Abstract
Atoh1 (also known as Math1, Hath1, and Cath1 in mouse, human, and chicken, respectively) is a proneural basic helix–loop–helix (bHLH) transcription factor that is required in a variety of developmental contexts. Atoh1 is involved in differentiation of neurons, secretory cells in the gut, and mechanoreceptors including auditory hair cells. Together with the two closely related bHLH genes, Neurog1 and NeuroD1, Atoh1 regulates neurosensory development in the ear as well as neurogenesis in the cerebellum. Atoh1 activity in the cochlea is both necessary and sufficient to drive auditory hair cell differentiation, in keeping with its known role as a regulator of various genes that are markers of terminal differentiation. Atoh1 is known in other fields as an oncogene and a tumor suppressor involved in regulation of cell cycle control and apoptosis. Aberrant Atoh1 activity in adult tissue is implicated in cancer progression, specifically in medullablastoma and adenomatous polyposis carcinoma. We demonstrate through protein sequence comparison that Atoh1 contains conserved phosphorylation sites outside the bHLH domain, which may allow regulation through post-translational modification. With such diverse roles, tight regulation of Atoh1 at both the transcriptional and protein level is essential.
Keywords: Math1, bHLH, transcription factor, cochlea, organ of Corti, hair cells
Atoh1 is a neurogenic transcription factor
Atoh1 is a transcription factor with diverse roles during development and has been implicated as both an oncogene and a tumor suppressor. In addition to numerous reports demonstrating that Atoh1 is required for terminal differentiation of auditory hair cells (HCs) in the developing ear, Atoh1 also functions in early specification events during neurogenesis and has a role in cell cycle regulation in maintenance of the intestine. In order to elucidate how Atoh1 can induce expression of radically different gene sets depending on the tissue in which it is expressed, it is necessary to understand how Atoh1 protein functions, how Atoh1 activity is regulated, and where the results of its activity in different contexts diverge. Here, we review Atoh1 protein structure, its varied roles in developmental and homeostatic contexts, and how regulation of both transcription and protein activity allow for diversity of function.
Atoh1 is the vertebrate homologue of Drosophila melanogaster Atonal
The well-characterized proneural genes of the Aschaete–Scute complex (ASC) are required for normal development of the fruitfly peripheral nervous system; however, a subset of sensory organs are unaffected by loss of ASC components (Dambly-Chaudiere 1987). These organs are the internal chordotonal organs, which include sensory bristles, hairs, and papillae. The ASC proteins are class II basic helix–loop–helix (bHLH) transcription factors that can form heterodimers with the ubiquitous transcription factor, Daughterless (Da) (Villares and Cabrera 1987). Loss of da results in a total loss of the peripheral nervous system, including the chordotonal organs, suggesting that there is another set of proneural bHLH transcription factors with the ability to form heterodimers with Da in addition to ASC (Caudy et al. 1988). The D. melanogaster gene atonal (ato) was identified as a candidate gene in 1993, cloned, and subsequently shown to code for a class II bHLH transcription factor necessary and sufficient for the formation of chordotonal organs (Jarman et al. 1993).
Atoh1 structure and function
Murine Atoh1 was cloned as a result of a screen for vertebrate ato homologues (Akazawa et al. 1995). A typical bHLH domain is 60 residues long, with a DNA-binding basic region plus a protein-binding region consisting of two α-helices separated by a variable loop region; the variable loop region allows heterodimeric interactions with class I members of the bHLH family such as E47 (Aguado-Llera et al. 2010). The mouse Atoh1 open reading frame consists of an intronless 1.053-kb coding region that produces a protein 351 amino acids long and 37 kDa in size (Figs. 1, A and 2). Atoh1 shares 70% sequence identity with atonal in the bHLH domain. Outside of this domain, there is little similarity. In contrast to Atonal in which the bHLH domain is situated at the carboxy terminus, in Atoh1, the bHLH domain is in the middle of the protein (Fig. 2A). Atoh1 is rich in proline (35/351 residues), implying a protein-binding function, and 33% of the carboxy terminus (aa325–351) consists of serines, leading to the prediction that phosphorylation is integral to the protein function and regulation. The serines at sites 328 and 331 are specifically predicted to be targets for protein kinase C (Akazawa et al. 1995) (Fig. 2A).
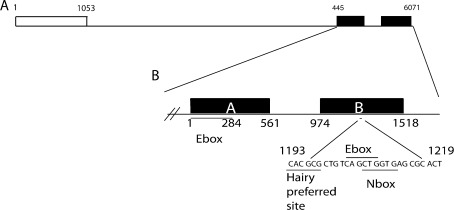
FIG. 1.
Gene structure of mouse Atoh1. A A schematic diagram of mouse Atoh1. The Atoh1 open reading frame (ORF), represented by an open box runs from position 1 to position 1053, consisting of a single exon. The two identified Atoh1 enhancer sites, represented by black boxes, are located approximately 3.4 kb 3′ to the ORF. B The two enhancer sites, site (A) and site (B), are separated by 413 bp of non-conserved sequence—the distance between enhancers (A) and (B) might influence enhancer activity. There is little sequence similarity between enhancer (A) and enhancer (B); enhancer (A) contains an E-box motif while enhancer (B) contains a Hairy preferred sequence, an E-box motif and an N box motif.
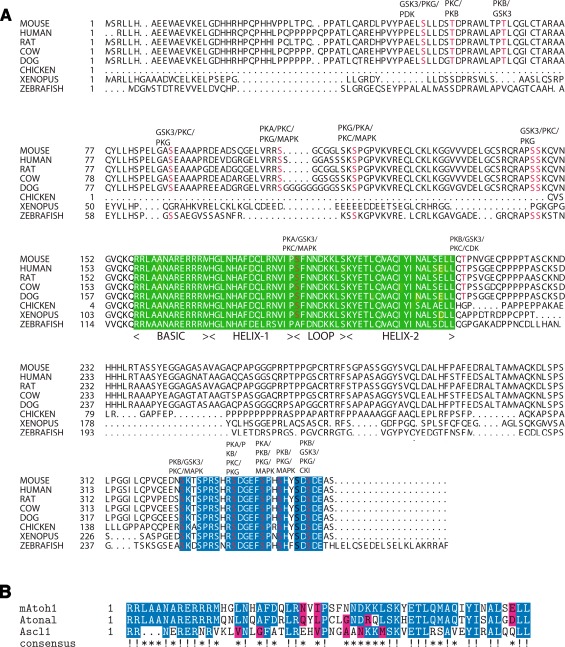
FIG. 2.
Cross-species comparison of the amino acid sequence of Atoh1 and comparison on the bHLH domains of mouse Atoh1, atonal, and mouse Ascl1. A An annotated comparison of the Atoh1 protein sequence in mouse, human, rat, cow, dog, chicken, X. tropicalis, and zebrafish. The mouse Atoh1 protein is 351 amino acids long and consists of three domains: the N terminal domain that is required for protein function but only conserved between mammals; the bHLH domain (green box) which is highly conserved across all eight species; and the C terminus, which contains a serine-rich domain (blue box) extending from amino acid 325–351 and is highly conserved across all eight species. There are several conserved, predicted phosphorylation sites (highlighted in red) annotated with the corresponding predicted kinase. Sequences were obtained from the NCBI database; predicted phosphorylation sites were generated using KinasePhos 2.0. B Comparison of the bHLH domains of mouse Atoh1, Drosophila atonal, and mouse Ascl1 demonstrates that the basic (DNA-binding) domains of Atoh1 and atonal are identical, and Ascl1 shares some common elements. In contrast, the helix–loop–helix domains of Atoh1 and atonal share 60% identity. Given the interchangeable nature of Atoh1 and atonal, these differences may be degenerate in terms of secondary structure and electron cloud topology. Comparison with Ascl1 demonstrates that, while overall similarity to Atoh1 is 46%, the basic domain diverges such that it is likely to select for different E-box motifs. Identical residues are marked in blue and indicated with an exclamation mark (!), residues with similar chemical properties are marked in red (as determined by STRAP chemical similarity alignment labeling), and asterisks mark regions that are somewhat conserved across the three sequences.
