Abstract
There are limitations on diagnostic methods to differentiate between active and latent tuberculosis (TB), and the prediction of latent progression to TB disease is yet complex. Traditionally, tuberculosis-specific host immune response was visualized using the tuberculin skin test. Nowadays, IFN-γ release assays (IGRA) provide a more specific and sensitive tool, by which exposure to Mtb could be determined. However, the merit of IGRA aids in diagnosing active TB is yet unclear. We adapted IGRA for use in mice, and quantifying bead-based flow cytometry techniques were used to assess cytokine profiles during the course of untreated infection and to investigate the value of IGRA and cytokines as biomarkers for therapy response. High variability of IGRA results during progression of active TB infection related to various phases of infection was obtained. However, a significant decrease in IGRA results and in levels of IFN-γ, IL-17, IP-10 or MIG was observed and appeared to be associated with successful therapy. This outcome does not support the value of IGRA to accurately diagnose active TB or to monitor infection progression. However, IGRA proved to be a useful biomarker to monitor therapy success. In addition, different cytokines might serve as biomarkers.
Introduction
Diagnosing tuberculosis (TB) remains complex and challenging. Differentiating between latent TB infections (LTBI) and active TB is of vital importance. Various tools for diagnosing TB are available. First, there is detection of Mycobacterium tuberculosis (Mtb) in sputum and other fluids (e.g. microscopic determination of bacilli, culture or amplification of Mtb-specific genome parts). Second, recognition of TB can be made in terms of disease presentation (e.g. patient anamnesis or chest radiography) or detection of a TB-specific host immune response (e.g. tuberculin skin test (TST) or interferon gamma (IFN-γ) release assays (IGRA). A limitation of the gold standard, that is, Mtb culture, is that it may take as long as 6 weeks, so more rapid detection of TB is needed. Therefore, numerous studies have been performed to identify biomarkers to detect TB infection, among which IGRA as potential biomarker [1–4]. The dynamics of IGRA during the course of untreated TB in not yet clear. The goal of the present study was to provide further insight in this respect and to investigate the value of IGRA as a biomarker to monitor therapy response.
IGRA are in vitro tests detecting an immune response to Mtb-specific antigens. IGRA was introduced in the last decade as replacement of the TST and should provide more specific test results in LTBI without cross-reactivity after a Bacillus Calmette-Guérin vaccination [5]. Currently, two different IGRA are available, QuantiFERON-TB Gold (QFT; Cellestis Ltd, Carnegie, Victoria, Australia) and T.SPOT.TB ELISpot (ELISpot; Oxford Immunotec, Abingdon, UK). Both tests are based on quantitative measurement of IFN-γ production by peripheral blood cells after stimulation with the Mtb-specific peptides early-secreted antigenic target-6 (ESAT-6) and culture filtrate protein-10 (CFP-10). Both IGRA showed excellent specificity that is unaffected by earlier BCG vaccination [5]. Several studies showed that T-SPOT.TB was more sensitive than QFT or TST [2, 5]. An important annotation to these findings is the absence of a gold standard to diagnose LTBI. Nevertheless, the CDC recommended that QFT can be used in all circumstances in which TST was used, including contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control [6]. This advice is incorporated in many national guidelines. At present the merit of IGRA for diagnosing active TB, additionally to their use in diagnosing LTBI, is still under debate.
From clinical studies the additional value of using respiratory samples next to blood samples for IGRA is not (yet) clear. It is hypothesized that an IGRA performed in a sample from the primary site of infection (e.g. the lungs) might differentiate between active TB and LTBI. In patients bronchoalveolar lavage fluid or pleural fluid is used in such a way [7]. In the present study we also addressed this matter, by comparing respiratory sample IGRA results with blood IGRA results. Additionally, we assessed the cytokine/ chemokine profile in serum samples from mice at the acute and chronic phases of TB infection and during therapy and investigated the diagnostic value of 17 immunological markers using a multiplex panel of bead-labelled cytokine and chemokine receptors.
In order to provide further insight into the dynamics of IGRA we adapted IGRA for use in our murine TB model [8]. A mouse model enables investigation of the value of this new test during the acute phase and chronic phase of infection and during standardized, well-controlled therapy. In animal models comparative studies at similar conditions of severity and duration of infection and therapy exposure can be performed, which is difficult in patients due to different underlying clinical conditions [9].
