Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare, highly aggressive hematopoietic malignancy that is characterized by cutaneous and bone marrow involvement and leukemic spread, representing 0.7% of primary cutaneous lymphomas1. Formerly known as CD4+/CD56+ hematodermic neoplasm or blastic NK-cell lymphoma2, BPDCN is derived from precursors of plasmacytoid dendritic cells and was only recently established as a distinct entity3.
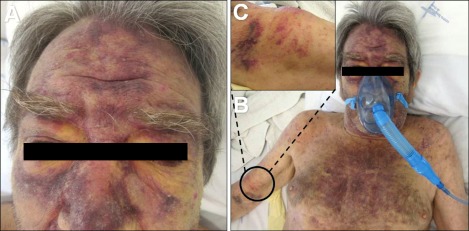
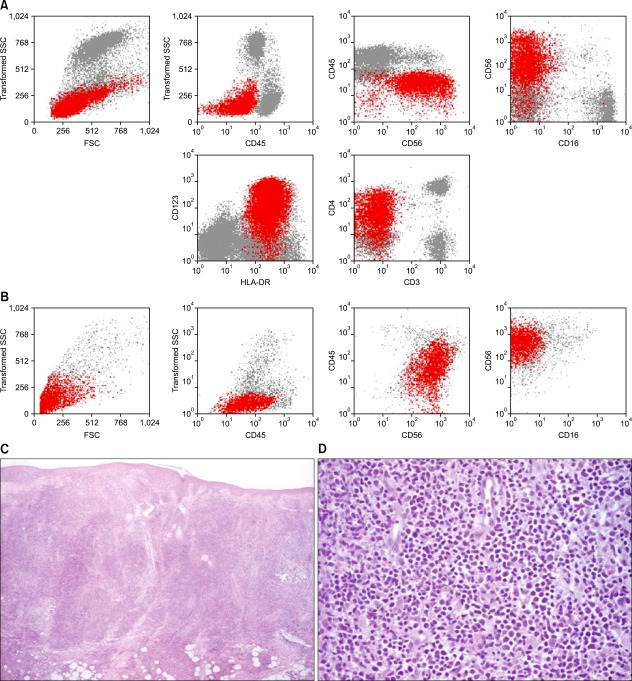
A 74-year-old man presented with a 4-month history of asymptomatic cutaneous lesions that initially appeared on his face but progressively spread to his thorax and arms. He had no systemic symptoms. His medical history included peripheral arterial disease and dyslipidemia, for which he was on acenocumarol and rosuvastatin. Physical examination revealed infiltrated violaceous plaques and nodules on his face and thorax and firm subcutaneous nodules on his arms along with reticulated purpuric macules (Fig. 1). Axillary and submandibular lymph nodes were bilaterally palpable. Laboratory data disclosed severe normochromic normocytic anemia (hemoglobin, 4.4 g/dl), thrombocytopenia (53×109/L), leukocytosis (16.48×109/L) with 44% of morphologically immature atypical cells and elevated β2-microglobulin (3.8 mg/L). Flow cytometry (FCM) of the skin cells and the peripheral blood showed 35% and 37%, respectively, of cells with the following immunophenotype (Fig. 2A, B): positive CD45 (dim), CD4, CD56, CD123, CD7 (dim), HLA-DR, and CD38. Other T/NK (CD1a, CD2, CD3, TCR, CD5, CD8, CD16, CD94, CD161), B (CD19, CD20, CD79a), myeloid (CD11b, CD13, CD14, CD15, CD33, CD64, myeloperoxidase) and immature cell (CD34) markers tested negative. Histopathology of a skin lesion showed a monomorphous diffuse infiltrate of large blastic cells with irregular nuclei occupying the entire dermis and infiltrating the subcutaneous tissue (Fig. 2C, D). Immunohistochemical analysis confirmed the presence of cells with the aforementioned immunophenotypic features. No chromosomal alterations were detected by cytogenetic analysis of peripheral blood cells. Computed tomography scans disclosed basal lung consolidation suggestive of malignant infiltration, mediastinal, hilar, axillary and retroperitoneal lymphadenopathy, hepatomegaly, and splenomegaly, findings that were compatible with widespread disease. Bone marrow analysis was not performed once its involvement was confirmed by the altered peripheral blood profile. Together these findings fulfilled the requirements for the diagnosis of BPDCN. The patient started palliative chemotherapy with cytarabine and mitoxantrone but died 3 weeks later of multi-organ failure.
Fig. 1.
(A, B) Features of the cutaneous lesions on the face and thorax. (C) Highlight of one firm subcutaneous nodule on the right arm.
Fig. 2.
(A, B) Flow cytometry dot plots of peripheral blood (A) and skin (B) cells showing blastic plasmacytoid dendritic cells (blastic plasmacytoid dendritic cell neoplasm, red dots), respectively, expressing CD45 (dim), CD4 (dim), CD56, CD123, and HLA-DR. (C, D) Histopathological examination of a cutaneous lesion. (C) Diffuse infiltrate occupying the entire dermis and infiltrating the subcutaneous tissue (hematoxylin and eosin stain [H&E]; original magnification, ×4). (D) Higher amplification of the large blastic cells with large irregular nuclei (H&E; original magnification, ×40). SSC: side scatter; FSC: forward scatter.