Comparison of the Atoh1 peptide sequence in mouse, human, Xenopus tropicalis, zebrafish, and chicken (Fig. 2A) shows that, outside the bHLH region and the conserved serine-rich domain at the C terminus, there is reduced similarity (Fig. 2A). Several conserved phosphorylation sites have been identified in the C terminus region, and it has been shown that both the bHLH domain and the N terminus are essential for transcriptional activity (Aragaki et al. 2008). This suggests additional protein interactions outside of the bHLH domain and post-translational modifications. In addition, comparison of the N terminus in mouse, human, rat, cow, and dog (predicted) demonstrate that this region is well conserved within mammals, while comparison of mammalian Atoh1 homologues with their non-mammalian homologues shows little similarity (Fig. 2A). Comparison of the region extending from the methionine marking the start of the protein to the last amino acid before the bHLH domain shows 82% match between mouse, human, rat, dog (predicted), and cow. Comparison of the same region of mouse Atoh1, zebrafish, and X. tropicalis gives a sequence similarity of 21%. Chicken was not included in the comparison because the sequence held in the NCBI database only includes 21 amino acids upstream of the bHLH domain.
The bHLH domain confers specificity of DNA binding and protein–protein interaction on Atoh1. Substituting the bHLH domain of Ascl1 (previously Mash1), the vertebrate ASC homologue, for the bHLH domain of Atoh1, induces differentiation of cells into alternative cell types (Nakada et al. 2004). Homology between ato and Atoh1 (Fig. 2B) is such that, when ato is substituted for Atoh1 in an Atoh1 null mouse, the Atoh1 phenotype is rescued, and over-expression of Atoh1 in ato Drosophila mutants results in partial rescue of the loss of chordotonal neurons (Ben-Arie et al. 2000; Wang et al. 2002). Note that atonal is the only Drosophila class II bHLH that has been shown to entirely recapitulate the function of its mammalian orthologue. Work with ato and an ato homologue in the silk moth, ato Bombyx mori (atoBM), suggests that the non-bHLH domains can play important roles. AtoBM shares little identity with ato outside the bHLH domain; however, like Atoh1, when expressed in place of ato in the fly, it can partially rescue the ato phenotype (Yu et al. 2011). Further rescue experiments show that the bHLH domains of ato and atoBM are not functionally equivalent, but the N terminus region of atoBM can substitute for that of ato (Yu et al. 2011). This is a surprising result given that the N terminus sequences are so divergent; however, it does hint that the N terminus contains a structural element that is more important than the ato-mediated rescue of the Atoh1 phenotype would suggest.
Class I (E proteins) and class II helix–loop–helix transcription factors can interact with E-box consensus sites. An E-box is a short hexameric DNA motif found in cis-acting gene regulatory regions that allows selective protein–DNA binding (Darlington et al. 2000; Kyriacou and Rosato 2000). Each class of bHLH (I–IV) has a preferred E-box sequence, and to add further specificity, nucleotides flanking the E-box select for individual bHLH proteins within each class (Powell et al. 2004; Powell et al. 2008). Like Atonal, Atoh1 binds directly to E-boxes (Jarman et al. 1993; Akazawa et al. 1995). Although Atoh1 can bind to E-boxes, it must form a heterodimer with a second bHLH transcription factor such as Da or E47 (E12) to activate target gene transcription. Typically, bHLH transcription factors bind to the E-box motif (5′ CANNTG 3′). The basic domain of Atoh1 contains a unique palindromic motif that can bind to E-box sequences, conferring specificity of DNA binding. Analysis of Atoh1 binding interactions during cerebellar development has identified the Atoh1 E-box-associated motif, AtEAM (5′ G/A, C/A, CA, G/T, C/A, TG, G/T, C/T 3′) (Chien et al. 1996; Klisch et al. 2011).
Identification of the AtEAM motif has facilitated genome-wide screening to find potential targets for Atoh1-mediated gene transcription (Klisch et al. 2011). Screening resulted in the identification of 601 genes that Atoh1 can potentially regulate (Klisch et al. 2011). Of the putative targets, 105 were involved in gene transcription; 33 in the cell cycle; 33 in chromosomal organization; 132 were metabolic genes; 15 were associated with Sonic Hedgehog (SHH) signaling, 12 with Notch signaling, 14 with TGF β signaling, 15 with Wnt signaling, and 11 with Jun N-terminal kinase (JNK)/mitogen-activating protein kinase (MAPK) signaling (Klisch et al. 2011). Overall, Atoh1 has been reported to be involved mainly in cell cycle maintenance and differentiation, as befits a proneural gene; however, the function of Atoh1 seems to vary among tissues and developmental contexts. In summary, Atoh1 is a transcription factor with diverse targets, but a high degree of specificity. Its DNA-binding basic domain binds specifically to the AtEAM and Atoh1 can associate with specific protein cofactors selected for by its helix–loop–helix domain and possibly its N terminus.
Atoh1 is required for hair cell development
Atoh1 expression in the developing organ of Corti
Three methods have been used to characterize the spatiotemporal pattern of Atoh1 in the mouse inner ear: reporter-gene studies, in situ hybridization (ISH), and immunohistochemistry. There is some discrepancy among the reports, some of which can be explained by the limitations of each approach.
ISH for Atoh1 shows that expression starts in the prosensory epithelium of the basal turn of the cochlea between E12.5 and E13, spanning the epithelium from the ventral floor to the luminal roof (Lanford et al. 2000; Yang et al. 2010). At E13.5, a row of Atoh1-expressing cells can be detected running from the base to the mid-base (Matei et al. 2005) (illustrated in Fig. 3A). By E15, transcripts are detected along the length of the duct, with a bias towards the luminal surface (Fig. 3C). By E17, Atoh1 is expressed only in the single row of inner hair cells (IHC) and three rows of outer hair cells (OHC) (Fig. 3C). This expression continues to P0, but by P3, Atoh1 starts to be downregulated, proceeding in a basal to apical gradient (Lanford et al. 2000; Yang et al. 2010). Effectively, the expression starts in the base, spanning several cells through the depth of the epithelium, and spreads to the apex. Concurrent with the appearance of HCs, Atoh1 transcripts are restricted to first a single row of IHCs and then to the OHCs as each row arises. It appears from the ISH data that Atoh1 is expressed not only in cells that will become HCs, but also in other cell types. Lineage tracing experiments provide evidence indicating that cells expressing Atoh1 can develop to form supporting cells in addition to HCs (Matei et al. 2005; Yang et al. 2010).
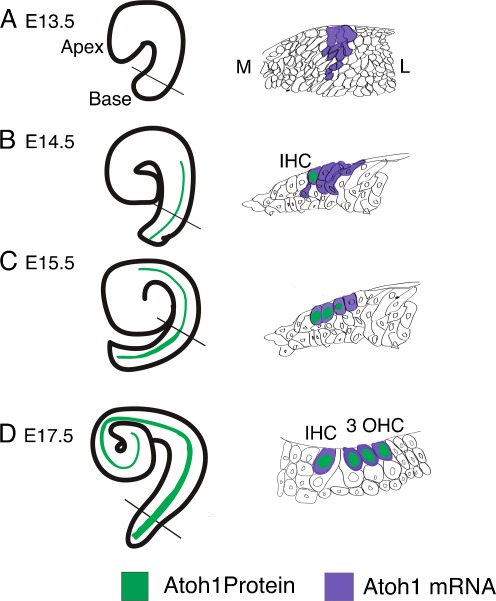
FIG. 3.
