Abstract
Rationale
Identification of malleable neurocognitive predictors of relapse among alcohol dependent individuals is important for the optimization of health care delivery and clinical services.
Objectives
Given that alcohol cue-reactivity can predict relapse, we evaluated cue-elicited high frequency heart rate variability (HFHRV) and alcohol attentional bias (AB) as potential relapse risk indices.
Method
Alcohol dependent patients in long-term residential treatment who had participated in mindfulness-oriented therapy or an addiction support group completed a spatial cueing task as a measure of alcohol AB and an affect-modulated alcohol cue-reactivity protocol while HFHRV was assessed.
Results
Post-treatment HFHRV cue-reactivity and alcohol AB significantly predicted the occurrence and timing of relapse by 6-month follow-up, independent of treatment condition and after controlling for alcohol dependence severity. Alcohol dependent patients who relapsed exhibited a significantly greater HFHRV reactivity to stress-primed alcohol cues than patients who did not relapse.
Conclusions
Cue-elicited HFHRV and alcohol AB can presage relapse and may therefore hold promise as prognostic indicators in clinical settings.
Keywords: alcohol dependence, attentional bias, cue-reactivity, heart rate variability, relapse
Introduction
As addiction science advances, more progress is made towards identifying predictors of relapse among alcohol dependent individuals. Prediction of alcohol relapse is an important goal inasmuch as it allows for the optimization of surveillance procedures embedded in health care service delivery systems. From a clinical standpoint, however, it is crucial to identify not only those variables that can predict relapse, but also those which are malleable to intervention. Thus, as recent multi-systems conceptualizations have mapped interrelated neurocognitive functions underlying the acquisition, maintenance, and reinstatement of addictive behaviors (Garland et al. 2011; George and Koob 2010), those factors which may be modulated by treatment deserve focal attention. Two important candidate variables are alcohol cue-reactivity and alcohol attentional bias (Carter and Tiffany 1999; Fadardi and Cox 2006; Field and Cox 2008; Glautier and Drummond 1994; Stritzke et al. 2004; Townshend and Duka 2007).
Physiological reactivity to alcohol cues is one potentially malleable relapse risk factor. Recurrent alcohol use conveys heightened incentive salience via mesocorticolimbic sensitization to conditioned stimuli associated with drinking (Robinson and Berridge 2001). Exposure to drinking cues can result in a conditioned appetitive response that imparts compulsivity to alcohol-seeking behaviors, motivating the alcohol dependent person to consume alcohol even after extended periods of abstinence (Robinson and Berridge 2008). Cues associated with drinking can elicit a broad array of physiological reactions, including central and autonomic nervous system responses (e.g., Heinz et al. 2009; Sinha et al. 2009). Physiological cue-reactivity is thought to reflect arousal as well as attentional and emotional processes (Carter and Tiffany 1999a; Tiffany, 1990). Although research on the relationship between physiological cue-reactivity and relapse is mixed (Perkins, 2009), a number of studies have found cue-reactivity (observed in the brain, viscera, or in peripheral physiology) to be a predictor of relapse (e.g., Braus et al. 2001; Drummond and Glautier 1994). For example, among alcoholics in a detoxification treatment program, greater salivary reactivity to olfactory alcohol cues predicted more frequent drinking at 3-month follow-up (Rohsenow et al. 1994). Another study of abstinent alcoholics found that the extent of cue-evoked activation in the anterior cingulate and medial prefrontal cortices and striatum predicted quantity of future alcohol consumption, whereas subjective craving did not (Grusser et al. 2004). Moreover, stress promotes relapse (Sinha 2007), and cue-reactivity is associated with relapse when it occurs within stressful contexts (Breese et al. 2005).
Stress-precipitated appetitive responses may be indexed by cue-elicited high-frequency heart rate variability (HFHRV), the beat-to-beat modulation of heart rate by parasympathetic activation of the vagus nerve (Berntson et al. 1997). According to the neurovisceral integration model (Thayer and Lane 2000, 2009), a network of central (e.g., prefrontal cortex – PFC, and anterior cingulate cortex - ACC) and autonomic (e.g., vagus nerve) nervous system structures exerts regulatory influences over perturbations to visceral homeostasis, such as those evoked in abstinent alcohol dependent individuals exposed to stress and alcohol cues. Homeostatic regulation of such perturbations may be reflected in HFHRV (Appel et al. 1989). During emotional provocations, neural activations in the PFC and ACC exert downstream influences on HFHRV (Lane et al. 2009), fine-tuning the cardiac pacemaker to mobilize energy resources in proportion to perceived motivational demands (Thayer and Lane 2009). As such, studies reveal cue-elicited increases in HRV associated with appetitive responses to methamphetamine and nicotine (Culbertson et al. 2010; Erblich et al. 2011) and increased HRV during exposure to food cues which abate upon consumption of a meal (Nederkoorn et al. 2000). Similarly, alcohol dependent persons participating in alcohol cue-reactivity paradigms evince elevated HRV (Ingjaldsson et al. 2003; Jansma et al. 2000; Rajan et al. 1998).
Increased HFHRV during exposure to emotionally-salient cues may be explained by two distinct yet potentially interrelated processes. On the one hand, cue-elicited HRV may indicate attempted regulation of stress (Butler et al. 2006; Pu et al. 2010) or appetitive responses (Segerstrom and Nes 2007). Failure to mount effective autonomic regulation of stress-primed appetitive responses may increase vulnerability to relapse. In that regard, Ashare et al. (2011) found that among abstinent smokers, higher HFHRV following stress exposure (interpreted by the authors to be indicative of blunted reactivity rather than the normative, situationally-appropriate decrease in HFHRV to stress) was predictive of increased odds of smoking relapse. On the other hand, HFHRV can also be elicited as a classically conditioned response to conditioned stimuli (Inagaki et al., 2005; Stockhorst et al. 2011). Plausibly, addicts may exhibit elevated cue-elicited HFHRV when they attempt to regulate a conditioned appetitive response elicited by stimuli associated with the substance of addiction.
