Abstract
Objective
Autism spectrum disorder (ASD) is increasingly viewed as a disorder of functional networks, highlighting the importance of investigating white matter and interregional connectivity. We used diffusion tensor imaging (DTI) to examine white matter integrity for the whole brain and for corpus callosum, internal capsule, and middle cerebellar peduncle in children with ASD and typically developing (TD) children.
Method
DTI data were obtained from 26 children with ASD and 24 matched TD children. Fractional anisotropy (FA), mean diffusivity (MD), and axial and radial diffusion were calculated for the whole brain, genu, body and splenium of the corpus callosum, genu, anterior and posterior limbs of the internal capsule, and middle cerebellar peduncle.
Results
Children with ASD had reduced FA and increased radial diffusion for whole brain white matter and all three segments of the corpus callosum and internal capsule, compared to TD children. Increased MD was found for the whole brain and anterior and posterior limbs of the internal capsule. Reduced axial diffusion was found for the body of corpus callosum. Reduced FA was also found for middle cerebellar peduncle.
Conclusions
Our findings suggest widespread white matter compromise in children with ASD. Abnormalities in the corpus callosum indicate impaired interhemispheric transfer. Results for internal capsule and middle cerebellar peduncle add to the currently limited DTI evidence on subcortico-cortical tracts in ASD. The robust impairment found in all three segments of the internal capsule is consistent with studies documenting impairment of elementary sensorimotor function in ASD.
Keywords: Autism, diffusion tensor imaging, corpus callosum, internal Capsule, middle cerebellar peduncle
Introduction
Autism spectrum disorder (ASD) is a pervasive neurodevelopmental disorder characterized by atypical behavioral profiles, with salient sociocommunicative impairments. Brain volumetric studies have shown abnormal white matter growth trajectories in children with ASD.1 Consistent and growing evidence from neuroimaging studies suggests that ASD is associated with abnormalities of distributed functional networks, rather than localized impairment 2–4. Brain connectivity and white matter have therefore become a focus of investigation. However, it remains open whether impairment in ASD is selective and limited to networks specifically important for socio-communicative functions, or non-selective and affecting many different networks and a wide range of cognitive and sensorimotor domains.
Diffusion tensor imaging (DTI) is a measure used to characterize the microintegrity of white matter. Various DTI indices such as fractional anisotropy (FA), mean diffusivity (MD), and axial and radial diffusion can be used. FA and axial diffusion provide quantitative information about the orientational coherence of white matter fiber tracts and are considered positive indices of axonal integrity. MD and radial diffusion estimate intravoxel water motility and are considered negative indices of myelination. Recent DTI studies in ASD have reported reduced fractional anisotropy in frontal,5–7 temporal8 and occipital lobes.9 Increases in mean diffusivity have also been reported in bilateral temporal8 and frontal lobes.5,6 A whole brain DTI study by Barnea-Goraly et al.9 observed reduced FA in bilateral anterior cingulate, prefrontal, and temporo-parietal white matter, but did not examine specific fiber tracts.
Several DTI studies6,10–12 have shown white matter compromise in parts of the corpus callosum, potentially related to earlier reports of reduced callosal size in ASD.13,14 Focus on the corpus callosum is motivated by evidence of atypical functional asymmetries and impaired interhemispheric information transfer in ASD.15–20 Evidence for subcortico-cortical tracts is more limited. Keller et al.11 found reduced FA in the retrolenticular portion of the right internal capsule in children and adults with ASD aged 10–35 years. Reduced FA in the posterior limb of the internal capsule was also reported in a small-sample ROI study of children ages 6–12 years.12 This contrasts with findings in toddlers (ages 1.8–3.3 years) by Ben-Bashat and colleagues21 who found increased FA in the internal capsule, which reached significance in the posterior limb, possibly indicating precocious maturation. A recent study by Cheng et al.7 reported reduced FA in the left posterior limb, but increased FA in the right posterior limb of the internal capsule for adolescents with ASD.
Findings for the cerebellar peduncles have also been limited and inconsistent. Decreased FA in the right superior cerebellar peduncle was reported by Catani et al.,22 but this study included only adults with Asperger’s disorder. In the study by Cheng et al., 7 results were more mixed with reduced FA in right inferior cerebellar peduncle, but increased FA in the middle cerebellar peduncles bilaterally, detected in adolescents with ASD.
While findings of white matter compromise thus predominate in the extant literature, there are some inconsistencies. In particular, it remains unclear whether patterns of white matter abnormality in ASD may differ between relatively late maturing cortico-cortical tracts and earlier developing subcortical tracts. In the current study, we therefore examined white matter integrity in children with ASD and typically developing (TD) children for the whole brain, for one of the major cortico-cortical tracts (the corpus callosum) and for two major subcortical tracts (internal capsule and middle cerebellar peduncle). While whole brain analysis provides a global measure of white matter, region of interest (ROI) analyses yield evidence related to specific functional circuitry. The corpus callosum is the major pathway for interhemispheric communication, which is suspected to be atypical in ASD.10,12–14,23–27 Subcortical tracts are relevant given the potential role of sensorimotor impairments in ASD 28 that may impact sociocommunicative development.29–31 The internal capsule is associated with sensorimotor functions32 and compromise to this structure may cause thalamocortical disconnection.33 The middle cerebellar peduncle is the largest of the fiber bundles connecting cerebellum and brainstem, conveying afferent signals to the cerebellum. Functional and anatomical abnormalities of the cerebellum have been reported in ASD.34,35 In the current study, DTI indices (FA, MD, axial and radial diffusion) were investigated to examine whether expected white matter compromise in ASD (reduced FA and axial diffusion; increased MD and radial diffusion) would be limited to cortico-cortical interhemispheric connectivity (corpus callosum) or would generalize to subcortico-cortical fiber tracts with predominantly sensorimotor functions.