A schematic diagram of the onset of Atoh1 expression in the mouse cochlea. A At E13.5, there is no detectable Atoh1 protein in the cochlea; however, mRNA expression has initiated at the base. Cross-section through the mid-base shows mRNA expression arises in the prosensory epithelia in a broad band spanning the depth of the epithelial layer. B At E14.5, Atoh1 protein is expressed in a single row of cells from the base to the mid-base of the cochlea. Cross-section through the mid-base shows Atoh1 protein is in one cell that will become an inner hair cell (IHC) but mRNA is expressed more broadly. C At E15.5, Atoh1 protein is in four rows of cells running from the base to the mid-base, three rows from the mid-base narrowing to two rows of Atoh1-positive cells towards the apex. Cross-section through the mid-base shows Atoh1 protein is detected in three cells, with Atoh1 mRNA beginning to be restricted to cells closer to the luminal surface. D At E17.5, four rows of cells extend to the mid-base, three rows continue towards the apex that are Atoh1-protein-positive. Cross-section through the mid-base shows Atoh1 protein is found in one IHC and three OHCs. Atoh1 mRNA is now restricted to the HCs. M medial; L lateral; IHC inner hair cell; OHCs outer hair cells. Solid black line indicates level of cross-section.
Atoh1 immunohistochemistry confirms that at E14.5 Atoh1 is expressed in the basal region of the cochlea in two discrete rows spanning the sensory epithelium and corresponding to IHCs and OHCs as they begin to differentiate. At E15.5, Atoh1 is restricted to the characteristic pattern of labeling one row of IHCs and three rows of OHCs in the basal region, two rows corresponding to the single row of IHCs and one row of OHC in the medial region, and one column of cells in the apical region (Chen et al. 2002).
Substituting the Atoh1-coding region for β-galactosidase (βGal) provides a means of labeling cells where the Atoh1 promoter is active. Mice heterozygous for Atoh1 and βGal first show a short band of βGal-positive cells at E12.5 in the prospective sensory epithelia (Bermingham et al. 1999). At E13.5, it is expressed in a short band of cells extending from the base of the cochlea (Matei et al. 2005). By E14.5, it is detected in the cochlea where it displays an apical to basal gradient of intensity of staining (Bermingham et al. 1999). By E18.5, βGal activity is detected in four rows of hair cells in the base, narrowing through to a single row in the apex (Bermingham et al. 1999; Woods et al. 2004; Fritzsch et al. 2005).
Expression of an EGFP driven by a fragment of the Atoh1 enhancer identifies a small number of GFP-positive cells in the otic vesicle at E11.5 (Matei et al. 2005). Reverse transcription polymerase chain reaction demonstrates increasing amounts of Atoh1 RNA in mouse sensory epithelium from E12.5 (Zine et al. 2001); however, ISH does not detect Atoh1 transcripts in the presumptive organ of Corti (OC) until E13 (Lanford et al. 2000). It could be that transcript levels are too low for DIG-labeled riboprobes to be detected and visualized by alkaline phosphatase-conjugated antibodies; perhaps radiolabeled probes would give more information at these earlier time points. There is also some discrepancy between the ISH data and data obtained with an EGFP-reporter made by fusing Atoh1 3′ enhancer to beta-globin basal promoter. Whereas Atoh1 RNA is detected spanning the putative sensory epithelium at the base from E13, becoming restricted to the HCs as they differentiate, in the EGFP-reporter mouse, Atoh1 is only expressed in cells that differentiate into HCs (Chen et al. 2002). Between E13.5 and E14.5, a single row of EGFP-positive cells (corresponding to presumptive IHCs) is detected on the medial border of the postmitotic nascent sensory epithelium (Chen et al. 2002) (Fig. 3B). As the cochlea matures, this row extends apically, and at around E14.5, a second row of GFP-positive cells begins to extend from the base. By E15.5, GFP is visible at the base in the characteristically patterned single row of IHCs and three rows of OHCs (Chen et al. 2002) (Fig. 3C). Comparison of RNA expression versus reporter-gene protein activity shows that RNA is first detected in a greater population than is fated to differentiate into hair cells and is gradually restricted to the HCs, whereas protein activity is confined to cells in the process of differentiating into HCs. It is predicted that there is at least one other enhancer, as yet unidentified, that could be modulating the expression detected by ISH that is not recapitulated by the EGFP-reporter. This could indicate that the dynamics of the changing pattern as development progresses involve interplay between Atoh1 and extrinsic factors and that Atoh1 can modulate its own expression. In brief, analyses of βGal-insertion and GFP-reporter lines agree that expression of Atoh1 starts between E13 and E14 as four rows of cells that extend towards the apex serially as development progresses (Fig. 3), continuing until P4 at which time Atoh1 promoter/enhancer is no longer active. ISH analysis suggests that Atoh1 is produced in a broader pattern a day earlier than the onset of GFP expression, then refined over time to one row of IHCs and three rows of OHCs (Fig. 3).
Atoh1 and auditory hair cells
It has been demonstrated that Atoh1 is necessary for the development of auditory HCs. The cochlea of a homozygous Atoh1 mutant mouse lacks differentiated HCs. Histological analysis and electron micrographs of cochlear tissue throughout development and at P0 show that, within the zone usually assigned to the OC, HCs are absent (Bermingham et al. 1999; Pan et al. 2010). Further analysis using immunohistochemistry and ISH to identify individual cell types at P0 showed that not only are there no cells expressing HC markers such as myosin 6 and calretinin, but that there are also no cells expressing markers of the various organ of Corti supporting cells (SCs), including inner pillar cells (probed for Jagged1, Fgfr3, TC2, and p75ntr), Deiters’ cells (probed for Jagged1, glial fibrillary acidic protein, TC2, and Fgfr3) and phalangeal cells (probed for Jagged1 and glial fibrillary acidic protein) (Woods et al. 2004). Although at P0 SCs are not found, examination of Atoh1 null cochleae at E16.5 demonstrates that there are cells in the region that would correspond to the OC that are immunoreactive for the SC markers Jagged1, Prox1, and Sox2 (Dabdoub et al. 2008). Given the caveat that there are no true SC markers, and that Jagged1, Sox2, and Prox1 are expressed first in prosensory cells and then in SCs, it is not clear whether the cells that are immunoreactive for these markers are cells that remain in the prosensory undifferentiated state or are differentiated SCs.
In the organ of Corti of Atoh1-null mice, Caspase 3 activity shows a wave of apoptosis between E14 and E18 that mimics the wild-type front of HC differentiation (Chen et al. 2002). It has been observed, however, that cells that look histologically similar to SCs are present at P0 (Bermingham et al. 1999), and the structure of the tissue is consistent with wild-type patterning at E16.5, E18.5, and P1 (Fritzsch et al. 2005; Dabdoub et al. 2008; Pan et al. 2010). It has been demonstrated that specification of the sensory epithelium is independent of Atoh1 (Chen et al. 2002; Dabdoub et al. 2008); this would mean that, during early development of the OC, SC markers such as Sox2, Prox1, and Jagged1 would be detected in Atoh1-null animals, as these factors are required for early patterning. Furthermore, it has been demonstrated that cells expressing Atoh1 can develop into either HCs or SCs (Matei et al. 2005; Yang et al. 2010), and examination of the homozygous βGal knock-in mouse at E18.5 shows Atoh1 promoter activity in a greater population of sensory epithelial cells than would have differentiated into HCs (Fritzsch et al. 2005). Notch signaling has been shown to regulate the segregation of prosensory cells into either HC fate (Notch-signaling cells) or SC fate (Notch-receiving cells) through the process of lateral inhibition (Lanford et al. 1999). In the absence of Atoh1, it is possible that, after the initial specification of SCs, the cells begin to differentiate, but the lack of Atoh1 causes the lateral inhibition loop between Atoh1-expressing cells and Hes-expressing cells to break down. The reduced levels of Hes might cause SCs either to undergo apoptosis or to revert to flat epithelium (a layer of epithelium lacking characteristic organization of the OC and immunoreactivity for markers of HCs and SCs).
