CPG neuron B40. When active, B65 excites B20 and inhibits B40. SCP is shown to be pivotal to the ability of EN stimulation to organize the egestive state and suppress the ingestive state by implementing feedforward pathways. Specifically, SCP directly enhances the activity of B65 and B20 and inhibits B40 activity, while further facilitating B20 activity and suppressing B40 activity by its excitation of B65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nusbaum MP, Marder E. A modulatory proctolin-containing neuron (MPN). II. State-dependent modulation of rhythmic motor activity. J Neurosci. 1989;9:1600–1607. doi: 10.1523/JNEUROSCI.09-05-01600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blitz DM, Christie AE, Coleman MJ, Norris BJ, Marder E, Nusbaum MP. Different proctolin neurons elicit distinct motor patterns from a multifunctional neuronal network. J Neurosci. 1999;19:5449–5463. doi: 10.1523/JNEUROSCI.19-13-05449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morgan PT, Perrins R, Lloyd PE, Weiss KR. Intrinsic and extrinsic modulation of a single central pattern generating circuit. J Neurophysiol. 2000;84:1186–1193. doi: 10.1152/jn.2000.84.3.1186. [DOI] [PubMed] [Google Scholar]
- 61.Koh HY, Vilim FS, Jing J, Weiss KR. Two neuropeptides colocalized in a command-like neuron use distinct mechanisms to enhance its fast synaptic connection. J Neurophysiol. 2003;90:2074–2079. doi: 10.1152/jn.00358.2003. [DOI] [PubMed] [Google Scholar]
- 62.Alania M, Sakharov DA, Elliott CJH. Multilevel inhibition of feeding by a peptidergic pleural interneuron in the mollusc Lymnaea stagnalis. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190:379–390. doi: 10.1007/s00359-004-0503-x. [DOI] [PubMed] [Google Scholar]
- 63.Beenhakker MP, Nusbaum MP. Mechanosensory activation of a motor circuit by coactivation of two projection neurons. J Neurosci. 2004;24:6741–6750. doi: 10.1523/JNEUROSCI.1682-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64•.White RS, Nusbaum MP. The same core rhythm generator underlies different rhythmic motor patterns. J Neurosci. 2011;31:11484–11494. doi: 10.1523/JNEUROSCI.1885-11.2011. The ability of CPGs to generate distinct output patterns commonly results from changes in the intrinsic and synaptic properties of the CPG neurons and/or changes in the set of participating CPG neurons. In contrast, in this paper, two different versions of the gastric mill (chewing) rhythm in the crab STNS result not from changes within the circuit but from distinct, long-lasting changes in the activity patterns of the same two projection neurons that drive both rhythms. These changes in projection neuron activity result from distinct modulatory actions by two different extrinsic inputs, one of which (POC neurons) releases the peptide CabTRP Ia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blitz DM, Nusbaum MP. State-dependent presynaptic inhibition regulates central pattern generator feedback to descending inputs. J Neurosci. 2008;28:9564–9574. doi: 10.1523/JNEUROSCI.3011-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jing J, Cropper EC, Hurwitz I, Weiss KR. The construction of movement with behavior-specific and behavior-independent modules. J Neurosci. 2004;24:6315–6325. doi: 10.1523/JNEUROSCI.0965-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coffman KA, Dum RP, Strick PL. Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex. Proc Natl Acad Sci USA. 2011;108:16068–16073. doi: 10.1073/pnas.1107904108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wood DE, Stein W, Nusbaum MP. Projection neurons with shared cotransmitters elicit different motor patterns from the same neural circuit. J Neurosci. 2000;20:8943–53. doi: 10.1523/JNEUROSCI.20-23-08943.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stein W, DeLong ND, Wood DE, Nusbaum MP. Divergent co-transmitter actions underlie motor pattern activation by a modulatory projection neuron. Eur J Neurosci. 2007;26:1148–1165. doi: 10.1111/j.1460-9568.2007.05744.x. [DOI] [PubMed] [Google Scholar]
