Abstract
The 4th ASM Conference on Cell-Cell Communication in Bacteria was held in Miami, FL, from 6 to 9 November 2011. This review highlights three key themes that emerged from the many exciting talks and poster presentations in the area of quorum sensing: sociomicrobiology, signal transduction mechanisms, and interspecies communication.
INTRODUCTION
We as humans can learn a lot from our microbial neighbors, who have developed the capacity to work together for the common good through regulatory processes such as quorum sensing (QS). This mechanism of cell-cell communication enables the coordinated expression of a number of “public goods” whose production benefits the population as a whole. At the 4th American Society for Microbiology (ASM) Conference on Cell-Cell Communication in Bacteria (CCCB), held 6 to 9 November 2011 in Miami, FL, researchers from around the globe came to share their latest findings and insights to help further advance the field. The conference was attended by over 175 scientists, from junior to senior ranks, presenting 44 talks and 99 posters. Three broad themes emerged from the conference. First, the concept of sociomicrobiology was emphasized, building from the keynote address given by E. Peter Greenberg. This includes the idea that even in the microbial world, there are some individuals that are cooperative and altruistic while others exhibit detrimental behaviors and cheat. Second, new discoveries about the diverse array of signaling molecules, the receptors for these ligands, and ways to disrupt the signaling pathway were highlighted. This included discussion of some novel technologies for studying microbial signaling processes. Third, our growing understanding of the complexities of interspecies and interkingdom signaling was discussed in the context of both symbiotic and pathogenic relationships involving animals, plants, and microbes. Here we offer a brief synopsis of these three key themes of the conference.
PART 1. SOCIOMICROBIOLOGY
During more than 40 years of research in QS, much has been learned about its mechanistic basis, the individual parts, and their regulatory relationships, but comparatively little is known about its evolutionary and ecological context. Why and under what circumstances is QS an evolutionarily stable strategy, and what is its role under ecologically relevant conditions? A session dedicated to ecology and evolution provided answers to some of these questions. A related session on bacterial development and antibiotic signaling provided insight into the effects of QS on population fitness in well-described microbial model systems.
(i) Conceptual framework.
Andy Gardner from the University of Oxford, United Kingdom (A. Gardner, CCCB-11, abstr. S7:1), opened the evolution and ecology session with a broad introduction to evolutionary theory and social adaptation (22). He began with basic considerations on “design” by William Paley (British philosopher, 1743 to 1805), who focused on the differences between a rock and a watch. Paley argued that living organisms are more similar to watches than rocks, as the individual parts that comprise a watch appear to be designed to work together for a purpose. Paley's work had a strong influence on Charles Darwin. In his The Origin of Species (1859), Darwin proposes natural selection as a basis for a “design” that maximizes the fitness of individuals (15). Subsequent work by Fisher (1930) and Price (1970) further formalized the process of adaptation (20, 52). Gardner then put adaptation in the context of social behavior and Hamilton's kin selection theory (1964), which offers an answer to a central question in social evolutionary biology (24): how can one explain the evolution of social interactions (including bacterial group behaviors, such as cell-cell communication) if the entity of selection is the individual? Hamilton argues that individuals are designed to maximize their inclusive, not just their own, fitness. By helping close relatives reproduce, individuals still pass on their genes to the next generation, albeit indirectly, and it is this indirect fitness benefit that favors cooperative behaviors. This theoretical framework then provides a formal justification for the use of intentional terminology (conflict, cooperation, altruism, etc.) and defines programs of scientific inquiry. It is legitimate to ask “why” as well as “how” for ultimate and proximate explanations of biological phenomena.
Sam Brown from the University of Edinburgh, United Kingdom (S. P. Brown, CCCB-11, abstr. S7:2), expanded on the value of integrating functional and mechanistic inquiry in the context of QS. Asking “how” as well as “why” may reveal important novel insights into the constraints of the regulatory system at biological scales ranging from the subcellular to the population level and raise intriguing questions about function. For example, is QS about sensing density or is it about sensing diffusion (26, 55)? Why are QS regulatory networks often so complicated? Why is there often more than one QS signaling system in a bacterium? Brown focused on the question of multiple QS systems, using the las and rhl acyl-homoserine lactone (HSL) signaling systems in Pseudomonas aeruginosa as an example. He proposed a functional connection between the presence of multiple QS systems and the ability to distinguish between high density and limited diffusion as triggers for QS target gene expression. Two QS signals (QSS) with distinct stabilities and diffusion properties might help resolve information about the social and physical environment. He provided data on the differences in the pH-dependent stabilities of the two quorum signals, supporting previous work (78), and proposed a framework with which to build a dynamic QS model that captures signal concentrations on an intra- and intercellular scale under different environmental conditions.