Method
Participants
Twenty-six children with ASD (24 males, 2 female) and twenty-four TD children (23 males, 1 female), matched for age and nonverbal IQ were included (see Table 1 and Table S1, available online, for details). The ASD group consisted of 15 children with autistic disorder and 11 children with Asperger’s disorder. Clinical diagnoses were made by an expert clinical psychologist (author AJL) using the Autism Diagnostic Interview–Revised (ADI-R)36 and the Autism Diagnostic Observation Schedule (ADOS).37 Children with associated medical conditions were excluded.
Table 1.
Demographic data for autism spectrum disorders (ASD) and typically developing (TD) groups.
| ASD (n = 26) | TD (n = 24) | Group comparison | |
|---|---|---|---|
| Mean (sem) Range |
Mean (sem) Range |
p | |
| Age (years) | 12.7 (0.6) 9–18 |
13.0 (0.6) 9–19 |
0.76 |
| Verbal IQ | 105.6 (3.6) 71–147 |
108.2 (2.6) 74–130 |
0.38 |
| Nonverbal IQ | 109.5 (3.3) 69–140 |
110.3 (2.5) 85–129 |
0.71 |
| Handedness | 23 right, 3 left | 22 right, 2 left | 0.71 |
Note: Group comparisons were performed with t-test for continuous dependent variables (i.e., age, verbal IQ, nonverbal IQ) and chi-square test for handedness.
Seven of the 26 ASD participants were taking prescribed psychoactive medications at the time of study (see Table S1, available online). Five were on monotherapies, including a stimulant, an anticonvulsant, an atypical antipsychotic, and Selective Serotonin Reuptake Inhibitors (SSRI) (n=2). One participant was taking an SSRI and a stimulant, one a stimulant and a Serotonin and Norepinephrine Reuptake Inhibitor. Medication information was unavailable for five ASD participants.
TD children had no reported personal or family history of autism or any other neurological or psychiatric conditions. Independent-sample t-test confirmed that ASD and TD groups were matched on age, t(48)=0.3; p=.76; verbal IQ, t(48)=0.8; p=.38; and nonverbal IQ, t(48)=0.4; p=.71, as determined using the Wechsler Abbreviated Scale of Intelligence38 (WASI; Table 1).
The research protocol was approved by the Institutional Review Boards of the University of California, San Diego and San Diego State University. Written informed assent and consent was obtained from all participants at the time of their visit.
DTI Scanning Procedure
MRI scans were performed on a 3.0T scanner (GE Signa Excite HD), using a standard 8-channel head coil to acquire single-shot echoplanar diffusion weighted images (repetition time 10000ms, echo time 99.4ms, field-of-view 240mm2, slice thickness 5mm [no gap], 27 axial slices, 128×128 matrix). Two degrees of diffusion weighting (b = 0 and 2000 s/mm2) were used. Data were acquired in 15 non-linear directions with four repetitions. Head movement was minimized with foam pillows around participants’ heads.
DTI Data Analysis
DTI data were preprocessed using the diffusion toolbox in FSL.39 Eddy current correction was performed using default GE scanner settings and distortions due to magnetic field inhomogeneities were corrected using field maps derived from the phase difference images obtained from two images with different echo times.40 No significant group differences were detected for translational motion or rotational motion as determined by the root mean square of the translational and rotational motion in three cardinal directions. Two tensor-derived rotational invariants (FA, MD) and all three diagonal tensor elements (eigenvalues: λ1, λ2 and λ3) were saved for subsequent analysis. MD was calculated as the average of the three eigenvalues. To measure anisotropy, we calculated FA, as previously defined.41 FA is defined to a scale from 0 (“isotropic” diffusion, equal in all directions) to 1 (“anisotropic” diffusion in only one direction). Water diffusion parallel to white matter axons is referred to as axial diffusion (λ1) and the average of water diffusivities perpendicular to axonal fibers as radial diffusion ([λ2+λ3]/2).42
For whole brain white matter analysis, B0-images (zero diffusion weighted images) were first segmented into gray matter, white matter, and cerebrospinal fluid (CSF) using SPM5 (www.fil.ion.ucl.ac.uk/spm) (Figure S1, available online). Segmentation of white matter was based on >0.8 probability for white matter classification and exclusion of voxels from gray matter, CSF, and extracranial space.43,44 The white matter tissue map was then applied to the FA, MD, axial and radial diffusion maps.
Corpus callosum, internal capsule and middle cerebellar peduncle were identified as ROIs in order to study abnormalities in diffusion parameters in these highly organized tracts. ROIs were drawn in native space on B0 images by an operator blinded to the study group, using Analyze software (Mayo Clinic, Rochester, MN) (Figure 1). Individual ROIs were applied to the DTI maps and mean values were obtained after grouping them as three ROIs for corpus callosum (genu, body and splenium), three ROIs for internal capsule (genu, anterior and posterior limbs: combined for both hemispheres) and one ROI for middle cerebellar peduncle (combined for both hemispheres).
