Abstract
Previous microarray analyses revealed that in Bradyrhizobium japonicum, about 100 genes are induced by genistein, an isoflavonoid secreted by soybean. This includes the three genes freC, freA, and freB (systematic designations bll4319, bll4320, and bll4321), which are likely to form a genistein-, daidzein-, and coumestrol-inducible operon and to encode a multidrug efflux system. Upstream of freCAB and in the opposite orientation, FrrA (systematic designation Blr4322), which has similarity to TetR-type regulators, is encoded. A deletion of frrA leads to increased expression of freB in the absence of an inducer. We identified the correct translational start codon of frrA and showed that the gene is inducible by genistein and daidzein. The protein, which was heterologously expressed and purified from Escherichia coli, binds to two palindrome-like DNA elements (operator A and operator B), which are located in the intergenic region between freC and frrA. The replacement of several nucleotides or the insertion of additional spacer nucleotides prevented binding. Binding of FrrA was also affected by the addition of genistein. By mapping the transcription start sites, we found that operator A covers the transcriptional start site of freC and operator B is probably located between the −35 regions of the two divergently oriented genes. Operator A seems to be conserved in a few similar gene constellations in other proteobacteria. Our data indicate that in B. japonicum, besides NodD1 (the LysR family) and NodVW (a two-component response regulator), a third regulator type (a TetR family member) which responds to the plant signal molecules genistein and daidzein exists.
INTRODUCTION
Bacterial efflux systems impart resistance to a broad spectrum of toxic agents (3, 20, 25, 26). So far, only a few studies address efflux systems and their regulation within rhizobia, which are soil bacteria able to enter symbiosis with legumes. In Sinorhizobium meliloti strain 41, the nolG gene encodes an AcrB-like membrane protein of the resistance-nodulation-cell division (RND) superfamily (1, 9). Interestingly, the gene is associated with nodulation genes, which are under the control of a nod box promoter. The nod box is the binding site of NodD, a LysR-type regulator, which activates nodulation genes in the presence of flavonoids (33). In a recent comprehensive study of S. meliloti strain 1021, genes encoding efflux systems belonging to different families were analyzed (5). A deletion of smeAB resulted in an increased sensitivity to toxic compounds and to reduced nodulation competitiveness. The expression of smeAB is regulated by a TetR-type protein (SmeR), which is encoded within the same operon (5). In two strains of Rhizobium leguminosarum bv. viciae and bv. trifolii, nodT, which encodes an outer membrane efflux protein similar to TolC, is located downstream of nodN and nodJ, respectively (29, 36). In both cases, nodT seems to form an operon with the nodulation genes. In Rhizobium etli, the rmrA and rmrB genes, whose products are members of the major facilitator superfamily (MFS), are required for efficient nodulation of bean (6). The genes are inducible by the flavonoids naringenin and genistein. The regulator belonging to the TetR family is likely to be encoded upstream of rmrA (6); however, regulation of the system has not been further described. Flavonoid-inducible efflux systems are also known for Agrobacterium tumefaciens (22) and Erwinia amylovora (4), where they contribute to the competitiveness for root colonization or to virulence, respectively. In both cases, TetR-type regulators are encoded next to the genes encoding the efflux systems. In Bradyrhizobium japonicum, which is a symbiont of soybean, more than 20 members of the RND family are encoded (17). One of the systems (bdeAB) is regulated by the two-component response regulator RegR. The regulator is activated by the cognate sensor RegS, which responds to an unknown signal (17, 18). Another system (ragCD) is cotranscribed with the rpoH3 gene, which encodes a heat shock sigma factor (15).
Often, efflux systems are regulated by proteins of the TetR repressor family (27). Members of this family are easily identified by their conserved helix-turn-helix DNA-binding domain (40). In contrast, ligand-binding domains are very diverse. In general, the repressor binds as a dimer to an operator site, and repression is relieved by binding of a signal molecule (27, 40).
In a previous study, we found that in B. japonicum about 100 genes are induced by genistein (16). Three of the genes, bll4321, bll4320, and bll4319 (systematic nomenclature), are likely to form a flavonoid-inducible operon and to encode an efflux system of the RND superfamily. Here we characterize their putative transcriptional regulator, FrrA (systematic designation Blr4322), and its binding sites. Further, we show that DNA binding can be suppressed by the isoflavone genistein.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bradyrhizobium japonicum and Escherichia coli strains used in this study are listed in Table 1. In general, B. japonicum was grown in arabinose-gluconate (AG) medium (31) at 28°C under shaking (160 rpm). For conjugation, B. japonicum was grown in peptone salts yeast extract medium (28) at 28°C. E. coli was routinely grown in Luria-Bertani (LB) medium (32) at 37°C. For protein isolation, E. coli was cultured at 30°C in the presence of ampicillin (100 μg/ml). Cells were induced at an optical density at 600 nm (OD600) of about 0.7 with isopropyl-β-d-thiogalactopyranoside (IPTG) (100 μM), and the cells were harvested after 4 h.
Table 1.