A chimeric mouse was generated to circumvent the perinatal lethality of the homozygous null Atoh1 mutant, with surprising results. The chimeric Atoh1 null mouse exhibited normal patterning of the OC, with one row of IHCs and three rows of OHCs. Immunohistochemistry confirmed the presence of calretinin, myosin VIIa, and stereocilia (phalloidin staining) in all the alleged HCs (Du et al. 2007). The presence of βGal in clusters of these HCs demonstrates that they contain no endogenous Atoh1 and do not retain the ability to produce it. In addition, as is typical for HCs which are interspersed with supporting cells in the wild-type, there was no cell–cell contact between Atoh1-negative HCs and Atoh1-positive HCs (Du et al. 2007). These findings suggest that Atoh1 is required only for very early events in HC differentiation and might induce secreted factors that can potentiate lateral inhibition in the absence of endogenous Atoh1. Bearing in mind that a degree of tissue organization persists in the Atoh1 null cochlea at P1 (Fritzsch et al. 2005; Dabdoub et al. 2008; Pan et al. 2010), it is possible that these are HCs that have entered only initial stages of differentiation (Du et al. 2007). Furthermore, it was not possible to examine these putative HCs in mature cochleae as βGal is only expressed in cells with active Atoh1 promoter activity (Atoh1 is not expressed after P4), so it is not known whether these cells retain their identity into adulthood or are functional (Du et al. 2007). Examination of the Pax2-Cre conditional knock out of Atoh1 at P7 shows very few myosin VIIa-positive cells in receipt of the few remaining nerve fibers (Pan et al. 2010) while, at E18.5 and P0, there are no HCs. This implies that neural cells may supply extrinsic factors. Taken together, the chimeric mouse and the late-appearing HCs of the Pax2 conditional knock-out suggest some interesting possibilities for the mechanism by which expression of Atoh1 is required for HC differentiation; it could be that, rather than directly inducing HC genes, it has a structural role in the regulatory network-controlling patterning.
In mammals, HCs of the vestibular system can regenerate and ectopic expression of Atoh1 in mature animals results in supernumerary HCs (Forge et al. 1993; Lopez et al. 1997; Wang et al. 2010); however, Atoh1 has limited ability to generate ectopic HCs in the mature cochlea. Several groups have successfully generated ectopic HCs by transfecting cochlear explants or by in utero electroporation with Atoh1 under the control of a constitutively active promoter (Zheng and Gao 2000; Gubbels et al. 2008). Epithelial cells of the greater epithelial ridge readily take on a HC fate demonstrating that, even after HC differentiation has started, surrounding cells of the cochlea retain the competence to take on a sensory identity (Zheng and Gao 2000). Attempts to produce ectopic HCs in mature animals and to regenerate HCs in vivo following cochlear injury by either ototoxic drugs or excessive noise have had limited success. One group has shown that adenovirus-mediated expression of Atoh1 in mature guinea pig cochleae results in ectopic hair cells (Kawamoto et al. 2003) and infecting newly injured guinea pigs with an adenovirus carrying Atoh1 leads to regeneration of HCs; however, this approach failed to restore HCs in animals with longer-term injuries or more damaged cochleae (Izumikawa et al. 2005, 2008). Replication of these results and further investigation into the requirement for SC survival may yield useful information.
Atoh1 in differentiation, proliferation, and apoptosis of neurons and intestinal cells
Atoh1 in neurogenesis
As a gene first cloned as a candidate proneural factor, Atoh1 is well characterized as having a role in neurogenesis. Ectopic expression of Atoh1 in the multipotent teratocarcinoma cell line P19 converts undifferentiated cells into neurons and downregulates the cell cycle (Farah et al. 2000). In vivo Atoh1 is expressed and has been shown to be active in many regions of the developing nervous system including the dorsal neural tube, the rhombic lip, and the developing cerebellum. In dorsal neural tube, Atoh1 is co-expressed with markers for dorsal commissural interneurons; cross-inhibition with Neurogenin1 allows differentiation into distinct populations of neuronal sub-types (Helms and Johnson 1998; Gowan et al. 2001). Atoh1 also specifies the fate of the dorsal Lim1 neuronal lineage; in its absence, cells take on the fate of neighboring populations, roofplate, and dorsal Lim2 neurons (Bermingham et al. 2001; Gowan et al. 2001). Atoh1 null mice lack rhombic lip derivatives that contribute to the proprioceptive, vestibular, and auditory sensory network and to the parafacial respiratory group/retrotrapezoid nucleus that controls breathing (Machold and Fishell 2005; Wang et al. 2005; Rose et al. 2009a; Rose et al. 2009b). Atoh1 interacts with the transcription factor Tcf4 to induce differentiation of pontine neurons of the brainstem (Flora et al. 2007). In the cerebellum, Atoh1 is required for specification of cerebellar granule cells (Ben-Arie et al. 1997) and is the earliest marker of developing granule cells in the external germinal layer (EGL) (Machold and Fishell 2005; Wang et al. 2005). SHH and Notch signaling have been shown to cross-regulate with Atoh1 to allow differentiation of granule cells (Gazit et al. 2004). SHH signaling regulates the size of the pool of granule cell precursors; in response to SHH; cells exit the cell cycle and differentiate (Flora et al. 2009). Induction of Gli2 (a component of SHH) expression by Atoh1 renders cells responsive to SHH (Flora et al. 2009).
Atoh1 in intestine development and homeostasis
Atoh1 also has roles in homeostasis and dysplasia in the intestine. Mice null for Atoh1 lack goblet cells, and epithelial cells are maintained in a proliferative state (Yang et al. 2001). Atoh1 is required to specify a subgroup of secretory cells that give rise to paneth cells, goblet cells, and enteroendocrine cells through induction of the bHLH transcription factor Neurogenin 3 and the transcriptional repressor Gfi1 (Yang et al. 2001; Shroyer et al. 2005; Bjerknes and Cheng 2006). Overexpression of Atoh1 results in ectopic secretory cells (VanDussen and Samuelson 2010). Atoh1 can arrest the cell cycle during intestinal development by repressing the transcription factor RBP-Jκ, which is implicated in Notch-mediated cell proliferation. It is also suggested that, independently of Notch, Atoh1 can regulate the cell cycle proliferation genes p57kip and p27kip (Kazanjian et al. 2010; Kim and Shivdasani 2011; Peignon et al. 2011). Although Atoh1 has been implicated in regulation of p27kip in the gut and in medullablastoma (whether this regulation is direct is unclear), p27kip is not regulated by Atoh1 in the inner ear (Chen et al. 2002).
Atoh1 in dysplasia
Atoh1 activity in the developing cerebellum maintains cells in a proliferative state whereas, in the intestine, it causes cells to exit the cell cycle and differentiate. It follows that uncontrolled proliferation can result from Atoh1 gain-of-function in developing cerebellum but from Atoh1 loss-of-function in intestine.
In medullablastomas arising from the EGL, Atoh1 is an oncogene. In medullablastomas strongly expressing Atoh1, markers of differentiation such as TuJ1, p27kip1, NeuroD1, and Tag-1 are downregulated, whereas SHH target genes such as Gli1 and Gli2, which control the cell cycle are upregulated. This pattern of expression suggests that Atoh1 is required to maintain cells at an early stage of specification in order to provide time for clonal expansion (Briggs et al. 2008; Zhao et al. 2008; Ayrault et al. 2010).
In other tissues, Atoh1 acts not as an oncogene, but as a tumor suppressor gene in adenomatous polyposis carcinoma (APC), Atoh1 is downregulated (Leow et al. 2004; Bossuyt et al. 2009b). Whereas in neural development Atoh1 makes cells receptive to signals promoting proliferation, in gut development and homeostasis, Atoh1 represses proliferation. Atoh1 interacts with the N-methyltransferase, Dmnt, to antagonize tumor progression by (1) inducing differentiation into goblet cells, (2) halting proliferation, and (3) positively regulating apoptosis by modulating the JNK/MAPK pathway (Bossuyt et al. 2009b; VanDussen and Samuelson 2010).