Figure 1.

Representative slices showing the region of interest (ROI) placement (rectangle box) in the genu, body and splenium of the corpus callosum (A–C), genu and anterior and posterior limbs of the internal capsule (D) and middle cerebellar peduncle (E). Note: ROIs were drawn in native space on B0 images on three representative slices that were selected to allow positioning of ROIs in the body, genu, and splenium of the callosum and genu, anterior and posterior limbs of the internal capsule in both hemispheres. Axial slices showing maximal thickness of corpus callosum (for body, genu and splenium separately) and internal capsule were identified and then ROIs were placed on three contiguous slices. For the corpus callosum, two ROIs (70μl each) were placed on each slice (reference slice and slices inferior and superior to the reference slice) for body and splenium and one for the genu. For the internal capsule, on each of the three contiguous slices (reference slice and slices inferior and superior to the reference slice), one ROI (70μl) was placed on the genu and anterior and posterior limbs of the internal capsule in each hemisphere. Left and right middle cerebellar peduncles were selected from one slice showing maximal thickness, with a volume of 158μl each. All ROI placements were confirmed by viewing them on sagittal images for any partial volume effect.
Statistical analyses were performed using SPSS for Windows 16.0 (SPSS Inc., Chicago, IL). Between group differences in the DTI measurements of the whole brain, corpus callosum, internal capsule and middle cerebellar peduncle were analyzed with a one-way analysis of variance (ANOVA). Bonferroni corrections were performed for the number of ROIs (corpus callosum [genu, body and splenium], internal capsule [genu, anterior and posterior limbs] and middle cerebellar peduncle) to correct for multiple comparisons.
Results
For whole brain white matter, FA was significantly decreased (p=.004) whereas MD and radial diffusion were significantly increased (p=.01 and .007 respectively) in the ASD group compared to the TD group. The group difference in axial diffusion did not reach significance (p=.22; Figure 2).
Figure 2.
Fractional anisotropy (FA), mean diffusion (MD), axial and radial diffusion (mean±sem) for whole brain white matter. Note: ASD = Autism Spectrum Disorder.
*p<0.05 (corr.); **p<0.005 (corr.).
Pearson correlation analysis was performed to detect possible effects of age and IQ on the DTI indices. Age was negatively correlated with MD of the posterior limb of the internal capsule in both ASD and TD groups (p=.02), and with MD of the splenium of corpus callosum in the ASD group (p=.03) and marginally in TD group (p=.07). No other correlations were detected for age or for verbal or nonverbal IQ with any of the DTI indices (Table S2, available online). Therefore, age was used as covariate in the group comparison for MD only. The correlations between DTI indices and ADOS and ADI scores were also not significant.
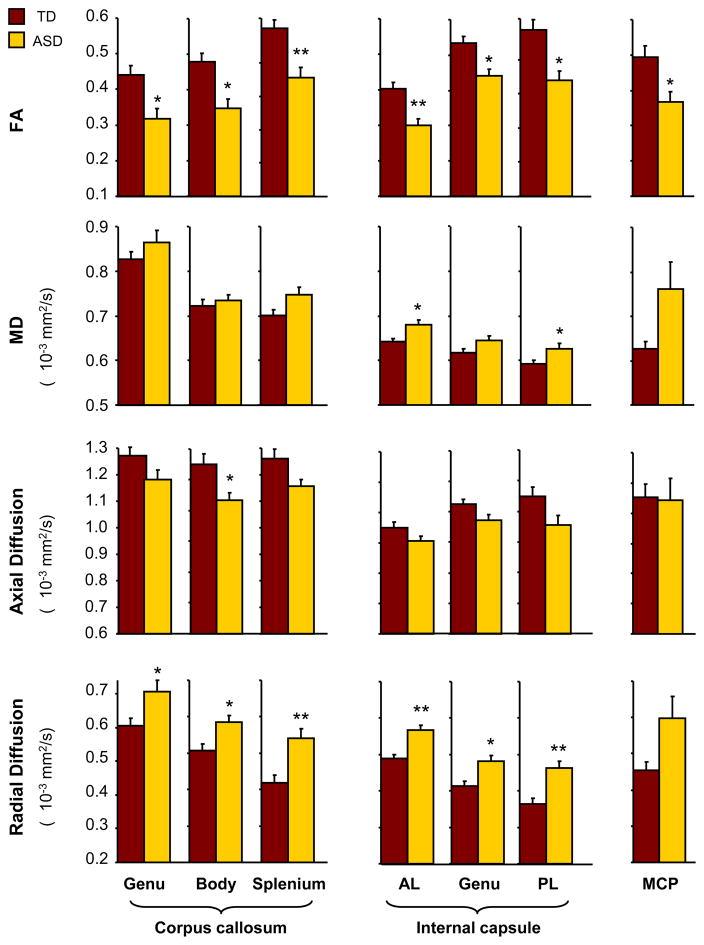
For all three callosal ROIs (genu, body, and splenium), we found significantly decreased FA (genu: p=.02, body: p=.007, splenium: p=.001; corrected for multiple comparisons) and increased radial diffusion (genu: p=.04, body: p=.03, splenium: p=.001) in the ASD group compared to the TD group. Axial diffusion for the body was significantly reduced in the ASD group (p=.03). Marginally increased MD and decreased axial diffusion were also detected for the splenium of the corpus callosum (p=.07). Mean increases in MD observed for genu and body of the corpus callosum were not significant (p>.05; Figure 3).