Strains, plasmids, and oligonucleotides used in this study
| Strain, plasmid, or oligonucleotide | Relevant characteristicsa | Reference or usage |
|---|---|---|
| B. japonicum | ||
| 110spc4 | Referred to as wild type; Spr | 28 |
| BJDΔ805 | Deletion of frrA; Spr Kmr | This study |
| BJD810 | frrA-lacZ at annotated translational start integrated into wild type; Spr Tcr | This study |
| BJD811 | frrA-lacZ at assumed translational start integrated into wild type; Spr Tcr | This study |
| BJD829 | Translational freB-lacZ fusion integrated into wild type; Spr Tcr | This study |
| BJDΔ805-829 | Translational freB-lacZ fusion integrated into frrA deletion strain; Kmr Spr Tcr | This study |
| BJDΔ840 | Deletion of freA and partial deletion of freC and freB; Spr Kmr | This study |
| E. coli | ||
| DH10B | F−mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80dlacZΔM15 ΔlacX74deoR recA1endA1 araD139Δ(araleu)7697galU galKλ−rpsL nupG; Smr | Invitrogen, Germany |
| BL21(DE3) | F−dcm ompT hsdS(rB− mB−) gal λ [pLysS], used for expression of proteins used to GST; Cmr | Stratagene |
| S17-1 | hsdR pro thi (RP4-2 km::Tn7 tc::Mu, integrated into the chromosome), used for mobilization of plasmids; Smr Spr | 35 |
| Plasmids | ||
| pSUP81-ZA | Mobilizable suicide vector for generation of translational lacZ fusions and for cointegration into the genome of B. japonicum; Tcr | 41 |
| pGEX4-T3 | Vector for generation of gst fusions; Apr | GE Healthcare, United Kingdom |
| pBJD809 | Derivate of pGEX4-T3 encoding a GST-FrrA fusion; Apr | This study |
| pBJD810 | Derivate of pSUP81-ZA containing a frrA-lacZ fusion at the annotated start codon of frrA; Tcr | This study |
| pBJD811 | Derivate of pSUP81-ZA containing a frrA-lacZ fusion at the confirmed start codon of frrA; Tcr | This study |
| pBJD829 | Derivate of pSUP81-ZA containing a freB-lacZ fusion; Tcr | This study |
| Oligonucleotides | ||
| bll4321_SP1b | GATGTCCGGCGTGAACGAAAC | Transcript mapping |
| bll4321_SP2_HindIIIc | TATAAAGCTTCGGATGGTTGTCGATTGCTTC | Transcript mapping |
| bll4321_SP3_HindIIIc | TATAAAGCTTGAAGGCTTCGATCTGCCGTG | Transcript mapping |
| blr4322_SP1b | CTCGGGTCCGCTTTCATAGAA | Transcript mapping |
| blr4322_SP2_HindIIIc | TATAAAGCTTCGTCCATACTGGTTGCGGAAA | Transcript mapping |
| anchor | AACTGTGTAAGCTTAGGTACG | Transcript mapping |
| dA-anchor | AACTGTGTAAGCTTAGGTACG(A)17B | Transcript mapping |
| dT-anchor | AACTGTGTAAGCTTAGGTACG(T)17V | Transcript mapping |
| 4319F | ATTATGCGGCCGCCGATGTTGGCCAGTATCTGA | Generation of freB-lacZ fusion |
| 4319R | ATTATAGGCCTGCACGTTCTCGTTCCTCACT | Generation of freB-lacZ fusion |
| 4322F0 | TTATTTCTAGAGCGCTTTGCCACCGAAGATGT | Generation of frrA-lacZ fusion |
| 4322R | TTATTAGGCCTTGCGCACAATGGCTGCGTCTCA | Generation of frrA-lacZ fusion |
| 4322R2 | TTATTAGGCCTTGATCAAGGGTGACTCTCCGTC | Generation of frrA-lacZ fusion |
| blr4322stfor | TTAGGATCCATGATCGAAACGATCGCCCATCC | Generation of gst-frrA fusion |
| blr4322rev | TTAGAATTCGTGCGGGCTAGAACGCGCCCC | Generation of gst-frrA fusion |
| opAd | TATCGAAACTGAACCGTTCAGTACTTGAC | EMSA |
| opBd | CAACGATACTGAACTGTTTAGTTTCGTTG | EMSA |
| controld | TGTCGTGCTCAGGGCGTCTCTTCAGTTCT | EMSA |
Ap, ampicillin; Cm, chloramphenicol; Km, kanamycin; Sm, streptomycin; Sp, spectinomycin; Tc, tetracycline. B, nucleotide C, G, or T; V, nucleotide A, C, or G. Restriction enzyme recognition sites used for cloning are underlined.
Gene-specific primer used for first-strand cDNA synthesis.
Gene-specific primer used for PCR.
The complementary oligonucleotide used for the generation of a double-stranded DNA is not shown.
Nodulation assays.
Soybean seeds (Glycine max cv. Amphor; obtained from Rustica Semences, Blagnac, France) were surface sterilized, inoculated, and grown as described previously (14). Nodules were counted 11 and 28 days after inoculation.
Construction of mutant strains and translational frrA-lacZ and freB-lacZ fusions.
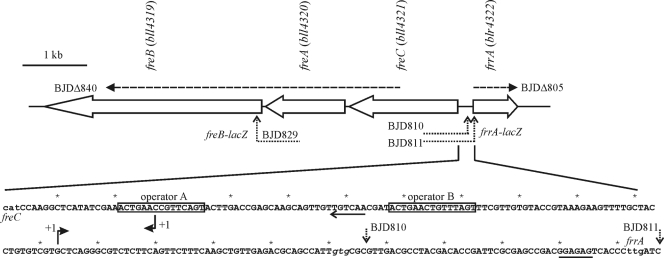
Plasmids, strains, and oligonucleotides are listed in Table 1. Deletion mutants were generated by homologous recombination as described previously (14). For deletion of frrA (strain BJDΔ805), 652-bp and 602-bp fragments upstream and downstream of frrA were amplified, cloned, and used for homologous recombination. By this, the chromosomal region from position 4774685 to 4775341 was replaced by a kanamycin resistance marker, resulting in strain BJDΔ805 (see Fig. 1). Similarly, in strain BJDΔ840, a 4.25-kb region (chromosomal nucleotide positions 4769322 to 4773572) encompassing bll4320 and about half of bll4319 and bll4322 is replaced by a kanamycin resistance marker (see Fig. 1). The deletions were verified by PCR using suitable primer combinations.
Fig 1.