(ii) Cooperation.
Multicellular development in Myxococcus xanthus provides a striking example for the apparent benefit of cooperation. This “poster child” of social microbiology goes through an elaborate developmental program culminating in the production of fruiting bodies (33, 70). These intricate structures consist of reproductive spores and asexual stalk cells. Lotte Sogaard-Andersen from the Max-Planck Institute, Marburg, Germany (L. Sogaard-Andersen et al., CCCB-11, abstr. S3:1), provided a comprehensive picture of the molecular mechanism of contact-dependent signal transduction in the organism. The relevant morphogen, termed “C signal,” is produced by starving M. xanthus cells and induces aggregation and sporulation (33). C signaling is distinct from the canonical QS circuitry with diffusible signals in that it requires intimate contact between cells. The active C signal is generated by proteolytic cleavage. A secreted protease, PopC, is responsible for this process. It was found that the secretion of PopC is dependent on the stringent response protein RelA, which produces alarmone guanosine tetraphosphate (ppGpp). The stringent response is a major regulatory circuitry that reprograms bacterial gene expression in response to nutrient starvation (51). The components providing the link between RelA/ppGpp and PopC were identified as PopD and FtsH: ppGpp directs activation of FtsH, an ATP-dependent protease that degrades PopD, which in turn inhibits PopC. Taken together, these data show that secretion-controlled proteolysis governs the activation of the C signal upon starvation in M. xanthus.
How, then, is C-signal accumulation converted into a developmental response? A poster from Lee Kroos's group at Michigan State University (R. Rajagopalan and L. Kroos, CCCB-11, abstr. 28) characterized two C-signal-dependent transcription factors, FruA and MrpC, that are important in this process. They had previously shown that both proteins bind target promoters cooperatively (41), allowing for the possibility to integrate spatial and nutritional information during fruiting body formation. In their poster, they show that the activity of MrpC, and to a lesser extent FruA, is sensitive to starvation. Protein abundance decreases rapidly upon nutrient repletion throughout the differentiation process, consistent with a role in commitment to development.
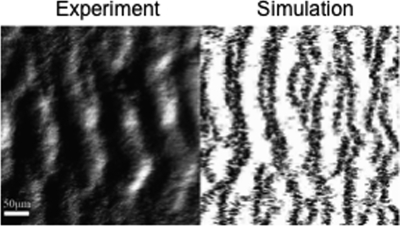
Another intriguing M. xanthus group behavior is the formation of macroscopic traveling waves, or ripples, as groups of cells glide together on a solid surface (Fig. 1). Interestingly, rippling occurs only when M. xanthus cells make direct contact with prey cells (7). Oleg Igoshin and Heidi Kaplan's laboratories at Rice University and the University of Texas—Houston (H. Zhang et al., CCCB-11, abstr. S7:5) found through a combination of computational modeling and experimentation that rippling can be explained by specific side-to-side cell-cell communication (C signaling), which induces cell reversals followed by a refractory nonreversal period. This periodic motility increases the rate of spreading over prey while at the same time permitting cells to remain in contact with prey longer.
Fig 1.
Cooperation in M. xanthus. Predatory ripples as seen experimentally (left panel) or in corresponding computer simulations (right panel). Images courtesy of Oleg Igoshin, Rice University; reproduced with permission (the work by O. Igoshin was supported by NSF Career grant MCB-0845919).
In addition to bacterial development, other intriguing morphological changes controlled by cell-cell signaling were described. George Salmond from the University of Cambridge, United Kingdom (J. Ramsay et al., CCCB-11, abstr. S3:6), reported on the “how” and speculated on the “why” of gas vesicle production in Serratia sp. strain ATCC 39006. Salmond's group has long studied the molecular biology of prodigiosin and carbapenem production in this organism and the control of these antibiotics by acyl-HSL QS (14, 76). Recent phenotypic screening of Serratia mutants revealed that these bacteria also produce gas vesicles—the first identified in an enterobacterium—and that gas vesicle production is controlled by QS—the first demonstration of the QS-dependent biogenesis of an intracellular organelle (53). While QS regulatory mutants are translucent and prodigiosin mutants are white, prodigiosin and gas vesicle double mutants are also translucent. QS-dependent gas vesicle production permits flotation and pellicle formation of Serratia cultures. Such bacterial “gang culture” might improve access to oxygen and, together with antibiotic production, provide a competitive advantage in the natural environment.
(iii) Competition and cheating.