Figure 3.
Fractional anisotropy (FA), mean diffusion (MD), axial and radial diffusion (mean±sem) for the genu, body and splenium of the corpus callosum; anterior limb (AL), genu and posterior limb (PL) of the internal capsule bilaterally; and middle cerebellar peduncle (MCP) bilaterally. Note: ASD = Autism Spectrum Disorder.
*, p<0.05 (corr.); **, p<0.005 (corr.).
In the internal capsule, radial diffusion for all three ROIs (genu, anterior and posterior limbs) were significantly higher (genu: p=.007, anterior limb: p=.001, posterior limb: p=.001) in the ASD than the TD group, whereas FA was significantly lower (genu: p=.007, anterior limb: p=.001, posterior limb: p=.007). MD of anterior and posterior limbs of the internal capsule was significantly higher in the ASD group (anterior limb: p=.02, posterior limb: p=.04). No significant difference was found for axial diffusion (p>.21).
FA for the middle cerebellar peduncle was significantly reduced in the ASD group (p=.02). Differences for the other diffusivities were not significant (p>.21). A summary of ASD and TD group comparisons for whole-brain white matter, corpus callosum (genu, body and splenium), internal capsule (genu and anterior and posterior limbs), and middle cerebellar peduncle is presented in Table 2. Secondary analyses showed that exclusion of 3 girls or 5 left-handers had only minimal effect on our findings.
Table 2.
Summary of autism spectrum disorder (ASD) and typically developing (TD) group comparisons for whole-brain white matter, corpus callosum (genu, body and splenium), internal capsule (genu and anterior and posterior limbs), and middle cerebellar peduncle.
| FA (df=48) | MD (df=48) | Axial diffusion (df=48) | Radial diffusion (df=48) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | p (uncorr) | F | p (uncorr) | F | p (uncorr) | F | p (uncorr) | ||
| Whole-brain white matter | 9.39 | .004 | 5.70 | .02 | 1.48 | .22 | 7.86 | .007 | |
| Corpus callosum | genu | 9.81 | .003* | 1.49 | .22 | 3.61 | .06 | 7.39 | .007* |
| body | 13.22 | .001* | .52 | .45 | 8.64 | .005* | 8.63 | .005* | |
| splenium | 14.46 | .0001* | 4.94 | .01 | 5.93 | .01 | 13.96 | .0001* | |
| Internal capsule | anterior limb | 16.55 | .0001* | 9.70 | .003* | 3.4 | .06 | 20.02 | .0001* |
| genu | 12.23 | .001* | 4.59 | .03 | 4.32 | .04 | 11.62 | .001* | |
| posterior limb | 13.24 | .001* | 6.28 | .007* | 4.84 | .03 | 15.31 | .0001* | |
| Middle cerebral peduncle | 9.32 | .004* | 4.37 | .04 | 1.29 | .26 | 4.67 | 0.30 | |
Note: FA = Fractional anisotropy; MD = mean diffusivity
p<.05, corrected for multiple comparisons.
Discussion and Conclusions
Our findings add to the evidence of widespread white matter compromise in ASD, as first suggested by preliminary observations in a small-sample study by Barnea-Goraly et al.9 We found reduced FA and increased MD and radial diffusion for whole brain white matter in our cohort of children and adolescents with ASD, indicating loss of directional coherence of fiber bundles, reduced fiber density, and impaired myelination or axonal integrity.45,46 In each of our ROIs, mean differences in the expected direction (indicating white matter compromise) were observed, although not all of them were statistically significant after multiple comparison correction.