Materials and methods
Animals
Specified pathogen-free female BALB/c mice, obtained from Charles River (Les Oncins, France) were infected at 10–12 weeks of age, as described previously [8]. The experimental protocols adhered to the rules specified in the Dutch Animal Experimentation Act and the published Guidelines on the Protection of Experimental Animals by the Council of the EC. The Institutional Animal Care and Use Committee of the Erasmus MC Rotterdam approved the present protocols.
Infection
Mice were infected through intratracheal instillation of a 40-μL suspension containing 9.4x104 CFU Mtb (H37Rv), as previously described [8].
Anti-tuberculosis drugs
Therapy was administered from 4 weeks after the infection onwards. The therapeutic regimen consisted of a combination of isoniazid, rifampicin and pyrazinamide during the initial phase of 9 weeks, followed by a combination of isoniazid and rifampicin during 4 weeks, as described previously [8]. Anti-tuberculosis drugs were administered in human pharmacokinetic-equivalent doses [10, 11].
Interferon-gamma release assay
Peptides ESAT-6 / CFP-10 were synthesized by Pepscan (Pepscan Therapeutics, Lelystad, The Netherlands). Blood samples were obtained from sacrificed mice by cardiac puncture at indicated time points. In mice receiving therapy, administration of anti-tuberculosis drugs was stopped 72 h before dissection. Within 15 minutes of collection of blood, the heparinized samples were diluted five times using a mixture of RPMI 1640 medium (BioWhittaker–Cambrex, East Rutherford, New Jersey, USA) enriched with 2 mM L-glutamine (Invitrogen, Carlsbad, CA, USA), 100 units of 100 μg/ml penicillin-streptomycin solution (Invitrogen), 10% heat-inactivated fetal calf serum (Invitrogen) and β-mercapto-ethanol 0.002‰ (Sigma M7522, Sigma Chemical, St. Louis, MO, USA). Samples were divided into three portions, and stimulated with either concanavalin A (Invitrogen) in a final concentration of 10 μg/ml (positive control) or with enriched RPMI 1640 medium (negative control) or with a peptide mixture of ESAT-6 and CFP-10 (Pepscan) both in a final concentration of 0.25, 0.5, 1 or 2 μg/ml (Mtb-specific antigen response). After incubation for 24, 48, 72 or 96 hrs, at 37°C and 5% CO2, 50 μl of the supernatant was used to assess the IFN-γ concentration in duplicate, employing the Mouse IFN-γ ELISA-kit II according to the manufactures protocol (Becton Dickinson, Franklin Lakes, NJ, USA) and a Bio-Rad Elisa Reader model 680 (Bio-Rad Laboratories, Veenendaal, the Netherlands). To prepare lung cell suspensions from sacrificed mice the lungs were aseptically removed and processed using a cell strainer with 70 μm nylon mesh (Becton Dickinson). Cell suspensions were subsequently processed according to the protocol described for blood.
Cytokine / chemokine concentrations in EDTA plasma
Quantification of cytokines / chemokines was performed using a bead-based flow cytometry technique (xMap; Luminex Corporation, Austin, TX, USA). The mouse soluble cytokine receptor multiplex panel used (Millipore Corporation, Billerica, MA, USA), consisted of a bead-labelled cytokine receptor against the following biomarkers: GM-CSF, IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12 (p40), IL-13, IL-17, IP-10, M-CSF, MCP-1, MIG, MIP-1α, MIP-1β and TNF-α. Tests were performed according to manufactures protocol. Samples were tested in duplicates. Results in median fluorescence intensity (MFI) values were converted to pg/mL using MILLIPLEX Analyst software (Millipore) and subsequently averaged. Statistical analysis was performed using a two-tailed, unpaired Mann-Whitney test. Only statistically significant (p-value ≤0.05) changes in cytokine concentrations were presented.