Cheaters are individuals that reap the benefits of cooperation without contributing or by contributing less (31, 73). They constitute a major problem for the explanation of cooperation. Cheaters have been found to arise in many microbial systems, including M. xanthus and P. aeruginosa (17, 23, 57, 69). They typically exploit the production of secreted public goods that are costly for the individual to produce. The limited dispersal of microbes through colonial growth appears to be one manifestation of kin selection that keeps cooperating individuals together and cheaters out (73). An additional evolutionary force that helps restrict cheating is pleiotropy (21, 74). In an interlinked genetic regulatory network, the potential benefit gained from the loss of cooperative behavior may be balanced by a hidden cost.
In his keynote talk, Pete Greenberg from the University of Washington (E. P. Greenberg, CCCB-11, abstr. OS:1) proposed “metabolic policing” as a specific pleiotropic mechanism that may restrict cheating in P. aeruginosa QS. In this organism, acyl-HSL QS controls many secreted public goods, such as extracellular proteases and toxins, but also controls some private goods, such as periplasmic nucleoside hydrolase (Nuh) (61). Extracellular proteases permit growth of P. aeruginosa on proteins such as casein as the sole C source. Nuh, on the other hand, metabolizes adenosine and permits growth on this nucleoside as the sole carbon source. Greenberg's group conducted in vitro evolution experiments with the P. aeruginosa wild type and found that QS mutant cheaters evolve in casein medium, in which nutrient acquisition is public, but not in adenosine medium, in which nutrient acquisition is private. Their results are in agreement with data from defined wild-type and mutant P. aeruginosa coculture experiments presented in a poster by Brett Mellbye from Martin Schuster's lab at Oregon State University (B. L. Mellbye and M. Schuster, CCCB-11, abstr. 30) (40). Interestingly, when P. aeruginosa was grown in medium containing both casein and adenosine, QS cheaters did not evolve. However, their emergence was not restrained when glucose, a QS-independent substrate, was substituted for adenosine. These data show that QS control of private goods may curtail cheating; additional experiments are planned to confirm that the underlying mechanism is metabolic policing through the simultaneous use of public and private nutrients.
A poster presentation by Avigdor Eldar from Tel-Aviv University, Israel (A. Eldar, CCCB-11, abstr. 98), argued that social cheating may be a relevant evolutionary force in generating QS signaling diversity in bacterial populations (19). Within-species pherotype diversity is found particularly in Gram-positive bacteria, including Staphylococcus aureus and Bacillus subtilis, in which peptide signals activate cognate receptors in the same strain but fail to activate, and sometimes inhibit, those of other strains (28, 65). QS cheaters often evolve through mutations in signal receptors that render them “signal blind” (17, 57). Subsequent mutations in the cognate signal synthase may alter signal structure in a way that again permits recognition by the mutant receptor, thereby essentially converting a cheater to a cooperator. Such coevolution may lead to the coexistence of different kin types in unstructured populations.
Eldar's work was nicely complemented by a talk from Ines Mandec Mulec from the University of Ljubljana, Slovenia (P. Stefanic et al., CCCB-11, abstr. S7:4), who put signaling diversity in an ecological context. She provided experimental evidence for the idea that pherotype divergence in B. subtilis is an adaptation to ecological diversity. B. subtilis strains isolated from microscale soil samples (1 cm3) belong to three distinct pherotypes, as determined by reporter strain assays (63). Interestingly, diversity identified at the microscale level is similar to that previously determined at the macroscale level. Phylogenetic clustering analysis based on housekeeping genes revealed ecotypes that were strongly associated with each pherotype, although there were also outliers. These rare pherotypes may act as cheaters that benefit from the common goods produced by the majority until they are themselves abundant, resulting in a stable coexistence of cooperators and cheaters. Considering the results from both studies, a combination of ecological divergence and social conflict may drive pherotype evolution in natural populations.