The frrA-freCAB gene region and its putative regulatory elements. In the upper section, open arrows symbolize orientations and sizes of genes. Dashed horizontal lines indicate the deleted region in the listed mutant strain. The arrowheads show the orientation of the kanamycin resistance marker. Dotted bent arrows mark the positions of putative translational lacZ fusions within frrA or freB. The horizontal part of these arrows depicts the region used for homologous recombination and integration of the reporter gene. In the lower section, the translational start codons of freC and frrA are indicated at the ends of the nucleotide sequence in lowercase letters. The annotated start codon of frrA (gtg), which is located 48 nt upstream of the experimentally determined start codon, is shown in italics. Dotted vertical arrows denote the integration sites of the lacZ reporter within the frrA open reading frame. A Shine-Dalgarno-like sequence upstream of the identified start codon of frrA is underlined. Bent arrows (+1) indicate transcriptional start sites and directions. Palindromic sequences are boxed. A horizontal arrow marks a potential −35 promoter element.
Oligonucleotides 4322F0 and 4322R1 were used to amplify a 672-bp fragment upstream of frrA, including the first two codons of the annotated translational start site of frrA. Cloning into pSUP81-ZA generated a translational frrA-lacZ fusion (plasmid pBJD810). Using primers 4322F0 and 4322R2, a 723-bp fragment was amplified and used to construct a translational frrA-lacZ fusion at the presumed translational start site (plasmid pBJD811). For the generation of the two constructs, XbaI and StuI sites of fragments and vector were used. With oligonucleotides 4319F and 4319R, 603 bp at the 3′ end of freA (bll4320), including the first six codons of freB, were amplified. The resulting fragment was restricted with NotI and StuI and cloned into the vector pSUP81-ZA, thereby generating a translational freB-lacZ fusion (plasmid pBJD829). Plasmids pBJD810, pBJD811, and pBJD829 were transferred into B. japonicum by biparental mating as described previously (14), which resulted in B. japonicum strains BJD810, BJD811, and BJD829, respectively. Cointegration of plasmids was verified by PCR. Deletion of frrA was done essentially as described previously (14) using a kanamycin resistance marker and flanking regions of frrA (about 600 bp) for homologous recombination, which resulted in strain BJDΔ805. Plasmid pBJD829 (freB-lacZ fusion) was integrated into the chromosome of BJDΔ805 as mentioned above. All lacZ fusions were verified by sequencing.
Determination of β-galactosidase activity.
AG medium was inoculated 1:100 from a freshly grown culture. For the induction of the frrA- and freB-lacZ fusion strains, flavonoids (10 μM, final concentration) were added to the main culture after 48 h, and cells were harvested after an additional incubation for 24 h. β-Galactosidase activity was determined using 4-methylumbelliferyl (4-MU)-β-d-galactopyranoside as the substrate as described previously (41). Flavonoids were obtained from TransMIT or Sigma.
Heterologous expression of FrrA as a glutathione S-transferase fusion protein.
frrA was amplified by PCR using the primers blr4322stfor and blr4322rev and cloned into the vector pGEX4-T3, resulting in plasmid pBJD809, which was transformed into BL21(DE3). The frrA part and the fusion site were verified by sequencing. For purification of the glutathione S-transferase (GST) FrrA fusion protein, cells from a 100-ml culture were harvested, washed with phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.7 mM KH2PO4, pH 7.4) and resuspended in 5 ml PBS. Cells were disrupted by sonication, and the GST fusion protein was purified using a “GSTrap FF” affinity column (GE Healthcare, United Kingdom) and a “HiTrap FF benzamidine” affinity column (GE Healthcare) as described previously (38). Eluates were collected and stored at 4°C until use. After removal of the GST fusion partner, which was done according to the manufacturer's protocol (GE Healthcare), FrrA contains two extra amino acid residues at the N-terminal end.
Analysis of oligomerization state of FrrA.
Oligomerization was analyzed by blue native gel electrophoresis, similarly to a method described previously (39). In short, a 5 to 18% acrylamide gradient gel was loaded with 1 μg purified protein. Electrophoresis was done at 4°C for 1 h at 30 V and then at 80 V until the samples reached the bottom of the gel. Proteins were visualized by silver staining.
Electrophoretic mobility shift assays (EMSA).
To create double-stranded DNA fragments, an equimolar mixture of complementary oligonucleotides was incubated for 5 min at 95°C and slowly cooled down to room temperature. Eighty picomoles protein and about 10 pmol DNA fragments were mixed in binding buffer (12 mM HEPES, 6 mM KCl, 3 mM MgCl2, 0.5 mM dithiothreitol [DTT], 4 mM Tris-HCl, 6 mM EDTA, pH 8.0) to give a final volume of 40 μl. After incubation at room temperature for 30 min, samples were separated by polyacrylamide gel electrophoresis (10% acrylamide-bisacrylamide [29:1]) in TBE buffer (89 mM Tris, 89 mM boric acid, 2 mM EDTA, pH 8.3) for 1 to 2 h at room temperature at 120 V. Salmon sperm DNA, which was added as a control in the first experiments (50 ng/μl, final concentration), did not influence the results and was omitted in later experiments. Gels prepared with unlabeled oligonucleotides were stained with ethidium bromide. Gels containing Cy5-labeled oligonucleotides were scanned with a Storm 860 imaging system (GE Healthcare).
Determination of transcriptional start sites by rapid amplification of cDNA ends (5′-RACE).
B. japonicum cells were harvested 8 h after induction with 1 μM genistein. Total RNA was isolated as described previously (8, 41). Reverse transcription was carried out with Superscript II reverse transcriptase (Invitrogen) at 42°C for 50 min using the gene-specific primer SP1 (Table 1). The resulting cDNA was treated with RNase H (20 min, 37°C) and purified with the MinElute reaction cleanup kit (Qiagen, Hilden, Germany). dA or dT tails were added to the 3′ end of the cDNA using terminal deoxynucleotidyltransferase according to the manufacturer's protocol (Promega, Mannheim, Germany). For second-strand synthesis, dA- or dT-anchor primers (Table 1) were used. The double-stranded DNA was amplified with the anchor primer and a second gene-specific primer, SP2 (Table 1). In the case of bll4321, an aliquot of the obtained PCR product served as the template for a seminested PCR using the anchor primer and a third gene-specific primer, SP3 (Table 1). The resulting PCR products were cloned into the HindIII site of pBluescript II SK(+) and sequenced. The transcriptional start site was considered validated if the longest sequence was obtained with three independent clones.