Results
Adaptation and interpretation of IGRA in mice with untreated TB infection
To optimize IGRA for use in our mouse TB model, in blood samples obtained from phosphate buffered saline (PBS)-inoculated control mice and TB-infected mice at different ages, the optimum concentration of antigens ESAT-6 and CFP-10 peptides and the optimum time period of incubation were determined. In blood from TB-infected mice a maximum IFN-γ production after stimulation with 1 μg/ml of each peptide was found and optimum time of antigen exposure was 72 h (data not shown). IFN-γ production level resulting from exposure of blood to Mtb-specific-antigens minus IFN-γ concentrations present in the negative controls was used as final read-out of the test and referred to as IGRA results, similar to the evaluation of IGRA results obtained in human blood.
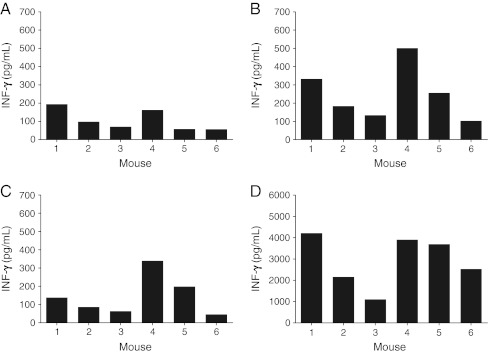
At week four of the TB infection, six individual mice were investigated; different responses were obtained (Fig. 1). A distinct pattern per mouse was observed. Three out of six mice showed that a high response in the positive control (Fig. 1d) also showed a high response on Mtb-specific antigens stimulation (Fig. 1b) and a high response in the negative control (Fig. 1a). These mice still showed a high IGRA result (Fig. 1c). Three out of six mice showed overall lower responses.
Fig. 1.
IFN-γ production in blood from six individual mice at 4 weeks after untreated TB infection. Blood samples were processed in IGRA. a Negative controls. b Mtb-specific-antigens responses after stimulation with ESAT-6 and CFP-10. c IGRA results (Mtb-specific-antigens responses minus the negative controls). d Positive controls. Data shown are mean IFN-γ concentrations of measurements in duplicate
IGRA in blood during the course of untreated TB infection in mice
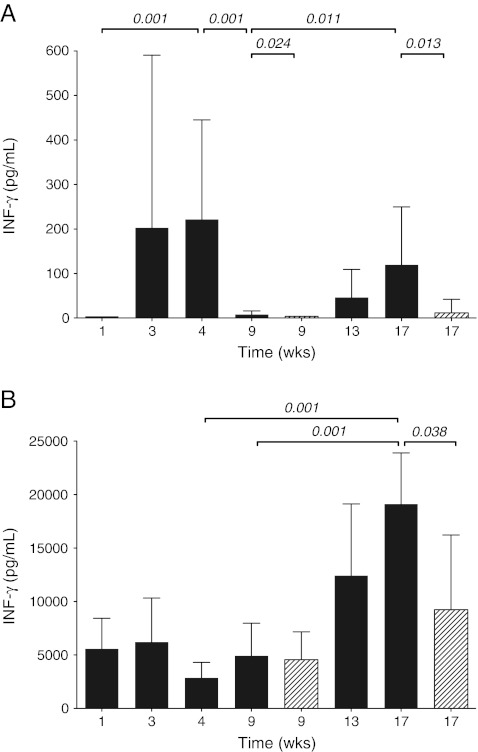
Dynamics in immune response, expressed in IGRA results during the course of infection, are shown in Fig. 2. In the acute phase of TB, the positive control response was present at week 1 and remained at a certain level (Fig. 2b). However, IGRA results were not observed at week 1, but were significantly induced at week 3 and week 4 (Fig. 2a). At the beginning of the chronic phase of the TB infection, IGRA results dropped dramatically between week 4 and week 9 (Fig. 2a), whereas positive control response did not decrease (Fig. 2b). In order to determine sequestration, IGRA were performed in suspensions of lung cells from infected mice at 4, 9 and 17 weeks of infection. High IGRA results in lung cell suspensions were observed at 4 weeks of infection, reflecting high activity of lung T-cells upon stimulation with Mtb-specific-antigens (data not shown). During the course of infection IGRA results in lung cells observed at week 4 decreased 15-fold at week 9 and another 1.5-fold at week 17 (data not shown). From 9 weeks after infection onwards IGRA results in blood gradually and significantly increase until 15-fold response at week 17 compared to 9 weeks of infection (Fig. 2a). Interestingly, also positive control response increased 4-fold in that period (Fig. 2b).