BPDCN is a rapidly evolving disease that primarily affects the elderly. The clinical presentation is quite constant, with 90% of patients presenting with asymptomatic solitary/multifocal cutaneous reddish-brown nodules or bruise-like lesions4,5. Bone marrow is involved in most cases, and practically any organ can be affected. The disease follows a short course and fulminant leukemia is the common terminal stage4-6. The diagnosis relies on the immunophenotypic features of the malignant cells. FCM is preferred over immunohistochemical analysis since it allows for the examination of more markers and their intensity determination7,8. The expression of CD4, CD56, and CD123 in the absence of T-cell, B-cell, or myeloid markers defines BPDCN4-8. The correct diagnosis implies an appropriate panel of antibodies; in contrast, insufficient knowledge on this entity and inadequate immunophenotypic investigation can lead to the misdiagnosis of a different leukemia8. Cytogenetic analysis is not helpful since no recurrent specific chromosomal aberrations were recognized7,8. The prognosis of patients with BPDCN is poor, with a median survival of 12~14 months regardless of treatment type9. Acute lymphoblastic leukemia-type treatment regimens are advised and a promising initial response may occur, but is followed by quick relapse6,9,10. Long-term remissions have been rarely reported in younger patients who received acute leukemia-type induction therapy and allogeneic stem cell transplantation9-11.
Although clarification of the immunophenotypic features of BPDCN has improved its recognition, this entity remains a diagnostic challenge. Cutaneous lesions are usually the only sign of the disease, so dermatologists should be knowledgeable about it and play a crucial role in uncovering this malignancy and avoiding diagnostic delays.
References
- 1.Petrella T, Bagot M, Willemze R, Beylot-Barry M, Vergier B, Delaunay M, et al. Blastic NK-cell lymphomas (agranular CD4+CD56+ hematodermic neoplasms): a review. Am J Clin Pathol. 2005;123:662–675. [PubMed] [Google Scholar]
- 2.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 3.Faccheti F, Jones DM, Petrella T. Blastic plasmacytoid dendritic cell neoplasm. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer; 2008. pp. 145–147. [Google Scholar]
- 4.Whittle AM, Howard MR. Skin lesions in plasmacytoid dendritic cell leukaemia. Br J Haematol. 2008;140:121. doi: 10.1111/j.1365-2141.2007.06821.x. [DOI] [PubMed] [Google Scholar]
- 5.Magro CM, Porcu P, Schaefer J, Erter JW, Furman RR, Shitabata PK, et al. Cutaneous CD4+ CD56+ hematologic malignancies. J Am Acad Dermatol. 2010;63:292–308. doi: 10.1016/j.jaad.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 6.Herling M, Jones D. CD4+/CD56+ hematodermic tumor: the features of an evolving entity and its relationship to dendritic cells. Am J Clin Pathol. 2007;127:687–700. doi: 10.1309/FY6PK436NBK0RYD4. [DOI] [PubMed] [Google Scholar]
- 7.Garnache-Ottou F, Feuillard J, Ferrand C, Biichle S, Trimoreau F, Seilles E, et al. Extended diagnostic criteria for plasmacytoid dendritic cell leukaemia. Br J Haematol. 2009;145:624–636. doi: 10.1111/j.1365-2141.2009.07679.x. [DOI] [PubMed] [Google Scholar]
- 8.Tsagarakis NJ, Kentrou NA, Papadimitriou KA, Pagoni M, Kokkini G, Papadaki H, et al. Acute lymphoplasmacytoid dendritic cell (DC2) leukemia: results from the Hellenic Dendritic Cell Leukemia Study Group. Leuk Res. 2010;34:438–446. doi: 10.1016/j.leukres.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Feuillard J, Jacob MC, Valensi F, Maynadié M, Gressin R, Chaperot L, et al. Clinical and biologic features of CD4(+) CD56(+) malignancies. Blood. 2002;99:1556–1563. doi: 10.1182/blood.v99.5.1556. [DOI] [PubMed] [Google Scholar]
- 10.Reimer P, Rüdiger T, Kraemer D, Kunzmann V, Weissinger F, Zettl A, et al. What is CD4+CD56+ malignancy and how should it be treated? Bone Marrow Transplant. 2003;32:637–646. doi: 10.1038/sj.bmt.1704215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jegalian AG, Buxbaum NP, Facchetti F, Raffeld M, Pittaluga S, Wayne AS, et al. Blastic plasmacytoid dendritic cell neoplasm in children: diagnostic features and clinical implications. Haematologica. 2010;95:1873–1879. doi: 10.3324/haematol.2010.026179. [DOI] [PMC free article] [PubMed] [Google Scholar]