Atypical hemispheric organization and interhemispheric transfer have been reported previously in ASD.47,48 The corpus callosum is the largest axonal pathway in the mammalian brain and the main fiber tract responsible for interhemispheric information transfer.49 Previous DTI and volumetric studies have reported reduced size and integrity of the callosum in children with ASD,13,14 which may relate to impaired cortical connectivity as suggested by functional imaging25,50 and neuropsychological studies.51 Abnormally reduced callosal size in non-autistic conditions has also been related to difficulties in bimanual coordination, stereoscopic vision, and transfer of procedural learning between the hemispheres.52 In the present study, we found reduced FA and increased radial diffusion in all three segments (body, genu and splenium) of the corpus callosum, which may suggest disruption of myelin sheaths responsible for maintaining axonal integrity. Our findings may relate to other types of studies suggesting atypical hemispheric asymmetries and impaired interhemispheric connectivity in ASD.15,17–20,53 These findings are largely in agreement with earlier reports of callosal compromise.6,7,10,12
Our study produced a slightly different pattern of callosal findings compared to previous studies that detected only partial compromise of the callosum.9,10,12 We observed reduced FA and increased radial diffusion for all three segments and reduced axial diffusion for the body of the corpus callosum. In contrast, in the most thorough previous DTI study of the corpus callosum, Alexander et al.10 failed to detect significantly reduced FA and axial diffusion in the callosal body, which contains frontal and parietal interhemispheric connections. Partially differing findings may relate to the wider inclusionary age range in the study by Alexander et al. (7–33 years), compared to the present study (9–18 years), and differences in volume and placement procedures for ROIs. However, the findings of global callosal compromise appear to be consistent with a recent meta-analysis of anatomical MRI studies suggesting significant volume reduction in all subdivisions of the corpus callosum.26
Our findings for internal capsule and middle cerebellar peduncle add to a limited body of DTI evidence on subcortico-cortical tracts in ASD. Impairment of the internal capsule was robust, with significantly reduced FA as well as increased radial diffusion for all three ROIs (anterior, genu, posterior). Neuropsychological abnormalities including memory impairment due to damage in genu and anterior limb of the internal capsule have been reported.54,55 Correlation between capsular injury and motor impairment after stroke has also been reported.56 The anterior limb of the internal capsule connects thalamus and prefrontal cortex,57 and together with the genu also transfer descending prefrontal input to the cerebrocerebellar circuit.58 Our finding of white matter compromise in the anterior limb and genu of the internal capsule may therefore be related to evidence implicating prefrontal cortex in ASD, such as altered serotonin synthesis59 and reduced activation for spatial working memory.60 Furthermore, loss of white matter integrity at the genu of the internal capsule may cause disconnection of reciprocal thalamocortical fibers in the anterior limb of the internal capsule that link the dorsomedial and anterior thalamic nuclei with prefrontal and anterior cingulate cortex.
Thalamocortical fibers provide crucial connectivity for auditory, visual, and somatosensory and attentional systems.61 It has been suggested that abnormal organization of cortical minicolumns, which represent the fundamental subunit of vertical cortical organization, may underlie the pathology of autism and be associated with altered thalamocortical connections, cortical disinhibition, and dysfunction of the arousal-modulating system of the brain.62 The posterior limb of the internal capsule is the region of the brain where most of the motor fibers come together, passing down to the spinal cord. Abnormalities in the posterior limb of the internal capsule in conditions other than autism is associated with motor and sensory deficits.32
In voxel-wise whole brain DTI studies, Barnea-Goraly et al.9 and Cheung et al.27 failed to detect significant FA reduction in the internal capsule, probably due to small sample size. Three other studies have reported localized effects in the internal capsule. Keller et al.11 detected reduced FA in the retrolenticular part of the right internal capsule, while Cheng et al.7 observed inconsistent effects in the posterior limb of the internal capsule, with reduced FA in the left, but increased FA in the right hemisphere in adolescents with ASD. Contrary to these previous studies, our findings suggest global and bilateral compromise of the internal capsule in children and adolescents with ASD, including genu as well as anterior and posterior limbs. This was confirmed by post hoc analyses for unilateral ROIs, showing significantly reduced FA in the ASD group for all three segments of the internal capsule in each hemisphere.
Our finding of reduced FA in the middle cerebellar peduncle was also consistent with impaired connectivity, but the standard error for all diffusion measures except FA was high for this ROI in the ASD group, suggesting heavy individual variability.22 The middle cerebellar peduncle is the largest of the peduncles and via this connection the cerebellum receives a copy of motor execution commands transmitted to lower motor neurons in the pyramidal tract. Structural and functional cerebellar abnormalities have been reported in ASD, possibly associated with motor and attentional impairments.63,64 Autopsy and neuroimaging studies have also reported the involvement of cerebellar abnormalities in a variety of conditions including attentional-deficit hyperactivity disorder,65 unipolar depression and bipolar disorder,66 obsessive-compulsive disorder,67 and schizophrenia.68 Our finding of reduced FA is consistent with a recent study showing reductions in cerebellar activation and cerebrocerebellar functional connectivity during finger movement,69 and with earlier reports of motor abnormalities implicating the cerebellum in ASD.70,71 Cerebellar involvement in ASD is more specifically suggested by postmortem studies showing reduced number of Purkinje neurons.72 Our finding of reduced anisotropy indicates involvement of the middle cerebellar peduncle in ASD, but the general profile of white matter compromise appears less consistent than for the other ROIs reported here.
Some of the limitations of the present study include the wide age range of our sample, which may have affected the specificity of our findings with regard to neurodevelopmental changes. Sample size, although comparable with most DTI studies of ASD to date, was relatively small. Although immediate drug effects on measures of anatomical connectivity are unlikely, it is possible that medication may have some beneficial long-term effects on anatomical connectivity, similar to the many types of behavioral intervention applied in children with autism. Such neuroanatomical effects are largely unknown and could not be the focus of the current investigation. Finally, links between DTI findings and ASD symptomatology remain unclear and may be affected by numerous diagnostic, clinical, demographic, and other factors of individual variability.