Fig. 2.
IFN-γ production in blood from mice (n = 7) during the course of untreated TB infection and TB-infected mice receiving therapy. Blood samples were processed in IGRA. Mice without therapy (dark gray bars) and mice receiving therapy (diagonally striped bars). a IGRA results (Mtb-specific-antigens responses minus the negative controls). b Positive controls. Data shown are mean IFN-γ concentrations of measurements in duplicate. The whiskers represent the standard deviation (SD). Statistical significance if present (p-value ≤0.05) is indicated
IGRA in blood of TB-infected mice receiving therapy
IGRA results in blood from mice both after 5 weeks and after 13 weeks of therapy were significantly decreased compared to untreated TB-infected mice (Fig. 2a). Positive control response did not significantly change after 5 weeks of therapy, but was lower after 13 weeks of therapy (Fig. 2b). When, after 13 weeks, the therapy was stopped, IGRA results did not increase again at 6 weeks after termination of therapy. Even at 13 weeks after therapy termination, when relapse of TB infection in terms of increased numbers of Mtb in the lung and histopathological changes was observed [8], IGRA results remained low (data not shown).
Cytokine or chemokine profile in plasma during the course of untreated TB infection in mice and TB-infected mice receiving therapy
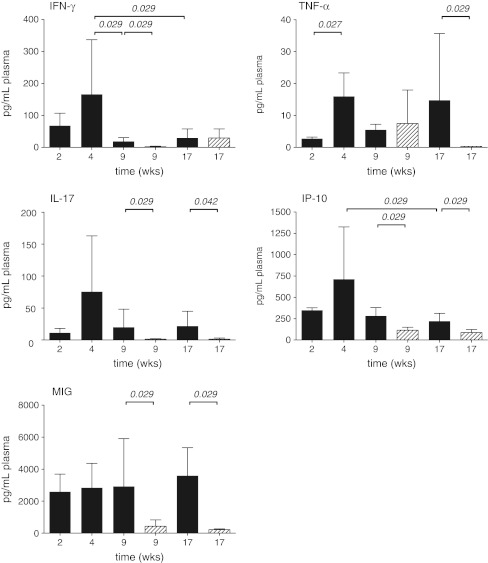
Five of the 17 cytokines and chemokines assessed showed a dynamic profile with significant changes during infection progression or following therapy. IFN-γ concentration in blood reached a maximum at 4 weeks after infection (Fig. 3). During the chronic phase of infection IFN-γ concentration significantly decreased. As found for IFN-γ also IL-17 and IP-10 showed an initial increase, which resulted in peak concentrations at 4 weeks after infection, followed by a decrease from that time (Fig. 3). Dynamics of TNF-α concentration showed a somewhat different pattern, with increased concentrations from week 4 onwards. MIG concentrations during the course of the untreated infection were constant (Fig. 3).
Fig. 3.
Concentrations of cytokines or chemokines in plasma from mice (n = 4) during the course of untreated TB infection and TB-infected mice receiving therapy: mice without therapy (dark gray bars) and mice receiving therapy (diagonally striped bars). Data shown are mean concentrations of cytokines or chemokines of measurements in duplicate. The whiskers represent the standard deviation (SD). Statistically significance if present (p-value ≤0.05) is indicated
In mice receiving therapy, concentrations of IFN-γ, IL-17, IP-10 and MIG were decreased after 5 weeks of therapy, which was also observed after 13 weeks of therapy except for IFN-γ. Decreased levels of TNF-α were only observed after 13 weeks of therapy (Fig. 3).
Discussion
In this study the dynamics of IGRA in TB infection and the effect of therapy were investigated in a mouse model. To this aim IGRA was optimized for use in mice. We observed a variety in IGRA responses between individual mice, which might be explained by differences in phase of TB infection in these mice and hence the number of reactive T-cells in blood. In a previous study, we showed that around 4 weeks after Mtb inoculation, the acute phase of infection progresses into the chronic phase [8]. At that time point mouse to mouse variation in T-cell response can be expected. Unfortunately, T-cell numbers in blood of TB-infected mice could not be determined, since the fluorescence-activated cell sorting technique using Mtb-infected blood samples could not be performed at our institute due to safety regulations.