To the extent that HFHRV is linked with central autonomic regulation of stress and appetitive processes, therapies that modify cue-elicited HFHRV responses may reduce the risk of relapse among alcohol dependent individuals. Indeed, several studies have demonstrated that behavioral interventions can significantly modify heart rate variability (Carney et al. 2000; Chang et al. 2008; Garakani et al. 2009; Wu & Lo, 2008). For example, in a controlled trial persons randomized to a meditation intervention evidenced significant changes in HFHRV correlated with altered activation in frontal midline brain regions compared to persons randomly assigned to five days of relaxation therapy (Tang et al., 2009). More germane to addiction, Garland et al. (2010) found that, relative to participants in a support group, alcohol dependent patients exhibited significantly greater heart rate variability recovery from stress-primed alcohol cue-exposure after participation 10 weeks of mindfulness training. Hence, behavioral interventions may modulate HFHRV cue-reactivity among alcohol dependent individuals and possibly mitigate the risk of relapse.
Alcohol attentional bias (AB; i.e., preferential attention to alcohol cues that have been conferred incentive salience) may also indicate appetitive responding and signal future relapse risk (Franken 2003). Alcohol dependent individuals exhibit automatic attentional orienting to and delayed disengagement from alcohol cues (Field and Cox 2008). Such alcohol AB is amplified by stress (Field and Powell 2007; Field and Quigley 2009) and is associated with craving (Field et al. 2009). Experimental manipulations that enhance alcohol AB have been shown to increase alcohol consumption (Field and Eastwood 2005). Yet, other investigations have failed to replicate this effect (Field et al., 2007; Schoenmakers et al., 2007). Some studies indicate that alcohol AB is associated with relapse risk. For example, Cox et al. (2002) found that, unlike control participants or alcohol abusers whose treatment was designated successful, alcohol abusers who relapsed or whose treatment was unsuccessful exhibited a significant increase in alcohol AB over the course of treatment. Other studies indicate that persons receiving treatment for alcohol use disorders have an AB away from alcohol cues (Townshend and Duka 2007), which is predictive of successful treatment outcomes (Fadardi and Cox 2009). Compared to placebo psychotherapy, AB modification in alcohol dependent inpatients significantly decreased alcohol AB, increased time-to-relapse, and predicted earlier time-to-discharge from treatment, a putative indicator of successful treatment (Schoenmakers et al. 2010). However, it should be noted that intervention-related changes in alcohol AB were not associated with time-to-relapse. Although the causal relation between alcohol AB and relapse remains indeterminate, extant evidence suggests potentially important linkages between these two factors.
Data for the present study were drawn from a randomized controlled pilot trial that examined the effects of a mindfulness-based intervention, Mindfulness-Oriented Recovery Enhancement (MORE), on cognitive, affective, and physiological variables implicated in alcohol dependence relapse (Garland et al. 2010). The previously published paper detailed significant effects of mindfulness training on proximal outcomes, including perceived stress, thought suppression, alcohol AB, and HFHRV responses to stress-primed alcohol cues, but did not examine relapse. In light of evidence suggesting that greater addiction AB and psychophysiological cue-reactivity foreshadow a return to substance use among abstinent patients, the primary aim of the present study was to examine whether post-treatment HFHRV cue-reactivity and/or alcohol AB predict the occurrence and timing of relapse in detoxified, alcohol-dependent individuals.
Materials and Methods
Participants
Eligible participants were alcohol-dependent adults in long-term residential treatment for substance-use disorders. The treatment facility offers a two-year program consisting of a therapeutic milieu, vocational training, and job placement. The goal of this program with respect to substance use is total abstinence. Although residents receive therapeutic milieu services (psychoeducation on topics related to addiction, process therapy groups) throughout the two-year program, the randomized controlled trial from which the present study is derived offered the opportunity to participate in an additional 10 week intervention consisting of either MORE or an addiction support group (ASG).
Potential participants met inclusion criteria if they were ≥18 years old, satisfied current (past month) Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) alcohol dependence criteria at the time of admission into the treatment facility, and had been in residential treatment for ≥18 months. In the facility where the study was conducted, 18 months marks the time of transition to employment, and thus represents a period of heightened relapse risk. Alcohol dependence criteria were assessed with a semi-structured psychiatric interview adapted from section I of the Mini-International Neuropsychiatric Interview (Sheehan et al. 1998). Diagnostic interviews were conducted by a licensed psychiatrist and licensed clinical social worker with training and expertise in making addiction diagnoses. Participants were recruited through a presentation about the study made at the treatment facility, as well as through flyers and referrals from staff. Residents were not required to participate in the study. Of the 71 residents who were eligible for study participation due to having resided in the program for ≥18 months, 10 declined to participate, and 3 were ineligible for the study due to their not meeting full criteria for alcohol dependence. Five residents dropped out after consenting but before starting the study treatments due to work scheduling conflicts.
Study participants (N = 53), the majority of whom were male (79.2%), African American (60.4%), and low-income (52.8% had earned < $20,000 in the year before entering treatment), had been in residential treatment for a mean of 22.3 ± 3.7 months. Participants reported relatively severe alcohol dependence: the mean number of current (past month) DSM-IV alcohol dependence criteria met by participants at the time of admission into the treatment facility was 6.5 ± 1.0, the mean total score on the Alcohol Use Disorders Identification Test was 32.0 ± 5.6, and the mean number of standard alcoholic drinks consumed per day in the year before entering treatment was 19.0 ± 10.9. Most participants (81%) reported daily use of at least one psychoactive drug in addition to alcohol before entering residential treatment, with cocaine being the most frequently used drug (32.1% of participants reported having used cocaine daily). All participants reported continuous abstinence from psychoactive substance use during their time in residential treatment, which ranged from 18 to 28 months. Reports of abstinence were corroborated by random breathalyzer and urinalysis testing conducted at the treatment facility, as well as through daily evaluation from program staff.
Procedures
Participants were recruited and screened via administration of the Alcohol Use Disorders Identification Test (AUDIT) and a psychiatric interview to ensure that all participants met current (past month) DSM-IV criteria for alcohol dependence at the time they entered into residential treatment. After completing a pre-treatment assessment protocol as described in Garland et al. (2010), participants were randomly assigned to participate in 10 weeks of MORE or ASG. Following completion of the 10 week treatment, participants were assessed with a spatial cueing task as a measure of alcohol AB and an affect-modulated cue-reactivity protocol while heart rate was recorded. All measures were administered in this same order across study participants. This study was approved by the University of North Carolina at Chapel Hill IRB and performed in accordance with the ethical standards set by the Declaration of Helsinki. All participants gave informed consent prior to inclusion in the study.