- 70••.Zhao S, Sheibanie AF, Oh M, Rabbah P, Nadim F. Peptide neuromodulation of synaptic dynamics in an oscillatory network. J Neurosci. 2011;31:13991–14004. doi: 10.1523/JNEUROSCI.3624-11.2011. The neuropeptide proctolin is shown to enhance the synaptic strength and short-term synaptic dynamics of the sole feedback synapse onto the pyloric pacemaker neurons in the isolated crab STG. Using voltage- and dynamic clamp manipulations, including injection of realistic waveforms into the presynaptic neuron, these proctolin actions are determined to be presynaptic, and include distinct influences on the graded and spike-mediated components of transmitter release. Unlike the previously documented proctolin actions on the excitability of pyloric neurons that change the pyloric motor pattern, the proctolin modulation of this synapse does not alter this motor pattern. However, it is necessary and sufficient for reducing the variability of the pyloric cycle period. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nadim F, Zhao S, Zhou L, Bose A. Inhibitory feedback promotes stability in an oscillatory network. J Neural Eng. 2011;8:065001. doi: 10.1088/1741-2560/8/6/065001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thirumalai V, Prinz AA, Johnson CD, Marder E. Red pigment concentrating hormone strongly enhances the strength of the feedback to the pyloric rhythm oscillator but has little effect on pyloric rhythm period. J Neurophysiol. 2006;95:1762–1770. doi: 10.1152/jn.00764.2005. [DOI] [PubMed] [Google Scholar]
- 73.Szabo TM, Chen R, Goeritz ML, Maloney RT, Tang LS, Li L, Marder E. Distribution and physiological effects of B-type allatostatins (myoinhibitory peptides, MIPs) in the stomatogastric nervous system of the crab Cancer borealis. J Comp Neurol. 2011;519:2658–2676. doi: 10.1002/cne.22654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coleman MJ, Meyrand P, Nusbaum MP. A switch between two modes of synaptic transmission mediated by presynaptic inhibition. Nature. 1995;378:502–505. doi: 10.1038/378502a0. [DOI] [PubMed] [Google Scholar]
- 75.Bartos M, Manor Y, Nadim F, Marder E, Nusbaum MP. Coordination of fast and slow rhythmic neuronal circuits. J Neurosci. 1999;19:6650–60. doi: 10.1523/JNEUROSCI.19-15-06650.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirby MS, Nusbaum MP. Peptide hormone modulation of a neuronally modulated motor circuit. J Neurophysiol. 2007;98:3206–3220. doi: 10.1152/jn.00795.2006. [DOI] [PubMed] [Google Scholar]
- 77.Coleman MJ, Nusbaum MP. Functional consequences of compartmentalization of synaptic input. J Neurosci. 1994;14:6544–6552. doi: 10.1523/JNEUROSCI.14-11-06544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Husson SJ, Mertens I, Janssen T, Lindemans M, Schoofs L. Neuropeptidergic signaling in the nematode Caenorhabditis elegans. Prog Neurobiol. 2007;82:33–55. doi: 10.1016/j.pneurobio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 79.Christie AE, Stemmler EA, Dickinson PS. Crustacean neuropeptides. Cell Mol Life Sci. 2010;67:4135–4169. doi: 10.1007/s00018-010-0482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nässel DR, Winther AME. Drosophila neuropeptides in regulation of physiology and behavior. Prog Neurobiol. 2010;92:42–104. doi: 10.1016/j.pneurobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 81.Van Loy T, Vandersmissen HP, Poels J, Van Hiel MB, Verlinden H, Vanden Broeck J. Tachykinin-related peptides and their receptors in invertebrates: a current view. Peptides. 2010;31:520–524. doi: 10.1016/j.peptides.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 82.Nässel DR, Wegener C. A comparative review of short and long neuropeptide F signaling in invertebrates: Any similarities to vertebrate neuropeptide Y signaling? Peptides. 2011;32:1335–1355. doi: 10.1016/j.peptides.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 83.Chen R, Hui L, Sturm RM, Li L. Three dimensional mapping of neuropeptides and lipids in crustacean brain by mass spectral imaging. J Am Soc Mass Spectrom. 2009;20:1068–1077. doi: 10.1016/j.jasms.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dickinson PS, Stemmler EA, Barton EE, Cashman CR, Gardner NP, Rus S, Brennan HR, McClintock TS, Christie AE. Molecular, mass spectral, and physiological analyses of orcokinins and orcokinin precursor-related peptides in the lobster Homarus americanus and the crayfish Procambarus clarkii. Peptides. 