Bioinformatics and statistical analyses.
The annotated genome of B. japonicum was retrieved from RhizoBase (http://genome.kazusa.or.jp/rhizobase), provided by the Kazusa DNA Research Institute. For BLAST searches, the resources of the National Center for Biotechnology Information were used (11). The secondary structure of FrrA was predicted with the I-Tasser server (30, 42). Palindrome searches were done with the corresponding tool of the JEMBOSS software package (19). For statistical analysis of nodule numbers, an unpaired t test was chosen. For analysis of β-galactosidase activities, an unpaired t test with Welch correction was used. Analyses were done with the software program GraphPad InStat (GraphPad Software, La Jolla, CA).
RESULTS
Determination of the translational start site of frrA.
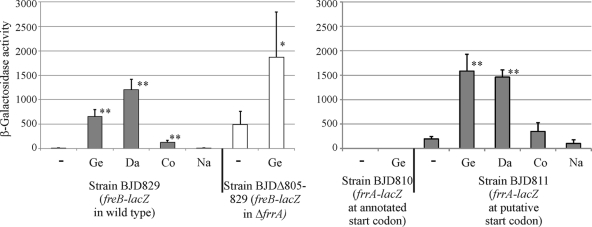
According to the annotated genome sequence, blr4322 (frrA) encodes a protein of 229 amino acids (12). However, the annotated start codon is not preceded by a well-conserved Shine-Dalgarno-like sequence. A better motif is found next to a second putative start codon located further downstream (Fig. 1). To determine the correct start site, translational lacZ fusions were created at both sites. β-Galactosidase activity values above the background level were obtained only with the lacZ gene at the second position (Fig. 1 and Fig. 2). Based on the new translational start site, the protein consists of 212 amino acids. Additionally, this test revealed that the expression of frrA is inducible by genistein and daidzein (Fig. 2).
Fig 2.
Induction of freB- and frrA-lacZ fusions by flavonoids. Columns labeled with asterisks depict values that are significantly higher than values obtained with the uninduced strain (∗∗, P < 0.01; ∗, P < 0.02). β-Galactosidase values obtained with genistein or daidzein are also significantly higher than those obtained after the addition of coumestrol (P < 0.01). Ge, genistein; Da, daidzein; Co, coumestrol; Na, naringenin; -, only the solvent for flavonoids (dimethyl sulfoxide [DMSO]) was added. β-Galactosidase activity (mean value ± SD) was calculated as pmol 4-MU formed per minute reaction time and cell density (OD600). Values were obtained from at least four independent cultures.
Expression of freB is inducible by genistein, daidzein, and coumestrol and regulated by FrrA.
In B. japonicum, the genes with the locus tags bll4321 (freC), bll4320 (freA), and bll4319 (freB) are likely to form an operon: the open reading frames overlap by 1 and 4 bases, respectively. A database search reveals that the genes encode an efflux system of the RND family; FreC has the characteristics of an outer membrane channel lipoprotein (E value 9.34e−90), FreA is a membrane fusion protein of the RND family (E value 5.34e−61), and FreB is a multidrug efflux protein (E value 0e+00). Previous microarray analysis showed that the genes are inducible by the flavonoid genistein (16). To confirm the microarray data, a translational freB-lacZ fusion was created (Fig. 1). The fusion was strongly inducible by the flavonoids genistein (75-fold) and daidzein (138-fold) and to a lesser extent by coumestrol (15-fold) but not by naringenin (Fig. 2). Therefore, we suggest the designations freC, freA, and freB (flavonoid-responsive efflux), with the gene-specific letter selected according to the similarity to the AcrAB-TolC system of E. coli.
blr4322 (frrA) is located about 200 bp upstream of freC and divergently oriented (Fig. 1). A database search indicated that the encoded protein is a transcriptional regulator of the TetR family. We integrated the freB-lacZ fusion into the genome of a blr4322 (frrA) deletion mutant. β-Galactosidase activity values were significantly higher in the absence of blr4322 than in its presence (Fig. 2), indicating that freB expression is repressed by Blr4322, and we suggest the designation FrrA (flavonoid-responsive regulator). Interestingly, in the frrA mutant background, freB expression was still inducible by genistein, albeit with a much lower fold change (about 4-fold; P < 0.02) than in the wild-type background (about 75-fold), suggesting the involvement of an additional regulator (see Discussion).
FrrA forms dimers and binds to palindrome-containing sequences close to putative promoter elements.
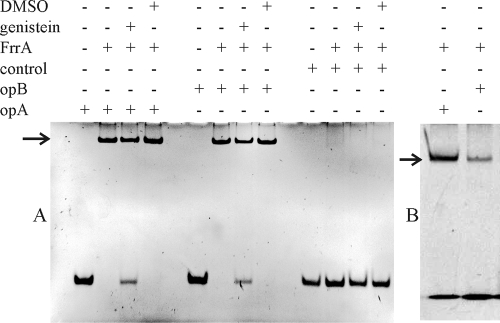
Based on the divergent configuration of the genistein-inducible genes freCAB and frrA, we expected that the intergenic region would contain a binding site for FrrA. Electrophoretic mobility shift assays (EMSA) with a PCR fragment encompassing the intergenic region confirmed this (data not shown). To test if the two palindromic sequences (operator A and B in Fig. 1) are involved in binding, targets of 29 bp were created with the oligonucleotides opA and opB and their complementary sequences (Table 1). EMSA showed that FrrA is shifting both palindrome-containing sequences but not a control oligonucleotide (Fig. 3). With a limiting protein amount, the shift is more pronounced with opA than with opB (Fig. 3B), suggesting a higher affinity of FrrA for operator A than for operator B, which contains a mismatch within the palindrome (Fig. 1). For both oligonucleotides, genistein prevents the shift at least partially (Fig. 3A), suggesting that FrrA acts as a repressor, which is released after binding of genistein. To test for specific sequence requirements for DNA binding, oligonucleotides with modifications in the operator A sequence were used for EMSA (Table 2). A replacement of four nucleotides within the palindrome prevented DNA binding even if the palindromic structure was maintained. An exchange of a single nucleotide had little effect. Interestingly, additional nucleotides at the spacer position also reduced binding of FrrA, indicating that the arrangement of the two palindromic half-sites is important and that FrrA might bind as a dimer, which would be typical for a TetR-type regulator (40). A result obtained by blue native gel electrophoresis (Fig. 4), which was confirmed by gel filtration (data not shown), indeed suggests that FrrA dimerizes.