Databases at the treatment facility were accessed 6-months post-treatment to determine if and when participants had relapsed, which, for the purposes of this study, was defined as one or more occasions of drinking any quantity of alcohol. Relapse was defined as such due to programmatic and logistical constraints at the treatment facility. In this particular treatment facility, if residents relapsed they were required to start the entire two-year program over again or were asked to leave the treatment program. Therefore, most participants who relapsed dropped out of treatment and could not be tracked. The treatment facility did not keep record of the quantity of alcohol used during the relapse episode(s), nor did they track the number of occasions of alcohol use. Hence, for this study, relapse was represented by a dichotomous variable. Given that participants were accompanied either by staff or other abstinent residents twenty four hours a day while residing in facility housing or on work assignment, it is probable that someone who had relapsed would have been identified, especially since they only had to be detected once in order to be considered relapsed. Once a relapse had been detected by clinical staff via behavioral observation and confirmed by breathalyzer, the event was entered into facility records, and a clinical response made.
MORE
The ten-session, manualized MORE intervention (Garland et al. 2010) involved instruction in the application of mindfulness techniques to address relapse triggers, craving, stress, and automatic appetitive behaviors. A licensed Master’s level social worker (MSW) with experience in mindfulness who was trained in cognitive-behavioral treatments for substance dependence delivered the MORE intervention.
ASG
To control for nonspecific therapeutic factors such as therapeutic alliance, group dynamic, and expectancy effects, the ten-session control condition consisted of MSW-led social support groups derived from the Matrix Model treatment manual (Rawson and McCann 2006).
Measures
Alcohol dependence severity
The Alcohol Use Disorders Identification Test (AUDIT) was used to measure severity of alcohol dependence. This widely used, internally consistent measure (α = .80), evidences convergent validity with biomarkers of alcohol consumption and predictive validity for distal consequences of drinking (Allen et al. 1997).
Alcohol attentional bias
A spatial cueing task created in E-Prime 2.0 (PST Inc., Pittsburgh, PA) and presented on an IBM T60 laptop with a 15” screen was used to measure alcohol AB (Garland et al. 2011). On each trial, first a fixation cross was presented for 500 ms. Next, two grayscale images were presented side by side for 200 ms: one image was neutral in content, the other was alcohol-related. Left/right position of the alcohol images was randomized and counterbalanced across 20 practice trials and 160 trials. Following a 50 ms inter-stimulus interval where the screen was completely blank, a target probe (two dots) replaced one of the images and a distracter probe (one dot) replaced the other image; probes appeared for 100 ms. Participants were instructed to indicate the location of the target probe by responding with a left or right button press on a keypad. Target probes randomly replaced alcohol and neutral images with equal frequency. The inter-trial interval was 500 ms.
On this task, alcohol AB is indicated by shorter reaction times to probes replacing alcohol-related images than probes replacing neutral images, which is held to reflect preferential attentional focus on alcohol (Field & Cox 2008). Alcohol AB as measured by this task is positively correlated with measures of alcohol dependence severity, including number of pre-treatment drinks per day and total scores on the AUDIT (Garland et al. 2011). Moreover, the parameters of this task accord with well-validated cognitive neuroscience methods used to probe attentional processes. In light of research that suggests attentional effects are more robust when targets appear with distracters relative to when targets are presented alone (for a review, see Carrasco 2006), in our spatial cueing task, stimuli (one or two dots) appear in both cue locations, requiring participants to discriminate between target and distracter probes. This task design was chosen to enhance AB detection and eliminate confounding contributions of automatic, reflexive attention that are not related to the emotional salience (e.g., alcohol-relatedness) of the image cues. Including a place marker in the opposite target probe location, requires the participant’s attention to be directed to the spatial location of the target probe and ensures that response selection cannot be based on reflexive detection of the probe through peripheral vision. Thus, the use of target and distracter probes requires greater attentional resources than detection of a single probe and thus this design may have more power to resolve attentional shifts elicited by alcohol cues. While the use of two probes may add an additional mental task compared to single-probe tasks, other forms of discrimination tasks, such as those requiring participants to report the direction of a target arrow, have found reliable attentional biases toward substance-related stimuli (Field et al. 2004; Field and Powell 2007).
Alcohol stimuli included 13 photos of alcoholic beverages and 7 photos of persons drinking alcohol. Neutral stimuli included 13 photos of kitchen items and 7 photos of persons in kitchen scenes. Stimulus sets were analyzed with respect to their spatial frequency (i.e., spectral peak and width) to ensure that they did not differ in terms of basic visual properties, which could elicit reflexive attentional capture regardless of image content. On measures of spectral peak (Neutral: 0.018, Alcohol: 0.017, t(38)=0.38, p=0.70) and width (Neutral: 59.20, Alcohol: 59.29, t(38)= 0.03, p=0.98), stimulus sets were not significantly different.
HFHRV cue-reactivity
HFHRV responses to stress-primed alcohol cues were measured during an affect-modulated psychophysiological cue-reactivity protocol. First, disposable Ag-AgCl electrodes were attached to participants’ right and left pectoral muscles. Electrocardiogram (ECG) data was sampled at 500 Hz and recorded continuously throughout the protocol on a Biopac MP150 data acquisition system (Biopac Systems, Goleta, CA). Next, participants were instructed to remain motionless, silent, and “not think about anything in particular” for a 5-minute baseline. Next, 30 aversive photographs from the International Affective Picture System (IAPS) were serially presented on a 15” laptop screen for 10 seconds each (total duration: 5 min). These photos presented a broad spectrum of unpleasant scenes and objects, and were selected to evoke emotional distress. Photos depicted subjects such as a striking snake, angry and sad facial expressions, persons threatened by guns and knives, starving children, acts of racism, and mutilated and burned human bodies. Lastly, 30 photographs of beer, wine, and distilled liquor (12 of which included individuals drinking or preparing to drink alcohol) were serially presented for 10 seconds each (total duration: 5 min). Participants were instructed to keep still and fixate on the image stream.