Our measurements at the regional and global level showed increased radial diffusion and reduced anisotropy in children with ASD, which could reflect histopathological events related to axonal loss and demyelination. Overall, our findings document that white matter abnormalities, in particular those indicated by reduced FA and increased radial diffusion, can be found broadly in the cerebrum, with consistent findings in all segments of the corpus callosum and the internal capsule. Our observations differ from those reported for younger children in two studies. Ben-Bashat and colleagues21 detected consistently greater FA in a large number of ROIs in children ages 1.8–3.3 years. Sundaram et al.5 studied slightly older children (with a mean age 4.8 years) and had mixed findings for tracts originating from frontal lobes, with reduced FA only in short-range fibers and greater length of long-range fibers in their ASD group compared to TD controls. The discrepancies in DTI findings between preschool children and older age groups suggest that patterns of white matter abnormalities in ASD are age-dependent. This appears analogous to volumetric MRI findings showing atypical developmental schedules of white matter growth in all forebrain lobes, possibly with the exception of the occipital lobe.73
Our results may indicate that impaired connectivity in ASD shows limited regional or network specificity and instead reflects global or widespread neurodevelopmental pathology. This is consistent with the view that ASD is not primarily a socio-communicative disorder (as suggested by the diagnostic criteria of the DSM-IV)74, but one affecting a wide variety of sensorimotor and cognitive domains.75,76 Especially the robust impairments we found in all three segments of the internal capsule are consistent with studies documenting impairment of elementary sensorimotor functions in ASD,30,31,77 whose potential role in sociocommunicative deficits further downstream in development remains to be fully understood.78
Future studies using DTI tractography will be needed for further examinaton of potential tract-specific impairments in ASD. In addition, combined studies of anatomical and functional connectivity will provide a broader basis for understanding atypical network organization in ASD.
Supplementary Material
Acknowledgments
This study was supported by the National Institutes of Health, R01-DC006155 and R01-MH081023, with additional funding from NIDCD 1T32 DC007361-03 (author BK).
Footnotes
Special thanks to the children and families who participated.
Disclosure: Drs. Shukla, Müller, and Lincoln, and Mr. Keehn report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003 Jul 16;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 2.Müller RA. From loci to networks and back again: anomalies in the study of autism. Ann N Y Acad Sci. 2008;1145:300–315. doi: 10.1196/annals.1416.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004 Oct 20;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008;18:2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar A, Sundaram SK, Sivaswamy L, et al. Alterations in Frontal Lobe Tracts and Corpus Callosum in Young Children with Autism Spectrum Disorder. Cereb Cortex. 2009 Dec 17; doi: 10.1093/cercor/bhp278. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Y, Chou KH, Chen IY, Fan YT, Decety J, Lin CP. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. Neuroimage. 2010 Jan 11; doi: 10.1016/j.neuroimage.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Lee JE, Bigler ED, Alexander AL, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett. 2007 Sep 7;424:127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 9.Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004 Feb 1;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007 Jan 1;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007 Jan 8;18:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- 12.Brito AR, Vasconcelos MM, Domingues RC, et al. Diffusion tensor imaging findings in school-aged autistic children. J Neuroimaging. 2009;19:337–343. doi: 10.1111/j.1552-6569.2009.00366.x. [DOI] [PubMed] [Google Scholar]
- 13.Egaas B, Courchesne E, Saitoh O. Reduced size of corpus callosum in autism. Arch Neurol. 1995;52:794–801. doi: 10.1001/archneur.1995.00540320070014. [DOI] [PubMed] [Google Scholar]
- 14.Hardan AY, Minshew NJ, Keshavan MS. Corpus callosum size in autism. Neurology. 2000 Oct 10;55:1033–1036. doi: 10.1212/wnl.55.7.1033. [DOI] [PubMed] [Google Scholar]
- 15.De FL, Hodge SM, Makris N, et al. Language-association cortex asymmetry in autism and specific language impairment. Ann Neurol. 2004;56:757–766. doi: 10.1002/ana.20275. [DOI] [PubMed] [Google Scholar]
- 16.Gage NM, Juranek J, Filipek PA, et al. Rightward hemispheric asymmetries in auditory language cortex in children with autistic disorder: an MRI investigation. J Neurodev Disord. 2009;1:205–214. doi: 10.1007/s11689-009-9010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbert MR, Harris GJ, Adrien KT, et al. Abnormal asymmetry in language association cortex in autism. Ann Neurol. 2002;52:588–596. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- 18.Herbert MR, Ziegler DA, Deutsch CK, et al. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain. 2005;128:213–226. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- 19.Rojas DC, Bawn SD, Benkers TL, Reite ML, Rogers SJ. Smaller left hemisphere planum temporale in adults with autistic disorder. Neurosci Lett. 2002 Aug 16;328:237–240. doi: 10.1016/s0304-3940(02)00521-9. [DOI] [PubMed] [Google Scholar]