Abnormal apoptotic response can also contribute to dysplasia. In APC carcinomas, ATOH1 acts through JNK/MAPK signaling to activate apoptosis, and in fly eyes, over-expression of ato leads to elevated apoptosis and an increase in the apoptotic marker Caspase 3 (Bossuyt et al. 2009a; Bossuyt et al. 2009b). Over-expression of Atoh1 in the brain results in elevated levels of apoptosis (Isaka et al. 1999), which is surprising given that in medullablastoma the apoptotic pathway is not activated. Atoh1-mediated cell death may also result from gene dosage effects rather than from specific targeting by Atoh1. Excess levels of Atoh1 in a cell may prompt the cell to enter the apoptotic pathway as a protective measure. Atoh1 activity (or lack thereof) in cancer is part of a multifactorial process. Epistasis and complementation can make it difficult to attribute cellular behaviors to specific genes. In the absence of NeuroD1 during cerebellar development, Atoh1 expression and proliferation are upregulated; however, because NeuroD1is missing, the cells cannot differentiate and are forced to undergo apoptosis (Pan et al. 2009). Thus, it could be argued that, in some of the tumors where Atoh1 apparently causes cell death or proliferation, there is another factor required downstream that is also abnormally expressed.
In summary, Atoh1 expression is found in various parts of the developing embryo and exerts varying influence depending on the cell type and developmental stage. It has been shown that targets of Atoh1-mediated transcription are tissue-dependent (Lai et al. 2011). While there are some targets of Atoh1 that are common to all tissues, some are restricted to specific populations; for example, Atoh1 binds sites within enhancers of Rassf4, Selm, Atoh1, and Grem2 in cerebellar tissue but does not drive their expression in the dorsal neural tube (Lai et al. 2011). Furthermore, targets validated in neural tissue are not necessarily transcribed in other developmental contexts; for example, only Rab15 and Selm have as yet been identified as targets regulated in both the inner ear and neural tissue (Lai et al. 2011).
Regulation of Atoh1
Genetic regulation
In mice, two enhancer sites found 3.4 kb 3′ of the Atoh1 coding region have been identified (Helms and Johnson 1998; Helms et al. 2000) (Fig. 1, B). These enhancers drive expression in the EGL of the cerebellum and in the ear at E14.5 and P0 (Helms et al. 2000). Enhancers A and B are 561 and 544 bp, respectively, separated by 300 bp.
There is no sequence similarity between A and B. Enhancer B contains an E-box selective for Atoh1 (Ebert et al. 2003), which is essential for Atoh1 expression in the neural tube. With the exception of dorsal neural tube expression, the two enhancers are redundant to each other. Interestingly, examination of HATH1 regulatory sequences shows that the human and mouse enhancers share 92% identity at enhancer A and 87% at enhancer B; this is a greater similarity than that between the orthologous proteins (Helms et al. 2000).
Whether expression of Atoh1 in vivo requires multiple combinations of different transcription factors or a single factor to effect changes in transcription, the size of enhancers A and B is suggestive of multiple protein-binding sites. Several proteins have been identified that directly interact with an Atoh1 enhancer as demonstrated by chromatin immunoprecipitation, electrophoretic mobility shift assays, or luciferase activity, for example, Zic1, cdx2, Hfn1a, β-catenin, and Hic1 (Akazawa et al. 1995; Ebert et al. 2003; Mutoh et al. 2006; Briggs et al. 2008; D'Angelo et al. 2010; Shi et al. 2010). Atoh1, Sox2, β-catenin, Hfn1a, and Cdx2 upregulate Atoh1 expression (Helms et al. 2000; Ebert et al. 2003; Gazit et al. 2004; Mutoh et al. 2006; D'Angelo et al. 2010; Shi et al. 2010; Neves et al. 2011). Hes1, Hes5, Sox2, Hic1, Ngn1, NeuroD1, and Zic1 repress Atoh1 at the level of transcription (Akazawa et al. 1995; Gowan et al. 2001; Ebert et al. 2003; Qian et al. 2006; Briggs et al. 2008; Dabdoub et al. 2008; Pan et al. 2009; Jahan et al. 2010). Intriguingly, Sox2 has the ability to both upregulate and downregulate Atoh1 depending on context (Dabdoub et al. 2008; Neves et al. 2011). In human APC carcinomas, it has been shown that ATOH1 is silenced through DNA methylation allowing tumors to escape cell cycle control and apoptosis (Bossuyt et al. 2009b).
Post-translational regulation
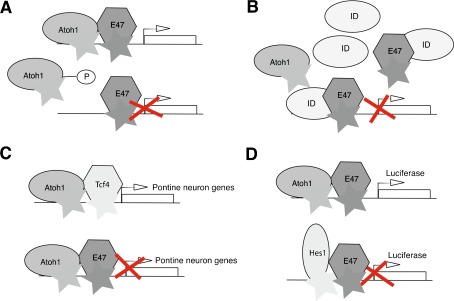
Activity of Atoh1 is also modulated at the protein level through (1) formation of heterodimers that restrict its transcriptional activities, (2) competitive-binding to E-boxes, and (3) possibly post-translational modification. The abundance of serine residues in the C terminus implies that phosphorylation might be used to modulate activity (Fig. 4A). Inhibitors of differentiation and DNA binding (Ids) 1, 2, and 3 all negatively regulate Atoh1 by competitive-binding to E proteins (Connerney et al. 2006; Jones et al. 2006) (Fig. 4B). Atoh1 can bind to the transcription factor Tcf4, creating heterodimers that specifically induce pontine nuclear neuron differentiation. This interaction is highly specific; substituting other protein partners such as E47 caused cells to take an alternative fate (Flora et al. 2007) (Fig. 4C). Co-transfection of C3H10T1/2 cells with Atoh1, an E-box luciferase-reporter and E47 resulted in luciferase activity, but, on addition of the transcription factor Hes1, luminescence was abolished, implying that Hes1 out-competes Atoh1 in binding E proteins (Akazawa et al. 1995) (Fig. 4D).
FIG. 4.
A schematic diagram illustrating some of the ways that Atoh1 activity is modulated at the protein level. A Phosphorylation may prevent interaction of Atoh1 with either DNA or with class I bHLH transcription factors such as E47, thereby preventing target gene transcription. B Ids, HLH proteins lacking the DNA binding basic region, may competitively bind E proteins displacing Atoh1. C Atoh1-mediated transcription is dependent on specific cofactors. Atoh1 coexpressed in 3 T3 cells with Tcf4, a class I bHLH transcription factor, results in differentiation of pontine neurons, but if E47 is substituted for Tcf4, Atoh1 does not promote transcription of pontine neuron genes. D Addition of E47 and Atoh1 to HEK293 cells carrying an E-box luciferase reporter results in luminescence; adding the transcription factor Hes1 abolishes luminescence, suggesting that Hes1 competes with Atoh1 in binding E proteins.
In the gut, canonical Wnt signaling maintains equilibrium between β-catenin and Atoh1. In the presence of Wnt signaling, β-catenin is allowed to accumulate in the nucleus causing cell proliferation; Atoh1 is phosphorylated and targeted for ubiquitin-mediated degradation. When no Wnt signal is received, β-catenin is targeted for degradation in place of Atoh1; Atoh1 is allowed to accumulate in the nucleus, and cells exit the cell cycle and differentiate (Leow et al. 2004; Aragaki et al. 2008; Bossuyt et al. 2009b; VanDussen and Samuelson 2010; Peignon et al. 2011). In medullablastoma cells, addition of BMP2 and 4 leads to a downregulation of Atoh1 (Zhao et al. 2008). In this case, Atoh1 may be targeted for degradation either through phosphorylation by BMP downstream effectors or by displacement of Atoh1 by Id proteins. Such interactions would be quite different from BMP’s transcriptional activation of Atoh1 in the rhombic lip. In the cochlea, BMP signaling is required for specification of the sensory epithelium (Li et al. 2005), but, during HC differentiation, is upstream of Ids 1, 2, and 3, which antagonize Atoh1 activity (Kamaid et al. 2010).
In summary, Atoh1 has been shown to be regulated at both transcriptional and post-translational levels. Multiple regulators of Atoh1 transcription have been identified. Protein associations in tandem with competitive inhibition are proposed to modulate Atoh1 activity at the protein level, and it is likely that phosphorylation-mediated degradation is employed to remove Atoh1 from the system; although, so far, direct evidence that Wnt signaling targets Atoh1 for destruction exists only for intestinal cells.