Data analysis
For AB data, trials with extreme RTs, defined as those with RTs 3 SD above or below the individual mean (Field et al. 2004), were discarded as outliers (mean = 2.5 ± 1.5 per participant); error trials were also discarded. For each participant, AB scores were calculated by subtracting their mean RT to target probes replacing alcohol photos from their mean RT to target probes replacing neutral photos, such that positive bias scores indicate an AB toward visual alcohol cues. All data are reported as means ± SD unless otherwise noted.
R-R intervals were detected in the ECG data using automated routines in Nevrokard aHRV software (Medistar, Stegne, Ljubljana, Slovenia). The R-wave file was then visually inspected to correct misidentified or omitted R-waves. Kubios 2.0 (Biosignal Analysis and Medical Imaging Group, University of Finland) was used for spectral analysis, applying a fast Fourier transform to extract HFHRV in the respiratory frequency band (0.15 – 0.40 Hz) from a de-trended, end-tapered interbeat interval time series (Berntson et al., 1997). HFHRV was averaged across the 5-minute baseline and alcohol cue-exposure periods. Overall HFHRV reactivity to alcohol cues was computed as the difference between alcohol cue-exposure levels and initial resting baseline levels, or delta (Δ). In ancillary analyses, overall HFHRV reactivity to stress cues was also computed as the difference between stress cue-exposure levels and initial resting baseline levels, or delta (Δ). Use of delta to compute cardiovascular reactivity has been shown to be a reliable means of measuring change induced by a variety of tasks presented in laboratory paradigms (Llabre et al. 1991).
Logistic regression analyses were performed to ascertain to what extent HFHRV cue-reactivity (to stress or alcohol cues), alcohol AB, treatment condition, and pre-treatment alcohol use disorder severity predicted any relapse to drinking occurring by the 6-month follow-up assessment. Our use of logistic regression accords with Hosmer & Lemeshow’s (2000) guideline of approximately 10 cases per independent variable. We utilized ANOVA to predict differences in HFHRV cue-reactivity with a dichotomous relapse variable. Lastly, we employed a Cox proportional hazards model to analyze the occurrence and timing of relapse, using the same set of covariates listed above. This model is appropriate for analyzing a data set containing censored cases (Allison 1995). The outcome variable assessed was the hazard rate of relapse within the 6-month follow-up window, and the unit of time for our analysis was one week. Participants remaining abstinent from alcohol by the end of the study window constituted the censored cases for this analysis. Odds ratios expressed the relative risk of relapse associated with each independent variable.
Results
Relapse
Over the course of the 10-week intervention groups, six people (11.3% of the initial sample) relapsed prior to completion of treatment and post-treatment measures. Approximately one-fifth (19.1%, n = 9) of the remaining 47 individuals relapsed over the 6-month follow-up period. The following analyses were conducted with this group of individuals who completed treatment and were assessed with post-treatment measures.
Prediction of relapse
A multiple logistic regression model with simultaneous entry of pre-treatment AUDIT scores, treatment group condition (MORE vs. ASG), post-treatment alcohol AB, and post-treatment HFHRV alcohol cue-reactivity1 significantly predicted the odds of relapse over the 6-month follow-up. Post-treatment alcohol AB and HFHRV cue-reactivity significantly and independently predicted the odds of relapse up to 6-months post-treatment, while neither pre-treatment alcohol use disorder severity nor treatment condition significantly predicted relapse. To examine the unique contribution of attentional bias and as predictors of relapse after controlling for variance associated with treatment condition and AUDIT scores, hierarchical regression was conducted with AUDIT scores and treatment condition entered as predictors in the first step, and attentional bias scores and HFHRV alcohol cue-reactivity entered in the second step. The addition of alcohol AB and HFHRV to the model significantly contributed to the prediction of relapse above and beyond AUDIT and treatment condition, χ2 = 10.16, df = 2, p=.006. Table 1 depicts logistic regression results.
Table 1.
Logistic regression analysis examining predictors of relapse up to 6-months post-treatment among a sample of treated alcohol dependent adults.
| Variable | B | SE | OR (95% CI) |
|---|---|---|---|
| Step 1 | |||
| Alcohol Use Disorder Severity | .07 | .08 | 1.07 (.92, 1.25) |
| Treatment Group | −.12 | .81 | .89 (.18, 4.33) |
| Step 2 | |||
| Alcohol use Disorder Severity | .05 | .10 | 1.05 (.87, 1.26) |
| Treatment Group | −1.11 | 1.20 | .33 (.03, 3.45) |
| Post-treatment Alcohol Attentional Bias | *.05 | .03 | 1.05 (1.00, 1.11) |
| Post-treatment HFHRV Reactivity to Alcohol Cuesa | *.10 | .05 | 1.10 (1.01, 1.20) |
NOTE: Omnibus test of model coefficients for simultaneous entry of all four predictors: χ2 = 10.99, df = 4, p =.03. Nagelkerke pseudo R2 for Step 1 = .03. Nagelkerke pseudo R2 for Step 2 = .40
Overall HFHRV reactivity to stress-primed alcohol cues was computed as the difference between alcohol cue-exposure levels and initial resting baseline levels, or delta (Δ). Positive values indicate increased HFHRV from baseline to cue-exposure, whereas negative values indicate decreased HFHRV from baseline to cue-exposure.