2009;30:297–317. doi: 10.1016/j.peptides.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma M, Szabo TM, Jia C, Marder E, Li L. Mass spectrometric characterization and physiological actions of novel crustacean C-type allatostatins. Peptides. 2009;30:1660–1668. doi: 10.1016/j.peptides.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86•.Vilim FS, Sasaki K, Rybak J, Alexeeva V, Cropper EC, Jing J, Orekhova IV, Brezina V, Price D, Romanova EV, et al. Distinct mechanisms produce functionally complementary actions of neuropeptides that are structurally related but derived from different precursors. J Neurosci. 2010;30:131–147. doi: 10.1523/JNEUROSCI.3282-09.2010. The influence of two RFamide peptide families, including the FRFamides and FMRFamides, is determined in the Aplysia feeding system. These peptides are found to originate from different precursor proteins and to localize mostly to different neurons, including distinct sets of identified sensory neurons within the feeding system. Each set of sensory neurons likely releases its peptide(s) peripherally, at the muscles they innervate, as well as centrally. These peptides also have distinct actions on the feeding circuit, and act via different intracellular pathways. However, despite localizing to different neurons and acting through distinct mechanisms, their combined actions are complementary and convert the ingestion motor pattern to an egestion pattern. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stemmler EA, Peguero B, Bruns EA, Dickinson PS, Christie AE. Identification, physiological actions, and distribution of TPSGFLGMRamide: a novel tachykinin-related peptide from the midgut and stomatogastric nervous system of Cancer crabs. J Neurochem. 2007;101:1351–1366. doi: 10.1111/j.1471-4159.2007.04520.x. [DOI] [PubMed] [Google Scholar]
- 88.Hui L, Zhang Y, Wang J, Cook A, Hui Y, Nusbaum MP, Li L. Discovery and functional study of a novel crustacean tachykinin neuropeptide. ACS Chem Neurosci. 2012 doi: 10.1021/cn200042p. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Poels J, Nachman RJ, Akerman KE, Oonk HB, Guerrero F, De Loof A, Janecka AE, Torfs H, Vanden Broeck J. Pharmacology of stomoxytachykinin receptor depends on second messenger system. Peptides. 2005;26:109–114. doi: 10.1016/j.peptides.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 90.Poels J, Verlinden H, Fichna J, Van Loy T, Franssens V, Studzian K, Janecka A, Nachman RJ, Vanden Broeck J. Functional comparison of two evolutionary conserved insect neurokinin-like receptors. Peptides. 2007;28:103–108. doi: 10.1016/j.peptides.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 91•.Poels J, Birse RT, Nachman RJ, Fichna J, Janecka A, Vanden Broeck J, Nässel DR. Characterization and distribution of NKD, a receptor for Drosophila tachykinin-related peptide 6. Peptides. 2009;30:545–556. doi: 10.1016/j.peptides.2008.10.012. Unlike the previously characterized Drosophila tachykinin (DTK) receptor (DTKR), which activates intracellular signaling in the presence of any of the 6 identified DTK peptides (DTK 1–6), the newly characterized DTK receptor NKD is responsive only to DTK-6. Interestingly, DTK-6 is the only identified DTK with an alanine in place of glycine in the C-terminal motif that defines this peptide family. This distinguishing feature of NKD is shared with the previously characterized stable fly DTK receptor, STKR (see [89]) [DOI] [PubMed] [Google Scholar]
- 92.Wood DE, Nusbaum MP. Extracellular peptidase activity tunes motor pattern modulation. J Neurosci. 2002;22:4185–4195. doi: 10.1523/JNEUROSCI.22-10-04185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Isaac RE, Bland ND, Shirras AD. Neuropeptidases and the metabolic inactivation of insect neuropeptides. Gen Comp Endocrinol. 2009;162:8–17. doi: 10.1016/j.ygcen.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 94.Carhan A, Tang K, Shirras CA, Shirras AD, Isaac RE. Loss of Angiotensin-converting enzyme-related (ACER) peptidase disrupts night-time sleep in adult Drosophila melanogaster. J Exp Biol. 2011;214:680–686. doi: 10.1242/jeb.049353. [DOI] [PubMed] [Google Scholar]