Fig 3.
Electrophoretic mobility shift assay with purified FrrA protein and 29-bp oligonucleotides. The shifted DNA is marked by an arrow. The double-stranded oligonucleotides consist of opA, opB, and their complementary sequences. As a negative control, a double-stranded oligonucleotide of the same length but without the operator sequence was used. DMSO is the solvent for genistein and was added as a control. Genistein (250 μM) partially prevented the shift. (A) Protein and DNA concentrations were about 2 μM and 240 nM, respectively. (B) Protein and DNA concentrations were about 625 nM and 375 nM, respectively. Different intensities of the shifted bands suggest that FrrA has a higher affinity for operator A than for operator B.
Table 2.
Binding of FrrA to modified operator A sequences
| Oligonucleotidea | Sequenceb | Band shift with FrrAc |
|---|---|---|
| opA | tatcgaaACTGAACCGTTCAGTacttgac | ++ |
| opA_29_1+2+14+15 | tatcgaaCATGAACCGTTCATGacttgac | − |
| opA_29_6+7+9+10 | tatcgaaACTGACACTGTCAGTacttgac | − |
| opA_29_1+2 | tatcgaaCATGAACCGTTCAGTacttgac | − |
| opA_29_3×C | tatcgaaACTGAACCCCCGTTCAGTacttgac | − |
| opA_29_9 | tatcgaaACTGAACCCGTTCAGTacttgac | + |
| opA_29_6+7 | tatcgaaACTGACACGTTCAGTacttgac | + |
| opA_29_7 | tatcgaaACTGAAACGTTCAGTacttgac | ++ |
| opA_29_2 | tatcgaaAATGAACCGTTCAGTacttgac | ++ |
The oligonucleotides were labeled with Cy5 at their 5′-ends. The unlabeled complementary oligonucleotides used for the generation of a double-stranded DNA are not shown.
The palindromic sequence is written in uppercase letters, the spacer nucleotides are underlined, and introduced modifications are shaded.
A band shift was not detectable (−), appeared similar to that of the oligonucleotide opA (++), or was hardly visible (+).
Fig 4.
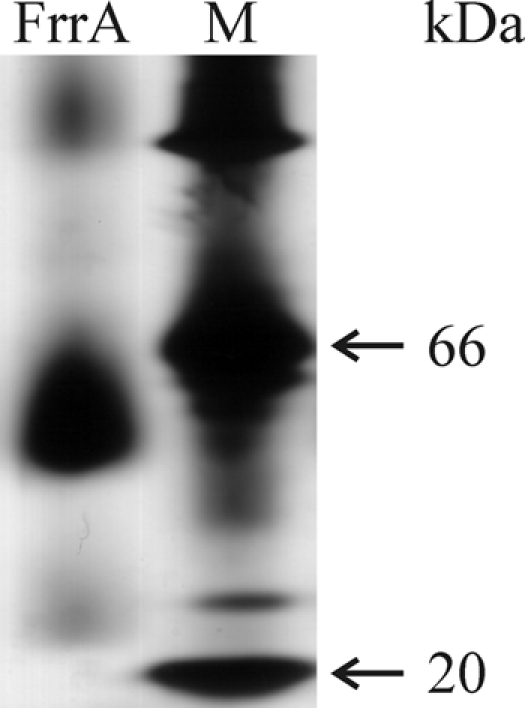
Mobility of FrrA in blue native acrylamide gel electrophoresis. The calculated molecular mass of the monomer is 23.7 kDa. The position of the dominant band in the FrrA-labeled lane suggests that the protein dimerizes. M, high-molecular-mass marker.
To find out if the binding sites are close to promoter elements, the transcription start sites of freC and frrA were determined by 5′-RACE. The transcriptional start site of freC is within operator A (Fig. 1), suggesting a significant contribution of this operator to the regulation of freC. A TTGACA motif located around position −35 might constitute part of the promoter. Operator B is located 40 nucleotides (nt) upstream of the transcriptional start sites of frrA and freC and might influence binding of the RNA polymerase to both promoters.
Operator A-like sequences are conserved only in proteobacteria.
The operator A and B motifs (“ACTGAACGGTTCAGT” and “ACTGAACTGTTTAGT”) were used in a nucleotide database search. The operator A motif (but not the operator B motif) was found in both orientations in intergenic regions of three other alphaproteobacteria, one betaproteobacterium, and one deltaproteobacterium (Table 3). In all cases, the first gene downstream of the operator A motif encodes a protein that is likely to be part of an efflux pump of the RND or MFS family. In all cases but one, a TetR-type regulator is encoded in the opposite orientation upstream of the palindrome. This conserved constellation of regulator, palindrome-like sequence, and transport protein-encoding gene suggests that the operator A motif is also a regulatory element in other proteobacteria. The identity of the binding site is reasonably reflected by conservation of the DNA-binding motif of the TetR-type regulators; 11 out of 22 amino acids are conserved in all 5 regulators (Fig. 5).
Table 3.