p<.05
Between-groups comparisons in HFHRV reactivity and alcohol AB
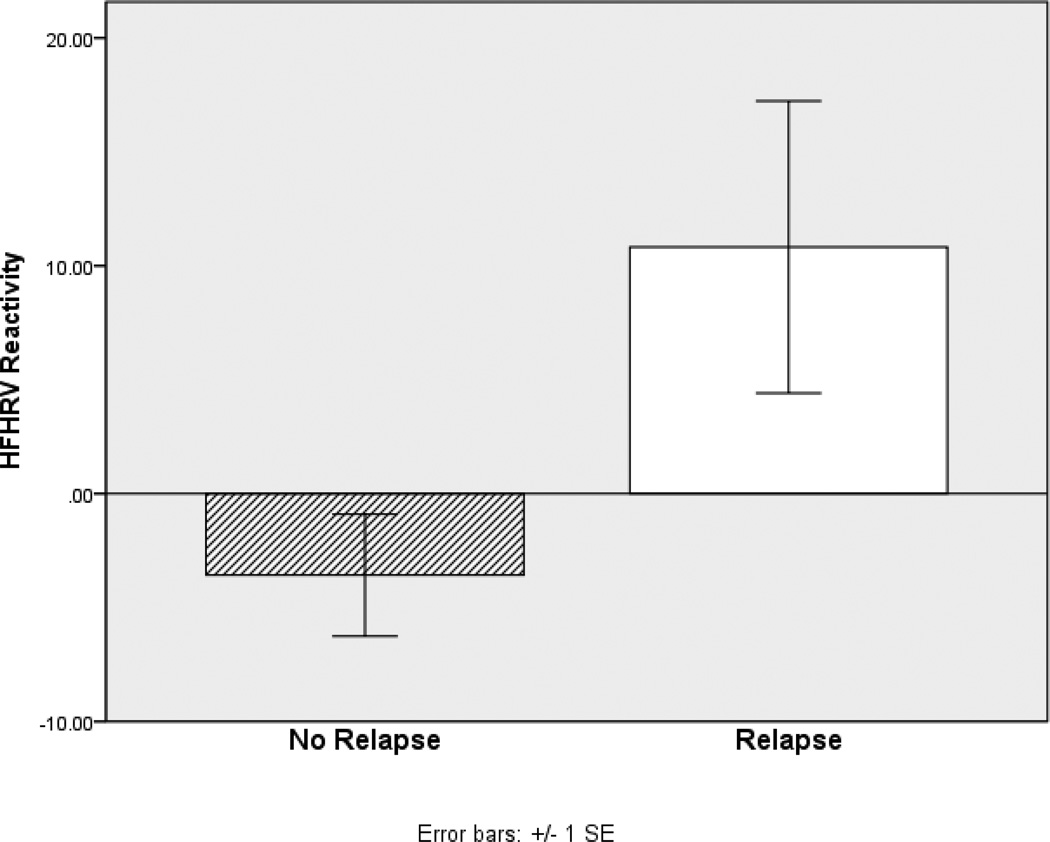
Using bivariate analyses, participants who relapsed were compared to those who did not relapse with regard to HFHRV cue-reactivity and alcohol AB. ANOVA indicated that participants who relapsed exhibited significantly greater HFHRV reactivity to alcohol cues than those who did not relapse by the 6-month follow-up, F(1,36) = 5.85, p = .02, d = .85 (see Figure 1). Similarly, participants who relapsed exhibited significantly greater HFHRV reactivity to stress cues than those who did not relapse by the 6-month follow-up, F(1,36) = 7.70, p = .009, d = 1.05. Specifically, those who relapsed experienced a mean increase in HFHRV from baseline through both stress- and alcohol cue-exposure, whereas those who did not relapse experienced a mean decrease in HFHRV through both stress- and alcohol cue-exposure.
Figure 1.
Differences in HFHRV reactivitya to alcohol cue-exposure between alcohol dependent patients who did and did not relapse by 6 month follow-up
a Overall HFHRV reactivity to alcohol cues was computed as the difference between alcohol cue-exposure levels and initial resting baseline levels, or delta (Δ). Positive values indicate increased HFHRV from baseline to cue-exposure, whereas negative values indicate decreased HFHRV from baseline to cue-exposure.
In contrast, bivariate analysis revealed that there were no statistically significant differences in alcohol AB between relapse and no-relapse groups, F(1,38) = 1.73, p = .20, d = .50, although participants who relapsed had higher mean alcohol AB (M = 11.40, SD = 19.72) than those who did not relapse (M = 1.06, SD = 21.00).
Estimators of the Timing of Relapse
A Cox proportional hazards model with treatment condition, pre-treatment AUDIT scores, post-treatment alcohol AB, and post-treatment HFHRV alcohol cue-reactivity2 significantly predicted the occurrence and timing of relapse, χ2 = 14.50, df = 4, p = .006. According to survival analysis via Cox regression, after controlling for treatment condition and pre-treatment AUDIT scores, post-treatment HFHFV alcohol cue-reactivity significantly predicted the occurrence and timing of relapse, OR = .92 (95% CI = .87, .97), p = .003. Larger HFHRV increases from baseline to alcohol cue-exposure were prospectively associated with shorter latency to relapse. Thus, individuals who experienced greater HFHRV cue-reactivity were more likely to relapse sooner than those who experienced less HFHRV reactivity. Controlling for treatment condition, a highly reactive individual was, on average, 4.31 times more likely to relapse than a less reactive individual.
In addition, controlling for treatment condition, pre-treatment AUDIT scores, and HFHRV cue-reactivity, post-treatment alcohol AB significantly predicted the rate and timing of relapse, OR = 1.04 (95% CI = 1.00, 1.08), p = .04. Thus, individuals with greater alcohol AB were more likely to relapse, and relapse sooner, than those with lower levels of alcohol AB.
Discussion
Among this sample of recovering alcohol dependent adults, extent of alcohol AB and HFHRV reactivity to stress-primed alcohol cues after 10 weeks of treatment significantly predicted the occurrence and timing of relapse in multivariate analyses. According to multiple logistic regression and Cox regression analyses, over a six month follow-up, individuals with greater post-treatment alcohol AB and HFHRV cue-reactivity were more likely to relapse, and tended to relapse sooner, than their counterparts with lower levels of alcohol AB and less HFHRV reactivity. Alcohol AB and HFHRV cue-reactivity predicted relapse independent of the effects of treatment and irrespectively of pre-treatment levels of alcohol dependence.