- 20.Rojas DC, Camou SL, Reite ML, Rogers SJ. Planum temporale volume in children and adolescents with autism. J Autism Dev Disord. 2005;35:479–486. doi: 10.1007/s10803-005-5038-7. [DOI] [PubMed] [Google Scholar]
- 21.Ben Bashat D, Kronfeld-Duenias V, Zachor DA, et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007 Aug 1;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 22.Catani M, Jones DK, Daly E, et al. Altered cerebellar feedback projections in Asperger syndrome. Neuroimage. 2008 Jul 15;41:1184–1191. doi: 10.1016/j.neuroimage.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 23.Casanova MF, El-Baz A, Mott M, et al. Reduced Gyral Window and Corpus Callosum Size in Autism: Possible Macroscopic Correlates of a Minicolumnopathy. J Autism Dev Disord. 2009 Jan 16; doi: 10.1007/s10803-008-0681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piven J, Bailey J, Ranson BJ, Arndt S. An MRI study of the corpus callosum in autism. Am J Psychiatry. 1997;154:1051–1056. doi: 10.1176/ajp.154.8.1051. [DOI] [PubMed] [Google Scholar]
- 25.Vidal CN, Nicolson R, DeVito TJ, et al. Mapping corpus callosum deficits in autism: an index of aberrant cortical connectivity. Biol Psychiatry. 2006 Aug 1;60:218–225. doi: 10.1016/j.biopsych.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Frazier TW, Hardan AY. A meta-analysis of the corpus callosum in autism. Biol Psychiatry. 2009 Nov 15;66:935–941. doi: 10.1016/j.biopsych.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung C, Chua SE, Cheung V, et al. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. J Child Psychol Psychiatry. 2009;50:1102–1112. doi: 10.1111/j.1469-7610.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- 28.Abrahamsen EP, Mitchell JR. Communication and sensorimotor functioning in children with autism. J Autism Dev Disord. 1990;20:75–85. doi: 10.1007/BF02206858. [DOI] [PubMed] [Google Scholar]
- 29.Brenner LA, Turner KC, Müller RA. Eye movement and visual search: are there elementary abnormalities in autism? J Autism Dev Disord. 2007;37:1289–1309. doi: 10.1007/s10803-006-0277-9. [DOI] [PubMed] [Google Scholar]
- 30.Leekam SR, Nieto C, Libby SJ, Wing L, Gould J. Describing the sensory abnormalities of children and adults with autism. J Autism Dev Disord. 2007;37:894–910. doi: 10.1007/s10803-006-0218-7. [DOI] [PubMed] [Google Scholar]
- 31.Rogers SJ, Hepburn SL, Stackhouse T, Wehner E. Imitation performance in toddlers with autism and those with other developmental disorders. J Child Psychol Psychiatry. 2003;44:763–781. doi: 10.1111/1469-7610.00162. [DOI] [PubMed] [Google Scholar]
- 32.Schmahmann JD, Pandya DN. Fiber pathways of the brain. Oxford University Press; 2006. [Google Scholar]
- 33.Wobrock T, Kamer T, Roy A, et al. Reduction of the internal capsule in families affected with schizophrenia. Biol Psychiatry. 2008 Jan 1;63:65–71. doi: 10.1016/j.biopsych.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 34.Palmen SJ, van EH, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- 35.Allen G, Courchesne E. Differential effects of developmental cerebellar abnormality on cognitive and motor functions in the cerebellum: an fMRI study of autism. Am J Psychiatry. 2003;160:262–273. doi: 10.1176/appi.ajp.160.2.262. [DOI] [PubMed] [Google Scholar]
- 36.Rutter M, Le Couteur A, Lord . Autism Diagnostic Interview - Revised. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- 37.Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule. Western Psychological Services; Los Angeles, CA: 2001. [Google Scholar]
- 38.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: The Psychological Corporation; 1999. Ref Type: Generic. [Google Scholar]
- 39.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 (Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 40.Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34:65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- 41.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 42.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 43.Wang C, Stebbins GT, Nyenhuis DL, et al. Longitudinal changes in white matter following ischemic stroke: a three-year follow-up study. Neurobiol Aging. 2006;27:1827–1833. doi: 10.1016/j.neurobiolaging.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Medina D, Toledo-Morrell L, Urresta F, et al. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol Aging. 2006;27:663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 45.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 46.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 47.Nyden A, Carlsson M, Carlsson A, Gillberg C. Interhemispheric transfer in high-functioning children and adolescents with autism spectrum disorders: a controlled pilot study. Dev Med Child Neurol. 2004;46:448–454. doi: 10.1017/s001216220400074x. [DOI] [PubMed] [Google Scholar]
- 48.Chiron C, Leboyer M, Leon F, Jambaque I, Nuttin C, Syrota A. SPECT of the brain in childhood autism: evidence for a lack of normal hemispheric asymmetry. Dev Med Child Neurol. 1995;37:849–860. doi: 10.1111/j.1469-8749.1995.tb11938.x. [DOI] [PubMed] [Google Scholar]
- 49.Seymour SE, Reuter-Lorenz PA, Gazzaniga MS. The disconnection syndrome. Basic findings reaffirmed. Brain. 1994;117 ( Pt 1):105–115. doi: 10.1093/brain/117.1.105. [DOI] [PubMed] [Google Scholar]
- 50.Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autistic brain: challenges for the “new psychophysiology”. Int J Psychophysiol. 2007;63:164–172. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: profile of a complex information processing disorder. J Int Neuropsychol Soc. 1997;3:303–316. [PubMed] [Google Scholar]