Dynamics in immune response expressed in IGRA results during TB showed a very slow start, which might be due to T-regulatory cell activity in the initial phase of infection, as described by Shafiani et al. [11]. However, a significant increase in IGRA results was observed up to 4 weeks after infection. The observed fall in IGRA results at 9 weeks after inoculation suggests that this might be due to migration of specific T-cells to the lung and/or due to strong regulation. IGRA results in lung cell suspensions during the active phase of infection showed indeed peak values at 4 weeks after infection. These data are in concordance with the study of Arko-Mensah et al. [1], showing that increased levels of (free) IFN-γ in the respiratory tract correlated with active pulmonary TB in mice. However, our observation that IGRA results in lung T-cells decreased after 4 weeks of infection (data not shown) discarded the hypothesis that migration of T-cells to the lung was responsible for decreased IGRA results in blood. Possibly the decline in IGRA result might be due to down-regulation of T-cells [12]. It may also be related to the IFN-γ depressing capacity of Mtb during the active infection, by inhibiting IFN-γ receptor signalling, as demonstrated in a study of Sahiratmadja et al. [13]. The observed increase in IGRA results and positive results from 9 weeks of infection onwards might reflect an increase in cellularity associated with severity of disease in mice during the advanced phase of TB.
The data obtained in this animal model of TB do not support the value of IGRA in blood to diagnose active TB. IGRA responses are highly variable according to various phases of active infection and might lead to false negative results during active TB. These findings are in line with a recent review by Hooper et al., who concluded that there is inadequate evidence to support the use of IGRA in the diagnosis or exclusion of active pleural TB in patients [14].
However, the data of the present study suggest that IGRA might be a useful biomarker to monitor therapy response. Our results are in line with the findings obtained by Lee et al., demonstrating that IGRA results decreased after successful treatment [15]. In our experimental TB model, it was also demonstrated that, next to IFN-γ, also IL-17 and IP-10 as cytokines in the pro-inflammatory reaction cascade, might be adequate markers indicative for successful therapy. The results of the dynamics of free IFN-γ concentrations as a biomarker during the different phases of infection are in concordance with results earlier described in other studies [8, 16]. However, it should be noted that the free IFN-γ concentration level in blood is also influenced by other factors not related to the TB infection. The TNF-α concentrations also revealed a strong increase during the acute phase of infection and sustained high concentrations throughout the chronic phase of infection. MIG concentrations showed no dynamics during the course of infection but revealed to be an adequate marker in therapy effect and in that perspective might contribute to proper follow-up of patients starting anti-TB therapy. These results indicate that besides IFN-γ also the pro-inflammatory cytokines like IL-17, IP-10, TNF-α and MIG are adequate biomarkers in the acute phase of infection. Recruiting T-cells, macrophages and neutrophils have a strong Th-1 reaction in this phase. In the present TB model changes in cytokine concentrations can be related to the TB infection. It should be realised that changes in pro-inflammatory cytokines can be caused by many other factors. However, the use of these cytokines to measure therapy efficacy seems achievable when using two paired samples taken before the start of therapy and after 3 weeks.
To our knowledge, only Beamer et al. reported studies on the use of IGRA in a mouse TB model. They showed that IGRA results from blood samples after exposure to Mtb-specific-antigens reflected IGRA results in lung cells [17]. They observed that a low IFN-γ response to Mtb-specific-antigens was associated with elevated risk of severe disease, compared to high responders. Their study using various mouse strains demonstrated that the course of TB was different and dependent on the mouse strain used, which is also reflected in their IGRA results [17].
Summarizing, the present study demonstrates a limited value of IGRA to monitor infection progression or even to detect active TB infection. These animal data do not support use of IGRA as an accurate diagnostic tool for active TB. By contrast, the value of IGRA in monitoring therapy response in the acute phase of TB infection is demonstrated. This may indicate that IGRA can be an adequate biomarker for therapy failure due to Mtb resistance. Also, a decrease in plasma concentrations of IFN-γ, IL-17, IP-10 or MIG was associated with successful therapy. As it is known that in TB patients the concentrations of cytokine biomarkers can be influenced by other non-TB-related factors, IGRA as a TB-specific biomarker is preferable to be used for monitoring therapy efficacy in TB patients.