Conclusion and perspectives
Regulation of Atoh1 is exquisite, with multiple layers of control. As a transcription factor with such diverse targets, highly precise doses at clearly defined stages of development are crucial. Within the developing cochlear sensory epithelium, Sox2, Prox1, NeuroD1, Hes1, and Hes5 may repress Atoh1 transcription; it can be antagonized at the protein level by Ids, Hes1, and Hes5. Atoh1 can be targeted for degradation by GSK3β in the gut and by BMP signaling during neural patterning. Although Atoh1 has been shown to be both necessary and sufficient to drive HC differentiation, interaction with co-factors is clearly necessary to explain why, in the gut, Atoh1 expression induced cells to take on a secretory lineage, as opposed to HCs or the variety of neuronal subtypes it can specify.
The molecular context within which Atoh1 is expressed is crucial to its activity. The proline-rich N terminus (Fig. 2A) suggests protein interactions in addition to those allowed by the HLH domain at its center. Atoh1 response to Wnt, BMP, and SHH signaling is also context-dependent. Table 1 summarizes the outcome of activating these pathways in different tissues, sometimes at the transcriptional level, sometimes at the protein level. BMP and Wnt signaling can both target Atoh1 for destruction. There are multiple conserved potential phosphorylation sites in the C terminus that may allow post-translation modification, thus facilitating regulation (Fig. 2A).
TABLE 1.
Atoh1 is differently modulated by signaling pathways depending on developmental context
| Signaling pathway | Tissue | Repression of Atoh1 | Up regulation of Atoh1 | Transcriptional regulation | Post translational regulation | Reference |
|---|---|---|---|---|---|---|
| Wnt | Gut | Yes | No | No | Yes | (Leow et al. 2004; Aragaki et al. 2008; Bossuyt et al. 2009b; VanDussen and Samuelson 2010; Peignon et al. 2011) |
| Pontine neurons | No | Yes | Yes | No | (Flora et al. 2007) | |
| Chicken ear | No | Yes | ? | No | (Stevens et al. 2003) | |
| BMP | Rhombic lip | No | Yes | Yes | No | (Alder 1999; Lee et al. 2000; Wine-Lee et al. 2004) |
| Granular neuron precursor expansion | Yes | No | No | Yes | (Zhao et al. 2008) | |
| Cochlea | Yes | No | No | Yes | (Li et al. 2005; Kamaid et al. 2010) | |
| SHH | Granular neuron precursor expansion | Yes | Yes | Yes | No | (Kenney and Rowitch 2000; Romer et al. 2004; Briggs et al. 2008; Zhao et al. 2008) |
| Cochlea | No | Yes | Yes | No | (Zhao et al. 2008; Hu et al. 2010) |
Damage to hair cells from any of multiple causes (age, toxic drugs, noise, and disease) is a common cause of hearing loss. Having identified Atoh1 as an important factor in HC differentiation there is much potential for exploring Atoh1-mediated HC regeneration. Non-mammals such as birds have the ability to regenerate HCs in the inner ear after injury by sound or drug (Cruz et al. 1987; Corwin and Cotanche 1988), but that is not the case in mammals. Ectopic expression of Atoh1 in mature cochleae has had limited success. This cannot be due to transcriptional regulation of Atoh1 since it is under the control of a constitutively active promoter. It is possible that the promoter used in the vector expressing Atoh1 has tissue specific limitations (Praetorius et al. 2010), but more likely that epigenetic changes in the cochlea render cells relatively insensitive to Atoh1, that Atoh1 is targeted for destruction, that Atoh1 is post-translationally modified ablating its function or that cofactors required for HC-specific transcription factor complexes are downregulated. Identification of protein co-factors of Atoh1 during development and protein-level repression of Atoh1 postnatally could give an answer. It may also be of value to search for phosphorylation sites conserved in species that do not regenerate HCs but not in species that do. The N terminus domain is well conserved among mammalian species, but not in non-mammals. Furthermore, there are several potential phosphorylation sites in the C terminus outside the serine-rich domain that may only be conserved across species that do not regenerate HCs.
Examination of the regions flanking the bHLH domain and a better understanding of protein-level regulation of Atoh1 could open more avenues of investigation into hearing loss, medullablastoma, colon cancer, as well as various developmental disorders.
Acknowledgments
We would like to thank Drs. Jane Johnson, Bernd Fritzsch, Allen Ryan, and Elizabeth Keithley and two anonymous reviewers for their valuable comments and insights. Funding for our work on Atoh1 is provided by an NIH R01 grant (DC011104) and the Shulsky Foundation.
Glossary
Abbreviations used in this article
- β Gal
β Galactosidase
- APC
Adenomatous polyposis coli
- ASC
Aschaete–Scute complex
- AtEAM
Atoh1 E-box associated motif
- Ato
Atonal
- bHLH
Basic helix–loop–helix
- EGL
External germinal layer
- HC
Hair cell
- Id
Inhibitor of differentiation and DNA binding
- IHC
Inner hair cell
- ISH
In situ hybridization
- OC
Organ of Corti
- OHC
Outer hair cell
- SC
Supporting cell
Contributor Information
Joanna Mulvaney, Email: adabdoub@ucsd.edu.
Alain Dabdoub, Email: adabdoub@ucsd.edu.
References
- Aguado-Llera D, Goormaghtigh E, Geest N, Quan XJ, Prieto A, Hassan BA, Gomez J, Neira JL. The basic helix-loop-helix region of human neurogenin 1 is a monomeric natively unfolded protein which forms a “fuzzy” complex upon DNA binding. Biochemistry. 2010;49:1577–1589. doi: 10.1021/bi901616z. [DOI] [PubMed] [Google Scholar]
- Akazawa C, Ishibashi M, Shimizu C, Nakanishi S, Kageyama R. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J Biol Chem. 1995;270:8730–8738. doi: 10.1074/jbc.270.3.1342. [DOI] [PubMed] [Google Scholar]
- Alder J, Lee KJ, Jessell TM, Hatten ME (1999) Generation of cerebellar granule neurons in vivo by transplantation of BMP-treated neural progenitor cells. Nat Neurosci 2:535–540 [DOI] [PubMed]
- Aragaki M, Tsuchiya K, Okamoto R, Yoshioka S, Nakamura T, Sakamoto N, Kanai T, Watanabe M. Proteasomal degradation of Atoh1 by aberrant Wnt signaling maintains the undifferentiated state of colon cancer. Biochem Biophys Res Commun. 2008;368:923–929. doi: 10.1016/j.bbrc.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Ayrault O, Zhao H, Zindy F, Qu C, Sherr CJ, Roussel MF. Atoh1 inhibits neuronal differentiation and collaborates with Gli1 to generate medulloblastoma-initiating cells. Cancer Res. 2010;70:5618–5627. doi: 10.1158/0008-5472.CAN-09-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, Matzuk MM, Zoghbi HY. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–172. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- Ben-Arie N, Hassan BA, Bermingham NA, Malicki DM, Armstrong D, Matzuk M, Bellen HJ, Zoghbi HY. Functional conservation of atonal and Math1 in the CNS and PNS. Development. 2000;127:1039–1048. doi: 10.1242/dev.127.5.1039. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Wang VY, Fernandez M, Banfi S, Bellen HJ, Fritzsch B, Zoghbi HY. Proprioceptor pathway development is dependent on Math1. Neuron. 2001;30:411–422. doi: 10.1016/S0896-6273(01)00305-1. [DOI] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. Neurogenin 3 and the enteroendocrine cell lineage in the adult mouse small intestinal epithelium. Dev Biol. 2006;300:722–735. doi: 10.1016/j.ydbio.2006.07.040. [DOI] [PubMed] [Google Scholar]