Conservation of operator A-like elements
| Strain | Locus taga | Motifb |
|---|---|---|
| Bradyrhizobium japonicum 110 | (TetR)bll4321(MDR) | ACTGAACGGTTCAGT |
| Ralstonia solanacearum strain MolK2* | RSMK02898(MDR) | ACTGAACCGTTCAGT |
| Xanthobacter autotrophicus Py2 | (TetR)Xaut_1512(MDR) | ACTGAACCGTTCAGT |
| Anaeromyxobacter sp. Fw109-5 | (TetR)Anae109_0569(MDR) | ACTGAACCGTTCAGT |
| Azospirillum sp. B510 | (TetR)AZLa05380(MDR) | AACTGAACGGTTCAGTT |
| Methylosinus trichosporium 0B3b | (TetR)MettrDRAFT_0112(MDR) | AACTGAACGGTTCAGTT |
The locus tag refers to the first gene downstream of the conserved motif. The putative function of the encoded protein or its involvement in multidrug resistance (MDR) is indicated in parentheses after the locus tag. If the conserved motif is bordered by divergently oriented genes, then the putative function of the protein encoded by the upstream-located gene is also given in parentheses. TetR, TetR-type regulator.
The motif used for database searches was that of B. japonicum. Palindrome-like sequences in intergenic regions were then checked for their sizes. The spacer nucleotide is underlined.
Fig 5.
Alignment of helix-turn-helix (HTH) motifs in TetR-type regulators putatively binding to the same operator sequence. The HTH motif was identified by the “helixturnhelix” tool of the JEMBOSS package (19). Helix 2 and helix 3 (underlined) refer to the helical regions as identified by I-Tasser (30). Helix 3 is the main determinant of sequence specificity (27). The accession numbers of the proteins are given. B. japonicum, Bradyrhizobium japonicum strain USDA110; M. trichosporium, Methylosinus trichosporium strain OB3b; Azospirillum sp., Azospirillum sp. strain B510; X. autotrophicus, Xanthobacter autotrophicus strain Py2; Anaeromyxobacter sp., Anaeromyxobacter sp. strain Fw109-5.
A deletion of genes encoding the regulator or the putative efflux system does not affect nodulation ability.
To assess the function of the efflux system and its regulator in symbiosis, the corresponding genes were replaced by a kanamycin resistance marker, resulting in mutants BJDΔ805 and BJDΔ840 (Table 1 and Fig. 1). In two independent test series, soybean seedlings were inoculated with the wild-type and mutant strains. For each strain, nodule numbers were determined from at least 15 plants at day 11 and at day 28 postinoculation. However, there was no difference in nodule number or plant appearance.
DISCUSSION
TetR-type regulators share several characteristics. They contain 9 conserved helical regions, including a helix-turn-helix DNA-binding motif at the N-terminal end, their binding sites are palindrome-like sequences of 15 bp or more, and they bind as dimers (27, 40). Results obtained by blue native gel electrophoresis (Fig. 4) suggest that FrrA is a dimer; a replacement of several nucleotides in a 15-bp palindromic sequence prevented binding of FrrA (Table 2); and bioinformatics analyses revealed the above-mentioned characteristics of a TetR-type regulator.
To our knowledge, this is the first report that the operator A and B sequences act as binding sites of a TetR-type regulator. In a database search, the operator A sequence was found only in other proteobacteria and in similar gene constellations, suggesting that this motif is used as an operator in these organisms as well. Interestingly, no member of the gammaproteobacteria turned up in the database search, although this subdivision of the proteobacteria is represented by the highest number of genomes. The G+C content of the palindrome is below 50%, and thus, the absence of the operator A motif in gammaproteobacteria might be of biological significance and not due to a statistical bias. Interestingly, the helix-turn-helix motifs of the putative TetR-type repressor proteins are less conserved than the binding sites (Fig. 5). Crystal structures revealed that mainly residues in helix 3 are involved in DNA contacts (27, 40). The conserved amino acid residues in helix 3 of FrrA (KAT.Y) are also present at high frequency in more than 2,000 TetR family members (27). Based on the sequence alignment in Fig. 5, several positions in helix 2 and in the loop region might also influence sequence specificity.
Replacement of two or four nucleotides within the palindrome of operator A led to a strongly decreased binding, or binding was not detectable. However, FrrA still binds if single nucleotides are replaced. Binding to operator B, which contains a mismatch within the palindrome, also reflects this. Operator A and operator B also differ in the spacer base pair, which is GC and AT, respectively. This suggests a tolerance at the central position, which is in line with the finding that the central base pair of the tetO operator does not contribute to sequence specificity of the tetracycline repressor (21). However, all operator A motifs identified here have a central GC base pair, and no motifs with a central AT base pair were found in the database. This might indicate a bias, because all organisms that contain this motif have a high G+C content of at least 64%, or indicate that the spacer nucleotide influences complex formation between the regulator and the operator. Irrespective of its contribution to repressor binding, the central base pair serves an essential spacer function. The elongation of the spacer strongly diminishes or prevents binding (Table 2), indicating that the two half-sites have to be presented with the correct distance. This is in line with the finding that a correct spacing of operator half-sites is essential for the binding of the TetR-type regulator QacR (7).
The freC-frrA intergenic region contains two slightly different operator sites. Similar constellations are found in other systems as well (40). For example, two different operators are present in the case of the Tn10-encoded tetracycline resistance gene (10). Interestingly, the affinity of the Tn10-encoded TetR repressor is higher for operator 2 (which has a mismatch in the palindromic binding site) than for operator 1 (which has no mismatch). In contrast, the affinity of FrrA appears to be higher for the operator with the perfect palindrome (Fig. 3B). The higher affinity and the location of operator A, which encompasses the transcription start site of freC, suggest that the freCAB operon is repressed more strongly than frrA. This assumption is supported by the much higher β-galactosidase values obtained with the strain containing frrA-lacZ than with the strain containing the freB-lacZ fusion (about a 23-fold difference) in the uninduced stage. Operator B, which is located 40 bp upstream of the two divergently oriented transcription start sites, might influence the expression of both genes. A “TTGACA” motif, which is a known promoter element in B. japonicum (2), was found in the −35 region of freC. This suggests that the promoter is recognized by the primary RNA polymerase sigma factor. No strongly conserved promoter elements were found for frrA.