Although the current findings are preliminary in nature, this study is the first to demonstrate an association between HFHRV cue-reactivity and relapse among recovering alcohol dependent adults. Individuals who exhibited comparatively exaggerated alcohol cue-reactivity as indexed by elevated HFHRV during stress-primed alcohol cue-exposure appeared to be especially vulnerable to relapse. Although higher tonic HFHRV is typically viewed as an index of autonomic flexibility (Thayer and Lane 2000, 2009), elevated phasic HFHRV reactions may indicate the presence of severe perturbations from visceral homeostasis that require robust parasympathetic regulation via intensive vagal activation (Appel et al. 1989). Highly reactive individuals who are more perturbed by stress-primed alcohol cues may exhibit greater central autonomic network activation during regulation of appetitive responses (indicated by increased HFHRV) than persons who are less affected by such cues (Garland et al. in press). This interpretation is bolstered by research indicating that regulatory effort in the face of appetitive stimuli is associated with increased HRV (Segerstrom and Nes 2007). Erblich et al. (2011), who found increased smoking cue-elicited HRV among nicotine dependent individuals, suggest that exposure to drug-related stimuli may have both appetitive and aversive or stressful components, and thus homeostatic perturbations by addictive cues “may thus reflect autonomic regulation of the aversiveness associated with coping with the induced cravings” (p. 740). Given that current study participants were in long-term treatment for alcohol dependence, appetitive responses triggered by stress-primed alcohol cue-exposure may have been perceived as ego-dystonic. Individuals who experienced the strongest alcohol cue-reactivity (and therefore were most likely to relapse) may have had to exert the greatest degree of regulatory effort to cope with their reactions, reflected in increased HFHRV.
Alternatively, increased cue-elicited HFHRV may reflect classical conditioning and neurocognitive processing of cues associated with reward. Studies with humans using classical conditioning paradigms have identified conditioned HFHRV responses to conditioned stimuli (Stockhorst et al. 2011). Furthermore, rats in an appetitive conditioning paradigm exposed to a conditioned stimulus associated with the “reward expecting state” exhibited significant increases in high-frequency, vagally-mediated HRV (Inagaki et al. 2005). A more parsimonious explanation of current study findings is that visual alcohol cues presented in the cue-reactivity paradigm may have elicited elevated HFHRV as a conditioned response. Thus, alcohol dependent persons who continue to exhibit strong conditioned responses to alcohol cues after treatment (as reflected by greater HFHRV cue-reactivity) may be at elevated risk for relapse. Hypothetically, persons whose appetitive response towards alcohol has been largely extinguished would experience attenuated HFHRV cue-reactivity and be less likely to relapse than those who were less responsive to treatment.
Furthermore, results of the present investigation complement those of a growing body of studies demonstrating a relationship between alcohol AB and treatment outcomes (Cox et al. 2002; Fadardi and Cox 2009; Schoenmakers et al. 2010). When treatment condition, alcohol dependence severity, and post-treatment HFHRV reactivity were controlled in multivariate analyses, we found that individuals exhibiting greater alcohol AB were especially prone to relapse. Inasmuch as the 200 ms alcohol AB indexes initial attentional orienting towards alcohol cues (Field and Cox 2008), reflexive capture of attention by alcohol-related stimuli may indicate an automatic appetitive response towards alcohol, perhaps mediated by long-lasting structural alterations in the ventral striatum (Robinson and Berridge 2001). Alcohol AB is held to be a cognitive mediator of alcohol dependence maintenance and relapse (Cox, Fadardi, Pothos, 2006; Franken 2003; Garland et al. 2011). When attention is automatically oriented toward alcohol cues by virtue of their heightened incentive salience, this preferential processing of appetitive stimuli diverts cognitive resources away from coping attempts and may ultimately subvert the intention to abstain from drinking (Tiffany and Conklin 2000). Thus, maintenance of elevated alcohol AB following treatment may signal heightened relapse risk. However, it should be noted that although individuals who relapsed had a higher mean alcohol AB than those who did not, in bivariate analyses there were no statistically significant, between-groups differences in alcohol AB. Discrepant results between bivariate and multivariate analyses can occur when then there is a high degree of within-groups variation relative to between-groups variation (Lo, Li, Tsou, & See, 1995). The large variability in alcohol AB observed among study participants may reflect individual differences in alcohol dependence severity and HFHRV reactivity. Indeed, post-treatment alcohol AB was modestly correlated with post-treatment HFHRV reactivity (r = .25) and pre-treatment alcohol dependence severity (r = .28). When these associations were controlled in multivariate analyses, alcohol AB was a statistically significant predictor of relapse. These findings should be considered tentative and require replication in future studies.
Current study findings are congruent with those of two recent investigations of smoking relapse among a sample of abstainers, which found AB towards cigarette cues and cigarette cue-reactivity independently predicted the odds of relapsing (Powell et al. 2011), and that higher HRV following stress exposure was associated with the inability to resist smoking following stress (Ashare et al. 2011). Ashare et al. (2011) interpreted these findings to be evidence of “blunted reactivity,” in contrast to the expected, situationally-appropriate decrease in HRV to stress that is typically observed in healthy adults. Thus, the inability to flexibly adapt to perturbations induced by stress and alcohol cues, as evidenced by a lack of a situationally-appropriate reduction in HRV following cue exposure, may presage relapse. These findings, coupled with our own, suggest that measures of addiction AB and cue-reactivity may have prognostic value in clinical settings. Future research may establish whether autonomic measures of cue-reactivity more accurately predict relapse than self-report measures. Field studies are needed to ascertain the real-world value of employing such measures as a complement to more traditional means of assessing relapse risk.
The study had several limitations. First, our research team did not employ an independent systematic biochemical assessment of abstinence from alcohol to verify reports of relapse registered in the treatment facility database. Yet, the treatment facility engaged in confirmatory screening with breathalyzers when a participant was suspected by staff of having relapsed, and engaged in random screening with breathalyzers and urinalysis at periodic intervals, reducing the probability that study participants were drinking alcohol while receiving treatment. In addition, we were limited to a dichotomous measure of relapse, and thus were unable to examine the frequency and quantity of alcohol consumption of those persons who relapsed following treatment. It is possible that predictors of relapse as a dichotomous variable may differ from predictors of the frequency and quantity of alcohol consumption. Also, because the presentation order of stress and alcohol cues was not counterbalanced, the differential contribution of each type of stimulus to HFHRV cue-reactivity and relapse cannot be ascertained. HFHRV alcohol cue-reactivity in the absence of priming by stress cues may not predict relapse to the same extent as the measure of HFHRV reactivity used in the present study. Our additional analysis, testing the effects of HFHRV responses to stress alone (prior to alcohol cue-exposure), showed a marginally significant effect of the response to stress, suggesting that the priming by stress is a potentially important component of the cue exposure procedure. Future studies should be designed in such a way that it is possible to study the effects of stress cue-reactivity and AB separately. Lastly, the generalizability of study findings may be somewhat limited as alcohol dependent individuals outside of treatment settings likely exhibit different cue responses than those evidenced by this sample of patients in long-term residential treatment. Furthermore, the number of study participants who relapsed was small, which may have limited statistical power. Future research should replicate these findings in a larger investigation of cue-reactivity using counterbalanced stimulus presentation, and incorporating a multidimensional measure of relapse along with biochemical corroboration and the corroboration of collateral informants.