- Bossuyt W, Geest N, Aerts S, Leenaerts I, Marynen P, Hassan BA. The atonal proneural transcription factor links differentiation and tumor formation in Drosophila. PLoS Biol. 2009;7:e40. doi: 10.1371/journal.pbio.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossuyt W, Kazanjian A, Geest N, Kelst S, Hertogh G, Geboes K, Boivin GP, Luciani J, Fuks F, Chuah M, VandenDriessche T, Marynen P, Cools J, Shroyer NF, Hassan BA. Atonal homolog 1 is a tumor suppressor gene. PLoS Biol. 2009;7:e39. doi: 10.1371/journal.pbio.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs KJ, Corcoran-Schwartz IM, Zhang W, Harcke T, Devereux WL, Baylin SB, Eberhart CG, Watkins DN. Cooperation between the Hic1 and Ptch1 tumor suppressors in medulloblastoma. Genes Dev. 2008;22:770–785. doi: 10.1101/gad.1640908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudy M, Grell EH, Dambly-Chaudiere C, Ghysen A, Jan LY, Jan YN. The maternal sex determination gene daughterless has zygotic activity necessary for the formation of peripheral neurons in Drosophila. Genes Dev. 1988;2:843–852. doi: 10.1101/gad.2.7.843. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.00114. [DOI] [PubMed] [Google Scholar]
- Chien CT, Hsiao CD, Jan LY, Jan YN. Neuronal type information encoded in the basic-helix–loop–helix domain of proneural genes. Proc Natl Acad Sci. 1996;93:13239–13244. doi: 10.1073/pnas.93.23.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connerney J, Andreeva V, Leshem Y, Muentener C, Mercado MA, Spicer DB. Twist1 dimer selection regulates cranial suture patterning and fusion. Dev Dyn. 2006;235:1345–1357. doi: 10.1002/dvdy.20717. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Cruz RM, Lambert PR, Rubel EW. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg. 1987;113:1058–1062. doi: 10.1001/archotol.1987.01860100036017. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW (2008) Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci U S A 105:18396–18401 [DOI] [PMC free article] [PubMed]
- Dambly-Chaudiere C, Ghysen A. Independent subpatterns of sense organs require independent genes of the Aschaete–Scute complex (ASC) in Drosophila larvae. Genes Dev. 1987;1:297–306. doi: 10.1101/gad.1.3.297. [DOI] [Google Scholar]
- D'Angelo A, Bluteau O, Garcia-Gonzalez MA, Gresh L, Doyen A, Garbay S, Robine S, Pontoglio M. Hepatocyte nuclear factor 1alpha and beta control terminal differentiation and cell fate commitment in the gut epithelium. Development. 2010;137:1573–1582. doi: 10.1242/dev.044420. [DOI] [PubMed] [Google Scholar]
- Darlington TK, Lyons LC, Hardin PE, Kay SA. The period E-box is sufficient to drive circadian oscillation of transcription in vivo. J Biol Rhythms. 2000;15:462–471. doi: 10.1177/074873040001500603. [DOI] [PubMed] [Google Scholar]
- Du X, Jensen P, Goldowitz D, Hamre K. Wild-type cells rescue genotypically Math1-null hair cells in the inner ears of chimeric mice. Dev Biol. 2007;305:430–438. doi: 10.1016/j.ydbio.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Ebert PJ, Timmer JR, Nakada Y, Helms AW, Parab PB, Liu Y, Hunsaker TL, Johnson JE. Zic1 represses Math1 expression via interactions with the Math1 enhancer and modulation of Math1 autoregulation. Development. 2003;130:1949–1959. doi: 10.1242/dev.00419. [DOI] [PubMed] [Google Scholar]
- Farah MH, Olson JM, Sucic HB, Hume RI, Tapscott SJ, Turner DL. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development. 2000;127:693–702. doi: 10.1242/dev.127.4.693. [DOI] [PubMed] [Google Scholar]
- Flora A, Garcia JJ, Thaller C, Zoghbi HY. The E-protein Tcf4 interacts with Math1 to regulate differentiation of a specific subset of neuronal progenitors. Proc Natl Acad Sci. 2007;104:15382–15387. doi: 10.1073/pnas.0707456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora A, Klisch TJ, Schuster G, Zoghbi HY. Deletion of Atoh1 disrupts Sonic Hedgehog signaling in the developing cerebellum and prevents medulloblastoma. Science. 2009;326:1424–1427. doi: 10.1126/science.1181453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259:1616–1619. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Matei VA, Nichols DH, Bermingham N, Jones K, Beisel KW, Wang VY. Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev Dyn. 2005;233:570–583. doi: 10.1002/dvdy.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit R, Krizhanovsky V, Ben-Arie N. Math1 controls cerebellar granule cell differentiation by regulating multiple components of the Notch signaling pathway. Development. 2004;131:903–913. doi: 10.1242/dev.00982. [DOI] [PubMed] [Google Scholar]
- Gowan K, Helms AW, Hunsaker TL, Collisson T, Ebert PJ, Odom R, Johnson JE. Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron. 2001;31:219–232. doi: 10.1016/S0896-6273(01)00367-1. [DOI] [PubMed] [Google Scholar]
- Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455:537–541. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms AW, Johnson JE. Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development. 1998;125:919–928. doi: 10.1242/dev.125.5.919. [DOI] [PubMed] [Google Scholar]
- Helms AW, Abney AL, Ben-Arie N, Zoghbi HY, Johnson JE. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development. 2000;127:1185–1196. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- Hu X, Huang J, Feng L, Fukudome S, Hamajima Y, Lin J (2010) Sonic hedgehog (SHH) promotes the differentiation of mouse cochlear neural progenitors via the Math1-Brn3.1 signaling pathway in vitro. J Neurosci Res 88:927–935 [DOI] [PMC free article] [PubMed]
- Isaka F, Ishibashi M, Taki W, Hashimoto N, Nakanishi S, Kageyama R. Ectopic expression of the bHLH gene Math1 disturbs neural development. Eur J Neurosci. 1999;11:2582–2588. doi: 10.1046/j.1460-9568.1999.00699.x. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res. 2008;240:52–56. doi: 10.1016/j.heares.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Fritzsch B. Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PLoS One. 2010;5:e11661. doi: 10.1371/journal.pone.0011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman AP, Grau Y, Jan LY, Jan YN. atonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell. 1993;73:1307–1321. doi: 10.1016/0092-8674(93)90358-W. [DOI] [PubMed] [Google Scholar]
- Jones JM, Montcouquiol M, Dabdoub A, Woods C, Kelley MW. Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J Neurosci. 2006;26:550–558. doi: 10.1523/JNEUROSCI.3859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaid A, Neves J, Giraldez F. Id gene regulation and function in the prosensory domains of the chicken inner ear: a link between Bmp signaling and Atoh1. J Neurosci. 2010;30:11426–11434. doi: 10.1523/JNEUROSCI.2570-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanjian A, Noah T, Brown D, Burkart J, Shroyer NF. Atonal homolog 1 is required for growth and differentiation effects of Notch/γ-secretase inhibitors on normal and cancerous intestinal epithelial cells. Gastroenterology. 2010;139:918–928.e916. doi: 10.1053/j.gastro.2010.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney AM, Rowitch DH (2000) Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Molecular and Cellular Biology 20:9055–9067 [DOI] [PMC free article] [PubMed]
- Kim TH, Shivdasani RA. Genetic evidence that intestinal notch functions vary regionally and operate through a common mechanism of math1 repression. J Biol Chem. 2011;286:11427–11433. doi: 10.1074/jbc.M110.188797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klisch TJ, Xi Y, Flora A, Wang L, Li W, Zoghbi HY. In vivo Atoh1 targetome reveals how a proneural transcription factor regulates cerebellar development. Proc Natl Acad Sci. 2011;108:3288–3293. doi: 10.1073/pnas.1100230108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriacou CP, Rosato E. Squaring up the E-box. J Biol Rhythms. 2000;15:483–490. doi: 10.1177/074873040001500605. [DOI] [PubMed] [Google Scholar]