Genistein, daidzein, and coumestrol but not naringenin are inducers of the freB gene. For frrA, a similar pattern was found. Although the increased expression of frrA was not statistically significant for coumestrol (a 1.7-fold increase), which is due to the higher β-galactosidase values of the uninduced control, this flavonoid might also act as a weak inducer of frrA. Interestingly, the same flavonoids induce, or in the case of naringenin do not induce, the nodulation genes (13). It is unlikely that the nod gene activators NodW or NodD1 are involved in the regulation of the freB or frrA gene. First, no nod box-like sequence is present in the intergenic region, and second, microarray analyses have shown that the freCAB genes are expressed independent of NodW and NodD1 (16; unpublished results). Based on our data, it is likely that FrrA is the main regulator of the freCAB and frrA genes; however, we cannot rule out the possibility that in addition a yet-unknown flavonoid-responsive activator is involved in regulation of these genes. Although FrrA and NodD1 exhibit no significant amino acid similarity, they are likely to interact with a similar set of flavonoids. Unfortunately, for both proteins no crystal structures, which would reveal the flavonoid-binding sites, are available.
The induction of an efflux system by flavonoids, e.g., genistein, might indicate that the flavonoid itself is somewhat detrimental to B. japonicum or that B. japonicum encounters a toxic compound at almost the same time that it encounters genistein and which is a substrate of the efflux system. Genistein is toxic for some bacteria (37) but seems to have little effect on rhizobia (23). However, B. japonicum also provokes an increased level of the phytoalexin glyceollin in soybean (34). B. japonicum is sensitive to glyceollin, but resistance can be induced by genistein (24). An efflux system like that encoded by freCAB might contribute to this resistance, and FrrA might ensure a timely expression. The wild type and a deletion mutant (strain BJDΔ840), which is lacking the freCAB genes, showed no difference in sensitivities toward genistein in liquid culture or on petri dishes (data not shown); glyceollin could not be tested because it was not commercially available to us. The same mutant did not exhibit any nodulation defect with soybean. Therefore, the function of the efflux system is not clear. Other efflux systems might compensate for a loss of this system. Lindemann et al. (17) identified more than 20 efflux systems in B. japonicum, and genes encoding three different efflux systems are induced by genistein independent of the nod gene regulator NodW (16). An argument for an existing overlapping function is the inducibility of the freB-lacZ fusion in the frrA mutant background. This induction suggests that another TetR-type regulator might be able to bind to the identified operator sites; however, we cannot exclude an additional involvement of an activator protein. According to the RhizoBase database, B. japonicum encodes 58 TetR-type regulators, and two of them (Blr2424 and Blr6623) are encoded next to genistein-inducible genes encoding two additional efflux systems (16). This indicates that B. japonicum is very versatile with respect to the adaptation to changing environmental conditions.
ACKNOWLEDGMENTS
We are grateful to Monika Weishaupt for technical assistance.
This work was supported by BMBF grant 0313805B (to M.W.).
Footnotes
Published ahead of print 2 March 2012
REFERENCES
- 1. Baev N, Endre G, Petrovics G, Banfalvi Z, Kondorosi A. 1991. Six nodulation genes of nod box locus 4 in Rhizobium meliloti are involved in nodulation signal production: nodM codes for D-glucosamine synthetase. Mol. Gen. Genet. 228:113–124 [DOI] [PubMed] [Google Scholar]
- 2. Beck C, Marty R, Kläusli S, Hennecke H, Göttfert M. 1997. Dissection of the transcription machinery for housekeeping genes of Bradyrhizobium japonicum. J. Bacteriol. 179:364–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blair JM, Piddock LJ. 2009. Structure, function and inhibition of RND efflux pumps in Gram-negative bacteria: an update. Curr. Opin. Microbiol. 12:512–519 [DOI] [PubMed] [Google Scholar]
- 4. Burse A, Weingart H, Ullrich MS. 2004. The phytoalexin-inducible multidrug efflux pump AcrAB contributes to virulence in the fire blight pathogen, Erwinia amylovora. Mol. Plant Microbe Interact. 17:43–54 [DOI] [PubMed] [Google Scholar]
- 5. Eda S, Mitsui H, Minamisawa K. 2011. Involvement of the SmeAB multidrug efflux pump in resistance to plant antimicrobials and contribution to nodulation competitiveness in Sinorhizobium meliloti. Appl. Environ. Microbiol. 77:2855–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. González-Pasayo R, Martínez-Romero E. 2000. Multiresistance genes of Rhizobium etli CFN42. Mol. Plant Microbe Interact. 13:572–577 [DOI] [PubMed] [Google Scholar]
- 7. Grkovic S, Brown MH, Schumacher MA, Brennan RG, Skurray RA. 2001. The staphylococcal QacR multidrug regulator binds a correctly spaced operator as a pair of dimers. J. Bacteriol. 183:7102–7109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hauser F, et al. 2007. Dissection of the Bradyrhizobium japonicum NifA+σ54 regulon, and identification of a ferredoxin gene (fdxN) for symbiotic nitrogen fixation. Mol. Genet. Genomics 278:255–271 [DOI] [PubMed] [Google Scholar]
- 9. Hernandez-Mendoza A, Quinto C, Segovia L, Perez-Rueda E. 2007. Ligand-binding prediction in the resistance-nodulation-cell division (RND) proteins. Comput. Biol. Chem. 31:115–123 [DOI] [PubMed] [Google Scholar]
- 10. Hillen W, Berens C. 1994. Mechanisms underlying expression of Tn10 encoded tetracycline resistance. Annu. Rev. Microbiol. 48:345–369 [DOI] [PubMed] [Google Scholar]