- 52.Jeeves MA. Stereo perception in callosal agenesis and partial callosotomy. Neuropsychologia. 1991;29:19–34. doi: 10.1016/0028-3932(91)90091-l. [DOI] [PubMed] [Google Scholar]
- 53.Gage NM, Juranek J, Filipek PA, et al. Rightward hemispheric asymmetries in auditory language cortex in children with autistic disorder: an MRI investigation. J Neurodev Disord. 2009;1:205–214. doi: 10.1007/s11689-009-9010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chukwudelunzu FE, Meschia JF, Graff-Radford NR, Lucas JA. Extensive metabolic and neuropsychological abnormalities associated with discrete infarction of the genu of the internal capsule. J Neurol Neurosurg Psychiatry. 2001;71:658–662. doi: 10.1136/jnnp.71.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tatemichi TK, Desmond DW, Prohovnik I, et al. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology. 1992;42:1966–1979. doi: 10.1212/wnl.42.10.1966. [DOI] [PubMed] [Google Scholar]
- 56.Pendlebury ST, Blamire AM, Lee MA, Styles P, Matthews PM. Axonal injury in the internal capsule correlates with motor impairment after stroke. Stroke. 1999;30:956–962. doi: 10.1161/01.str.30.5.956. [DOI] [PubMed] [Google Scholar]
- 57.Zou LQ, Xie JX, Yuan HS, Pei XL, Dong WT, Liu PC. Diffusion tensor imaging study of the anterior limb of internal capsules in neuroleptic-naive schizophrenia. Acad Radiol. 2008;15:285–289. doi: 10.1016/j.acra.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 58.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121 ( Pt 4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 59.Chugani DC, Muzik O, Rothermel R, et al. Altered serotonin synthesis in the dentatothalamocortical pathway in autistic boys. Ann Neurol. 1997;42:666–669. doi: 10.1002/ana.410420420. [DOI] [PubMed] [Google Scholar]
- 60.Luna B, Minshew NJ, Garver KE, et al. Neocortical system abnormalities in autism: an fMRI study of spatial working memory. Neurology. 2002 Sep 24;59:834–840. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- 61.Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002 Jan 17;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 62.Acosta MT, Pearl PL. The neurobiology of autism: new pieces of the puzzle. Curr Neurol Neurosci Rep. 2003;3:149–156. doi: 10.1007/s11910-003-0067-0. [DOI] [PubMed] [Google Scholar]
- 63.Penn HE. Neurobiological correlates of autism: a review of recent research. Child Neuropsychol. 2006;12:57–79. doi: 10.1080/09297040500253546. [DOI] [PubMed] [Google Scholar]
- 64.Allen G, Courchesne E. The cerebellum and non-motor function: clinical implications. Mol Psychiatry. 1998;3:207–210. doi: 10.1038/sj.mp.4000395. [DOI] [PubMed] [Google Scholar]
- 65.Castellanos FX, Giedd JN, Marsh WL, et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 66.Soares JC, Mann JJ. The anatomy of mood disorders--review of structural neuroimaging studies. Biol Psychiatry. 1997 Jan 1;41:86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 67.Jenike MA, Breiter HC, Baer L, et al. Cerebral structural abnormalities in obsessive-compulsive disorder. A quantitative morphometric magnetic resonance imaging study. Arch Gen Psychiatry. 1996;53:625–632. doi: 10.1001/archpsyc.1996.01830070073011. [DOI] [PubMed] [Google Scholar]
- 68.Katsetos CD, Hyde TM, Herman MM. Neuropathology of the cerebellum in schizophrenia--an update: 1996 and future directions. Biol Psychiatry. 1997 Aug 1;42:213–224. doi: 10.1016/S0006-3223(96)00313-7. [DOI] [PubMed] [Google Scholar]
- 69.Mostofsky SH, Burgess MP, Gidley Larson JC. Increased motor cortex white matter volume predicts motor impairment in autism. Brain. 2007;130:2117–2122. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- 70.Haas RH, Townsend J, Courchesne E, Lincoln AJ, Schreibman L, Yeung-Courchesne R. Neurologic abnormalities in infantile autism. J Child Neurol. 1996;11:84–92. doi: 10.1177/088307389601100204. [DOI] [PubMed] [Google Scholar]
- 71.Hallett M, Lebiedowska MK, Thomas SL, Stanhope SJ, Denckla MB, Rumsey J. Locomotion of autistic adults. Arch Neurol. 1993;50:1304–1308. doi: 10.1001/archneur.1993.00540120019007. [DOI] [PubMed] [Google Scholar]
- 72.Ritvo ER, Freeman BJ, Scheibel AB, et al. Lower Purkinje cell counts in the cerebella of four autistic subjects: initial findings of the UCLA-NSAC Autopsy Research Report. Am J Psychiatry. 1986;143:862–866. doi: 10.1176/ajp.143.7.862. [DOI] [PubMed] [Google Scholar]
- 73.Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- 74.American Psychiatry Association. Diagnostic and Statistical Manual of Mental Disorders-IV-TR. American Psychiatry Association; Washington, DC: 2000. [Google Scholar]
- 75.Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Res. 2009;2:125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Müller RA. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev. 2007;13:85–95. doi: 10.1002/mrdd.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takarae Y, Luna B, Minshew NJ, Sweeney JA. Patterns of visual sensory and sensorimotor abnormalities in autism vary in relation to history of early language delay. J Int Neuropsychol Soc. 2008;14:980–989. doi: 10.1017/S1355617708081277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hilton CL, Harper JD, Kueker RH, et al. Sensory Responsiveness as a Predictor of Social Severity in Children with High Functioning Autism Spectrum Disorders. J Autism Dev Disord. 2010 Jan 27; doi: 10.1007/s10803-010-0944-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.