Insofar as behavioral treatment promotes adaptive regulation of addictive behaviors, participation in treatment should alter indices of such regulation. HFHRV and alcohol AB may prove to be key indicators of successful self-regulation of appetitive responses (Garland 2011) and reliably predictive of susceptibility to future relapse.
Acknowledgements
The first author (E.L.G.) was supported in developing this manuscript by Grant Number R03DA032517-01 from the National Institute of Drug Abuse, Grant Number T32AT003378 from the National Center for Complementary and Alternative Medicine, a Francisco J. Varela Research Grant from the Mind and Life Institute, and a grant from the Florida State University Council on Research and Creativity.
Footnotes
Although the design of the study makes it impossible to totally separate the effects of alcohol AB and HFHRV stress cue-reactivity, we additionally tested the effects of HFHRV responses to stress alone (prior to presentation of the alcohol cues). In a multiple logistic regression model with pre-treatment AUDIT scores, treatment group condition (MORE vs. ASG), post-treatment alcohol AB, and post-treatment HFHRV stress cue-reactivity, alcohol AB did not significantly predict the odds of relapse over the 6-month follow-up. HFHRV reactivity to stress cues was a marginally significant predictor of relapse, OR = .90 (95% CI = .81, 1.00), p = .06.
Again, in order to test the effects of HFHRV responses to stress alone (prior to presentation of the alcohol cues) we conducted an additional Cox proportional hazards model with treatment condition, pre-treatment AUDIT scores, post-treatment alcohol AB, and post-treatment HFHRV stress cue-reactivity did not significantly predict the occurrence and timing of relapse, χ2 = 9.01, df = 4, p = .06. While the other variables in the model did not significantly predict the rate and timing of relapse, HFHRV reactivity to stress cues was a significant predictor, OR = .91 (95% CI = .83, .99), p = .04.
Contributor Information
Eric L. Garland, Florida State University, Tallahassee, FL 32306-2570
Ingmar H.A. Franken, Erasmus University, 3000 DR Rotterdam
Matthew O. Howard, University of North Carolina, Chapel Hill, NC 27599-3550
References
- Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT) Alcohol Clin Exp Res. 1997;21:613–619. [PubMed] [Google Scholar]
- Allison PD. Survival analysis using SAS: A practical guide. Cary, NC: SAS Institute, Inc; 1995. [Google Scholar]
- Appel ML, Berger RD, Saul JP, Smith JM, Cohen RJ. Beat to beat variability in cardiovascular variables: Noise or music? J Amer Coll of Cardiology. 1989;14:1139–1148. doi: 10.1016/0735-1097(89)90408-7. [DOI] [PubMed] [Google Scholar]
- Ashare R, Sinha R, Lampert R, Weinberger AH, Anderson GM, Lavery ME, Yanargisawa K, McKee S. Blunted vagal reactivity predicts stress-precipiated tobacco smoking. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufman PG, Malik M, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grusser S, Hermann D, Ruf M, Flor H, Mann K, Heinz A. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm. 2001;108:887–894. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Lê DA, O'Dell LE, Overstreet DH, Roberts AJ, Sinha R, Valdez GR, Weiss F. Stress enhancement of craving during sobriety: A risk for relapse. Alcohol Clin Exp Res. 2005;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Stein PK, Skala JA, Hoffman P, Jaffe AS. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosom Med. 2000;62:639–647. doi: 10.1097/00006842-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Chang RY, Koo M, Yu ZR, Kan CB, Chu IT, Hsu CT, Chen CY. The effect of t'ai chi exercise on autonomic nervous function of patients with coronary artery disease. J Altern Complement Med. 2008;14:1107–1113. doi: 10.1089/acm.2008.0166. [DOI] [PubMed] [Google Scholar]
- Cox WM, Hogan LM, Kristian MR, Race JH. Alcohol attentional bias as a predictor of alcohol abusers' treatment outcome. Drug Alcohol Depend. 2002;68:237–243. doi: 10.1016/s0376-8716(02)00219-3. [DOI] [PubMed] [Google Scholar]
- Cox WM, Fadardi JS, Pothos EM. The addiction-stroop test: Theoretical considerations and procedural recommendations. Psychol Bull. 2006;132:443–476. doi: 10.1037/0033-2909.132.3.443. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Glautier S. A controlled trial of cue exposure treatment in alcohol dependence. J Consul Clin Psychol. 1994;62:809–817. doi: 10.1037//0022-006x.62.4.809. [DOI] [PubMed] [Google Scholar]
- Fadardi JS, Cox WM. Alcohol attentional bias: drinking salience or cognitive impairment? Psychopharmacology. 2006;185:169–178. doi: 10.1007/s00213-005-0268-0. [DOI] [PubMed] [Google Scholar]
- Fadardi JS, Cox WM. Reversing the sequence: reducing alcohol consumption by overcoming alcohol attentional bias. Drug Alcohol Depend. 2009;101:137–145. doi: 10.1016/j.drugalcdep.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Eastwood B. Experimental manipulation of attentional bias increases the motivation to drink alcohol. Psychopharmacology. 2005;183:350–357. doi: 10.1007/s00213-005-0202-5. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Zetteler J, Bradley BP. Attentional biases for alcohol cues in heavy and light social drinkers: the roles of initial orienting and maintained attention. Psychopharmacology. 2004;176:88–93. doi: 10.1007/s00213-004-1855-1. [DOI] [PubMed] [Google Scholar]
- Field M, Munafo MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Powell H. Stress increases attentional bias for alcohol cues in social drinkers who drink to cope. Alcohol Alcohol. 2007;42:560–566. doi: 10.1093/alcalc/agm064. [DOI] [PubMed] [Google Scholar]
- Field M, Quigley M. Mild stress increases attentional bias in social drinkers who drink to cope: a replication and extension. Exp Clin Psychopharm. 2009;17:312–319. doi: 10.1037/a0017090. [DOI] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Garakani A, Martinez JM, Aaronson CJ, Voustianiouk A, Kaufmann H, Gorman JM. Effect of medication and psychotherapy on heart rate variability in panic disorder. Depress Anxiety. 2009;26:251–258. doi: 10.1002/da.20533. [DOI] [PubMed] [Google Scholar]