- 11. Johnson M, et al. 2008. NCBI BLAST: a better web interface. Nucleic Acids Res. 36:W5–W9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaneko T, et al. 2002. Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 9:189–197 [DOI] [PubMed] [Google Scholar]
- 13. Kosslak RM, Bookland R, Barkei J, Paaren HE, Appelbaum ER. 1987. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc. Natl. Acad. Sci. U. S. A. 84:7428–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krause A, Doerfel A, Göttfert M. 2002. Mutational and transcriptional analysis of the type III secretion system of Bradyrhizobium japonicum. Mol. Plant Microbe Interact. 15:1228–1235 [DOI] [PubMed] [Google Scholar]
- 15. Krummenacher P, Narberhaus F. 2000. Two genes encoding a putative multidrug efflux pump of the RND/MFP family are cotranscribed with an rpoH gene in Bradyrhizobium japonicum. Gene 241:247–254 [DOI] [PubMed] [Google Scholar]
- 16. Lang K, Lindemann A, Hauser F, Göttfert M. 2008. The genistein stimulon of Bradyrhizobium japonicum. Mol. Genet. Genomics 279:203–211 [DOI] [PubMed] [Google Scholar]
- 17. Lindemann A, et al. 2010. Host-specific symbiotic requirement of BdeAB, a RegR-controlled RND-type efflux system in Bradyrhizobium japonicum. FEMS Microbiol. Lett. 312:184–191 [DOI] [PubMed] [Google Scholar]
- 18. Lindemann A, et al. 2007. New target genes controlled by the Bradyrhizobium japonicum two-component regulatory system RegSR. J. Bacteriol. 189:8928–8943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mullan L. 2004. Jemboss reloaded. Brief Bioinform. 5:193–195 [DOI] [PubMed] [Google Scholar]
- 20. Nikaido H, Takatsuka Y. 2009. Mechanisms of RND multidrug efflux pumps. Biochim. Biophys. Acta 1794:769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Orth P, Schnappinger D, Hillen W, Saenger W, Hinrichs W. 2000. Structural basis of gene regulation by the tetracycline inducible Tet repressor-operator system. Nat. Struct. Biol. 7:215–219 [DOI] [PubMed] [Google Scholar]
- 22. Palumbo JD, Kado CI, Phillips DA. 1998. An isoflavonoid-inducible efflux pump in Agrobacterium tumefaciens is involved in competitive colonization of roots. J. Bacteriol. 180:3107–3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pankhurst CE, Biggs DR. 1980. Sensitivity of Rhizobium to selected isoflavonoids. Can. J. Microbiol. 26:542–545 [DOI] [PubMed] [Google Scholar]
- 24. Parniske M, Ahlborn B, Werner D. 1991. Isoflavonoid-inducible resistance to the phytoalexin glyceollin in soybean rhizobia. J. Bacteriol. 173:3432–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pos KM. 2009. Drug transport mechanism of the AcrB efflux pump. Biochim. Biophys. Acta 1794:782–793 [DOI] [PubMed] [Google Scholar]
- 26. Putman M, van Veen HW, Konings WN. 2000. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64:672–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramos JL, et al. 2005. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 69:326–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Regensburger B, Hennecke H. 1983. RNA polymerase from Rhizobium japonicum. Arch. Microbiol. 135:103–109 [DOI] [PubMed] [Google Scholar]
- 29. Rivilla R, Sutton JM, Downie JA. 1995. Rhizobium leguminosarum NodT is related to a family of outer-membrane transport proteins that includes TolC, PrtF, CyaE and AprF. Gene 161:27–31 [DOI] [PubMed] [Google Scholar]
- 30. Roy A, Kucukural A, Zhang Y. 2010. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5:725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sadowsky MJ, Tully RE, Cregan PB, Keyser HH. 1987. Genetic diversity in Bradyrhizobium japonicum serogroup 123 and its relation to genotype-specific nodulation of soybean. Appl. Environ. Microbiol. 53:2624–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 33. Schlaman HRM, Phillips DA, Kondorosi E. 1998. Genetic organization and transcriptional regulation of rhizobial nodulation genes, p 361–386 In Spaink H, Kondorosi A, Hooykaas PJJ. (ed), The Rhizobiaceae. Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- 34. Schmidt PE, Parniske M, Werner D. 1992. Production of the phytoalexin glyceollin-I by soybean roots in response to symbiotic and pathogenic infection. Bot. Acta 105:18–25 [Google Scholar]
- 35. Simon R, Priefer U, Pühler A. 1983. Vector plasmids for in vivo and in vitro manipulations of Gram-negative bacteria, p 98–106 In Pühler A. (ed), Molecular genetics of the bacteria plant interaction. Springer Verlag, Heidelberg, Germany [Google Scholar]
- 36. Surin BP, Watson JM, Hamilton WD, Economou A, Downie JA. 1990. Molecular characterization of the nodulation gene, nodT, from two biovars of Rhizobium leguminosarum. Mol. Microbiol. 4:245–252 [DOI] [PubMed] [Google Scholar]
- 37. Ulanowska K, Tkaczyk A, Konopa G, Wêgrzyn G. 2006. Differential antibacterial activity of genistein arising from global inhibition of DNA, RNA and protein synthesis in some bacterial strains. Arch. Microbiol. 184:271–278 [DOI] [PubMed] [Google Scholar]
- 38. Wenzel M, Friedrich L, Göttfert M, Zehner S. 2010. The type III-secreted protein NopE1 affects symbiosis and exhibits a calcium-dependent autocleavage activity. Mol. Plant Microbe Interact. 23:124–129 [DOI] [PubMed] [Google Scholar]
- 39. Wittig I, Braun H-P, Schägger H. 2006. Blue native PAGE. Nat. Protoc. 1:418–428 [DOI] [PubMed] [Google Scholar]
- 40. Yu Z, Reichheld SE, Savchenko A, Parkinson J, Davidson AR. 2010. A comprehensive analysis of structural and sequence conservation in the TetR family transcriptional regulators. J. Mol. Biol. 400:847–864 [DOI] [PubMed] [Google Scholar]
- 41. Zehner S, Schober G, Wenzel M, Lang K, Göttfert M. 2008. Expression of the Bradyrhizobium japonicum type III secretion system in legume nodules and analysis of the associated tts box promoter. Mol. Plant Microbe Interact. 21:1087–1093 [DOI] [PubMed] [Google Scholar]
- 42. Zhang Y. 2008. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]