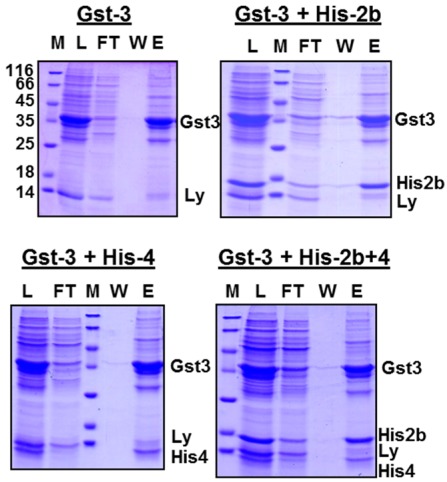
Figure 3.
Affinity-purification of EAV-protein oligomers. Gst-gp3, His-gp2b and His-gp4 were expressed individually in E. coli and solubilized from inclusion bodies, and refolded by dialysis. Urea extracts from inclusion bodies containing Gst-gp3 were dialysed alone (Gst-3) or were combined with His-gp2b (Gst-3+His-2b), with His-gp4 (Gst-3His-4) or with both (Gst-3+His-2b+4) prior to dialysis. The cleared dialysate was then agitated with glutathione-beads, beads were pelleted, put in a column, and washed 5 times with phosphate-buffered saline. Proteins were eluted with elution buffer. Aliquots of the dialysate (load), of the flow-through (FT), of wash 5 (W5) and of the eluates (E) were subjected to SDS-PAGE under reducing conditions and Coomassie-staining. M: Molecular mass markers, Ly: Lysozyme.