- Garland EL, Franken IH, Sheetz JJ, Howard MO. Alcohol attentional bias is associated with autonomic indices of stress-primed alcohol cue-reactivity in alcohol dependent patients. Exp Clin Psychopharm. doi: 10.1037/a0027199. (in press) [DOI] [PubMed] [Google Scholar]
- Garland EL. Trait mindfulness predicts attentional and autonomic regulation of alcohol cue-reactivity. J Psychophysiology. 2011;25:180–189. doi: 10.1027/0269-8803/a000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Boettiger CA, Gaylord SA, West Channon V, Howard MO. Mindfulness is inversely associated with alcohol attentional bias among recovering alcohol dependent adults. Cognit Ther and Res. 2011 doi: 10.1007/s10608-011-9378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Boettiger CA, Howard MO. Targeting cognitive-affective risk mechanisms in stress-precipitated alcohol dependence: An integrated, biopsychosocial model of allostasis, automaticity, and addiction. Med Hypotheses. 2011;76:745–754. doi: 10.1016/j.mehy.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Gaylord SA, Boettiger CA, Howard MO. Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: Results from a randomized controlled pilot trial. J Psychoactive Drugs. 2010;42:177–192. doi: 10.1080/02791072.2010.10400690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glautier S, Drummond DC. Alcohol dependence and cue reactivity. J Stud Alcohol. 1994;55:224–229. doi: 10.15288/jsa.1994.55.224. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grusser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol. 2009;14:108–118. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer D, Lemeshow S. Applied Logistic Regression. NY: Wiley & Sons; 2000. [Google Scholar]
- Inagaki H, Kuwahara M, Tsubone H. Changes in autonomic control of heart associated with classical appetitive conditioning in rats. Exp Animals. 2005;54:61–69. doi: 10.1538/expanim.54.61. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biol Psychiatry. 2003;54:1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44:213–222. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Lo SK, Li IT, Tsou TS, See L. Non-significant in univariate but significant in multivariate analysis: A discussion with examples. Changgeng Yi Xue Za Zhi. 1995;18:95–101. [PubMed] [Google Scholar]
- Jansma A, Breteler MH, Schippers GM, DeJong CA, Van Der Staak CP. No effect of negative mood on the alcohol cue reactivity of in-patient alcoholics. Addict Behav. 2000;25:619–624. doi: 10.1016/s0306-4603(99)00037-4. [DOI] [PubMed] [Google Scholar]
- Llabre MM, Spitzer SB, Saab PG, Ironson GH, Schneiderman N. The reliability and specificity of delta versus residualized change as measures of cardiovascular reactivity to behavioral challenges. Psychophysiology. 1991;28:701–711. doi: 10.1111/j.1469-8986.1991.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104:1610–1616. doi: 10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawkins L, West R, Powell J, Pickering A. Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacology. 2011;212:537–549. doi: 10.1007/s00213-010-1975-8. [DOI] [PubMed] [Google Scholar]
- Pu J, Schmeichel BJ, Demaree HA. Cardiac vagal control predicts spontaneous regulation of negative emotional expression and subsequent cognitive performance. Biol Psychol. 2010;84:531–540. [Google Scholar]
- Rajan I, Murthy PJ, Ramakrishnan AG, Gangadhar BN, Janakiramaiah N. Heart rate variability as an index of cue reactivity in alcoholics. Biol Psychiatry. 1998;43:544–546. doi: 10.1016/s0006-3223(97)00399-5. [DOI] [PubMed] [Google Scholar]
- Rawson R, McCann MJ. Counselor’s Treatment Manual: Matrix Intensive Outpatient Treatment for People With Stimulant Use Disorders. Washington, DC: DHHS Publication; 2006. [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: Some current issues. Philos Trans R Soc Lon B. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM, Wunschel SM, Abrams DB. Cue reactivity as a predictor of drinking among male alcoholics. J Consult Clin Psychol. 1994;62:620–626. doi: 10.1037//0022-006x.62.3.620. [DOI] [PubMed] [Google Scholar]
- Schoenmakers TM, de Bruin M, Lux IF, Goertz AG, Van Kerkhof DH, Wiers RW. Clinical effectiveness of attentional bias modification training in abstinent alcoholic patients. Drug Alcohol Depend. 2010;109:30–36. doi: 10.1016/j.drugalcdep.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Nes LS. Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychol Sci. 2007;18:275–281. doi: 10.1111/j.1467-9280.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M I N I ): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr Psychiatry Rep. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockhorst U, Huenig A, Ziegler D, Scherbaum WA. Unconditioned and conditioned effects of intravenous insulin and glucose on heart rate variability in healthy men. Physiol Behav. 2011;103:31–38. doi: 10.1016/j.physbeh.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Stritzke WG, Breiner MJ, Curtin JJ, Lang AR. Assessment of substance cue reactivity: advances in reliability, specificity, and validity. Psychol Addict Behav. 2004;18:148–159. doi: 10.1037/0893-164X.18.2.148. [DOI] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Fan Y, Feng H, Wang J, Feng S, Lu Q, Hu B, Lin Y, Li J, Zhang Y, Wang Y, Zhou L, Fan M. Central and autonomic nervous system interaction is altered by short-term meditation. Proc Natl Acad Sci USA. 2009;106:8865–8870. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95(Suppl 2):S145–S153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Avoidance of alcohol-related stimuli in alcohol-dependent inpatients. Alcohol Clin Exp Res. 2007;31:1349–1357. doi: 10.1111/j.1530-0277.2007.00429.x. [DOI] [PubMed] [Google Scholar]