Acknowledgments
Conflicts of interest
Nothing to declare.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Arko-Mensah J, Rahman MJ, Julian E, et al. Increased levels of immunological markers in the respiratory tract but not in serum correlate with active pulmonary mycobacterial infection in mice. Clin Microbiol Infect. 2009;15:777–786. doi: 10.1111/j.1469-0691.2009.02734.x. [DOI] [PubMed] [Google Scholar]
- 2.Diel R, Loddenkemper R, Nienhaus A (2009) Evidence based comparison of commercial interferon-gamma release assays for detecting active tuberculosis—a meta-analysis. Chest 137(4):952–968 [DOI] [PubMed]
- 3.Jiang J, Shi HZ, Liang QL, et al. Diagnostic value of interferon-gamma in tuberculous pleurisy: a metaanalysis. Chest. 2007;131:1133–1141. doi: 10.1378/chest.06-2273. [DOI] [PubMed] [Google Scholar]
- 4.Lange C, Pai M, Drobniewski F, et al. Interferon-gamma release assays for the diagnosis of active tuberculosis: sensible or silly? Eur Respir J. 2009;33:1250–1263. doi: 10.1183/09031936.00019709. [DOI] [PubMed] [Google Scholar]
- 5.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177–84. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Tuberculosis Controllers Association and Centers for Disease Control and Prevention (CDC) Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep. 2005;54:1–47. [PubMed] [Google Scholar]
- 7.Jafari C, Thijsen S, Sotgiu G, et al. Bronchoalveolar lavage enzyme-linked immunospot for a rapid diagnosis of tuberculosis: a Tuberculosis Network European Trialsgroup study. Am J Respir Crit Care Med. 2009;180:666–673. doi: 10.1164/rccm.200904-0557OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Steenwinkel JE, De Knegt GJ, Ten Kate MT, et al. Immunological parameters to define infection progression and therapy response in a well-defined tuberculosis model in mice. Int J Immunopathol Pharmacol. 2009;22:723–734. doi: 10.1177/039463200902200318. [DOI] [PubMed] [Google Scholar]
- 9.Bakker-Woudenberg IA. Experimental models of pulmonary infection. J Microbiol Methods. 2003;54:295–313. doi: 10.1016/S0167-7012(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal IM, Zhang M, Williams KN, et al. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 2007;4:e344. doi: 10.1371/journal.pmed.0040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shafiani S, Tucker-Heard G, Kariyone A, et al. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med. 2010;207:1409–1420. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joosten SA, van Meijgaarden KE, Savage ND, et al. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci USA. 2007;104:8029–8034. doi: 10.1073/pnas.0702257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahiratmadja E, Alisjahbana B, de Boer T, et al. Dynamic changes in pro- and anti-inflammatory cytokine profiles and gamma interferon receptor signaling integrity correlate with tuberculosis disease activity and response to curative treatment. Infect Immun. 2007;75:820–829. doi: 10.1128/IAI.00602-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooper CE, Lee YC, Maskell NA. Interferon-gamma release assays for the diagnosis of TB pleural effusions: hype or real hope? Curr Opin Pulm Med. 2009;15:358–365. doi: 10.1097/MCP.0b013e32832bcc4e. [DOI] [PubMed] [Google Scholar]
- 15.Lee SW, Lee CT, Yim JJ. Serial interferon-gamma release assays during treatment of active tuberculosis in young adults. BMC Infect Dis. 2010;10:300. doi: 10.1186/1471-2334-10-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Juarrero M, Kingry LC, Ordway DJ, et al. Immune response to Mycobacterium tuberculosis and identification of molecular markers of disease. Am J Respir Cell Mol Biol. 2009;40:398–409. doi: 10.1165/rcmb.2008-0248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beamer GL, Flaherty DK, Vesosky B, et al. Peripheral blood gamma interferon release assays predict lung responses and Mycobacterium tuberculosis disease outcome in mice. Clin Vaccine Immunol. 2008;15:474–483. doi: 10.1128/CVI.00408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]