- Lai HC, Klisch TJ, Roberts R, Zoghbi HY, Johnson JE. In vivo neuronal subtype-specific targets of Atoh1 (Math1) in dorsal spinal cord. J Neurosci. 2011;31:10859–10871. doi: 10.1523/JNEUROSCI.0445-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Shailam R, Norton CR, Ridley T, Kelley MW. Expression of Math1 and HES5 in the cochleae of wild type and Jag2 mutant mice. J Assoc Res Otolarngol. 2000;1:161–171. doi: 10.1007/s101620010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Dietrich P, Jessell TM (2000) Genetic ablation reveals that the roof plate is essential for dorsal interneuron specification. Nature 403:734–740 [DOI] [PubMed]
- Leow CC, Romero MS, Ross S, Polakis P, Gao WQ. Hath1, down-regulated in colon adenocarcinomas, inhibits proliferation and tumorigenesis of colon cancer cells. Cancer Res. 2004;64:6050–6057. doi: 10.1158/0008-5472.CAN-04-0290. [DOI] [PubMed] [Google Scholar]
- Li H, Corrales CE, Wang Z, Zhao Y, Wang Y, Liu H, Heller S. BMP4 signaling is involved in the generation of inner ear sensory epithelia. BMC Dev Biol. 2005;5:16. doi: 10.1186/1471-213X-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez I, Honrubia V, Lee SC, Schoeman G, Beykirch K. Quantification of the process of hair cell loss and recovery in the chinchilla crista ampullaris after gentamicin treatment. Int J Dev Neurosci. 1997;15:447–461. doi: 10.1016/S0736-5748(96)00103-7. [DOI] [PubMed] [Google Scholar]
- Machold R, Fishell G. Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron. 2005;48:17–24. doi: 10.1016/j.neuron.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005;234:633–650. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh H, Sakamoto H, Hayakawa H, Arao Y, Satoh K, Nokubi M, Sugano K. The intestine-specific homeobox gene Cdx2 induces expression of the basic helix-loop-helix transcription factor Math1. Differentiation. 2006;74:313–321. doi: 10.1111/j.1432-0436.2006.00074.x. [DOI] [PubMed] [Google Scholar]
- Nakada Y, Hunsaker TL, Henke RM, Johnson JE. Distinct domains within Mash1 and Math1 are required for function in neuronal differentiation versus neuronal cell-type specification. Development. 2004;131:1319–1330. doi: 10.1242/dev.01008. [DOI] [PubMed] [Google Scholar]
- Neves J, Parada C, Chamizo M, Giraldez F. Jagged 1 regulates the restriction of Sox2 expression in the developing chicken inner ear: a mechanism for sensory organ specification. Development. 2011;138:735–744. doi: 10.1242/dev.060657. [DOI] [PubMed] [Google Scholar]
- Pan N, Jahan I, Lee JE, Fritzsch B. Defects in the cerebella of conditional Neurod1 null mice correlate with effective Tg(Atoh1-cre) recombination and granule cell requirements for Neurod1 for differentiation. Cell Tissue Res. 2009;337:407–428. doi: 10.1007/s00441-009-0826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti. Hear Res. 2010;275:66–80. doi: 10.1016/j.heares.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peignon G, Durand A, Cacheux W, Ayrault O, Terris B, Laurent-Puig P, Shroyer NF, Seuningen I, Honjo T, Perret C, Romagnolo B. Complex interplay between beta-catenin signalling and Notch effectors in intestinal tumorigenesis. Gut. 2011;60:166–176. doi: 10.1136/gut.2009.204719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LM, Lage PI, Prentice DRA, Senthinathan B, Jarman AP. The proneural proteins Atonal and Scute regulate neural target genes through different E-box binding sites. Mol Cell Biol. 2004;24:9517–9526. doi: 10.1128/MCB.24.21.9517-9526.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LM, Deaton AM, Wear MA, Jarman AP. Specificity of Atonal and Scute bHLH factors: analysis of cognate E box binding sites and the influence of Senseless. Genes Cells. 2008;13:915–929. doi: 10.1111/j.1365-2443.2008.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius M, Hsu C, Baker K, Brough DE, Plinkert P, Staecker H. Adenovector-mediated hair cell regeneration is affected by promoter type. Acta Otolaryngol. 2010;130:215–222. doi: 10.3109/00016480903019251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D, Radde-Gallwitz K, Kelly M, Tyrberg B, Kim J, Gao W-Q, Chen P. Basic helix–loop–helix gene Hes6 delineates the sensory hair cell lineage in the inner ear. Dev Dyn. 2006;235:1689–1700. doi: 10.1002/dvdy.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, Connelly M, Stewart CF, Gould S, Rubin LL, Curran T (2004) Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/-)p53(-/-) mice. Cancer cell 6:229–240 [DOI] [PubMed]
- Rose MF, Ahmad KA, Thaller C, Zoghbi HY. Excitatory neurons of the proprioceptive, interoceptive, and arousal hindbrain networks share a developmental requirement for Math1. Proc Natl Assoc Sci. 2009;106:22462–22467. doi: 10.1073/pnas.0911579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MF, Ren J, Ahmad KA, Chao H-T, Klisch TJ, Flora A, Greer JJ, Zoghbi HY. Math1 is essential for the development of hindbrain neurons critical for perinatal breathing. Neuron. 2009;64:341–354. doi: 10.1016/j.neuron.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Cheng YF, Wang XL, Edge AS. Beta-catenin up-regulates Atoh1 expression in neural progenitor cells by interaction with an Atoh1 3′ enhancer. J Biol Chem. 2010;285:392–400. doi: 10.1074/jbc.M109.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroyer NF, Wallis D, Venken KJ, Bellen HJ, Zoghbi HY. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 2005;19:2412–2417. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CB, Davies AL, Battista S, Lewis JH, Fekete DM (2003) Forced activation of Wnt signaling alters morphogenesis and sensory organ identity in the chicken inner ear. Dev Biol 261:149–164 [DOI] [PubMed]
- VanDussen KL, Samuelson LC. Mouse atonal homolog 1 directs intestinal progenitors to secretory cell rather than absorptive cell fate. Dev Biol. 2010;346:215–223. doi: 10.1016/j.ydbio.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villares R, Cabrera CV. The Achaete-Scute gene complex of D. melanogaster: conserved domains in a subset of genes required for neurogenesis and their homology to myc. Cell. 1987;50:415–424. doi: 10.1016/0092-8674(87)90495-8. [DOI] [PubMed] [Google Scholar]
- Wang VY, Hassan BA, Bellen HJ, Zoghbi HY. Drosophila atonal fully rescues the phenotype of Math1 null mice: new functions evolve in new cellular contexts. Curr Biol. 2002;12:1611–1616. doi: 10.1016/S0960-9822(02)01144-2. [DOI] [PubMed] [Google Scholar]
- Wang VY, Rose MF, Zoghbi HY. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron. 2005;48:31–43. doi: 10.1016/j.neuron.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Wang GP, Chatterjee I, Batts SA, Wong HT, Gong TW, Gong SS, Raphael Y. Notch signaling and Atoh1 expression during hair cell regeneration in the mouse utricle. Hear Res. 2010;267:61–70. doi: 10.1016/j.heares.2010.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wine-Lee L, Ahn KJ, Richardson RD, Mishina Y, Lyons KM, Crenshaw EB, 3rd (2004) Signaling through BMP type 1 receptors is required for development of interneuron cell types in the dorsal spinal cord. Development 131:5393–5403 [DOI] [PubMed]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7:1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Yang H, Xie X, Deng M, Chen X, Gan L. Generation and characterization of Atoh1-Cre knock-in mouse line. Genesis. 2010;48:407–413. doi: 10.1002/dvg.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Zhou Q, Zhang C, Pignoni F (2011) Identification of Bombyx Atonal and functional comparison with the Drosophila Atonal proneural factor in the developing fly eye. Genesis (in press) [DOI] [PMC free article] [PubMed]
- Zhao H, Ayrault O, Zindy F, Kim JH, Roussel MF. Post-transcriptional down-regulation of Atoh1/Math1 by bone morphogenic proteins suppresses medulloblastoma development. Genes Dev. 2008;22:722–727. doi: 10.1101/gad.1636408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, Ribaupierre F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]