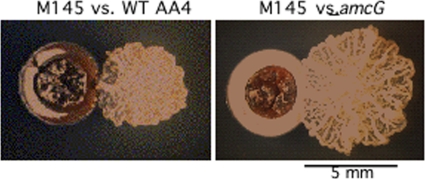
In addition to conflict within species, competitive interactions between different species were also investigated. Matt Traxler from Roberto Kolter's lab at Harvard Medical School (M. F. Traxler et al., CCCB-11, abstr. S3:4) reported on the effect of iron competition on actinomycete development (Fig. 2). The model actinomycete Streptomyces coelicolor, not unlike M. xanthus, undergoes an elaborate reproductive cycle that culminates in the production of spore-containing aerial hyphae (39). When screening for interactions with different actinomycete species, they found that Amycolatopsis sp. strain AA4 inhibits S. coelicolor hyphal development and that the production of an unusual siderophore, amychelin—specifically, its chelating activity—is responsible for this inhibition. An Amycolatopsis amychelin mutant did not inhibit hyphal growth, and in the absence of Amycolatopsis, iron-depleted and -replete conditions inhibit and accelerate formation of hyphae, respectively. Interestingly, Amycolatopsis amychelin-deficient colonies themselves displayed a striking iron starvation phenotype which was partially complemented in the presence of S. coelicolor, suggesting that Amycolatopsis can, in turn, utilize siderophores produced by S. coelicolor. Their results indicate that iron sequestration may be a strategy for an organism to delay the reproductive potential of a nearby competitor.
Fig 2.
Competition between actinobacteria. Development of aerial hyphae in S. coelicolor M145 (white areas on colonies) is arrested by the siderophore amychelin, produced by Amycolatopsis sp. AA4 (left panel). The ΔamcG strain, which is defective in amychelin production, does not inhibit aerial hypha formation in a nearby colony of S. coelicolor (right panel). Images courtesy of Matt Traxler and Roberto Kolter, Harvard Medical School; reproduced with permission.
PART 2. NEW DISCOVERIES ABOUT SIGNALING PATHWAYS
The number of publications from the QS field continues to grow at a high rate, and we have amassed a great deal of knowledge about the QS mechanisms of different bacteria. However, presentations across multiple sessions of the conference clearly demonstrated that much still remains to be discovered. What is the full repertoire of signals that microbes use for QS? How are these various ligands recognized by cell receptors, what structural changes result from ligand binding, and what are the downstream targets? How can the QS response be modulated, both artificially and naturally? What emerging technologies will enable us to continue to push the frontiers of our knowledge further? This section of the review serves to highlight some of the exciting advances presented at the conference that help answer these queries.
(i) Identification of new types/roles of signals.
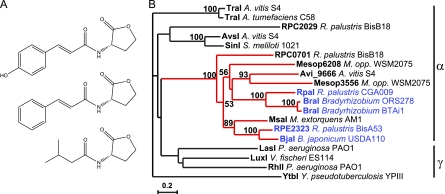
Traditionally, most QS signals in proteobacteria have been found to be produced by LuxI homologues. This family of enzymes synthesizes straight-chain fatty acid HSLs from fatty acid-acyl carrier protein intermediates and HSL derived from S-adenosylmethionine. In 2008, Carrie Harwood's group at the University of Washington—Seattle identified a noncanonical acyl-HSL, p-coumaroyl-HSL, that was produced by the phototroph Rhodopseudomonas palustris (58). In her talk (C. S. Harwood, CCCB-11, abstr. S2:1), Harwood described the discovery of two additional aryl-HSLs, cinnamoyl-HSL (1) and isovaleryl-HSL (A. Lindemann et al., CCCB-11, abstr. 31) (35), that were produced by Bradyrhizobium species utilizing the BtaI and BjaI synthases, respectively (Fig. 3A). This was made possible by the development of reporters that are sensitive to picomolar levels of the compounds. Thus, there appears to be a subfamily of LuxI homologues that has the capacity to use compounds other than straight-chain fatty acids as substrates for quorum-sensing signal production (Fig. 3B). Efforts are under way to establish the regulons under the control of these new aryl-HSL signals in proteobacteria.
Fig 3.
“Noncanonical” acyl-HSL signals and synthases. (A) Noncanonical acyl-HSL structures include p-coumaroyl-HSL (top), synthesized by Rhodopseudomonas palustris CGA009, cinnamoyl-HSL (middle), synthesized by Bradyrhizobium sp. strain ORS278 and BTAi1, and isovaleryl-HSL (bottom), synthesized by Bradyrhizobium japonicum USDA110 and R. palustris BisA53. (B) Phylogenetic tree of LuxI family members from selected alpha- and gammaproteobacteria. The subfamily tree of signal synthases containing LuxI homologs that synthesize noncanonical acyl-HSLs is highlighted in red, and the individual noncanonical acyl-HSL synthases for which products have been identified are highlighted in blue. A. vitis, Agrobacterium vitis; A. tumefaciens, Agrobacterium tumefaciens; S. meliloti, Sinorhizobium meliloti; M. opp., Mesorhizobium opportunistum; M. extorquens, Methylobacterium extorquens. Figure courtesy of Amy Schaefer and Caroline Harwood, University of Washington—Seattle; panel B is modified from reference 35 with permission.
Many proteobacteria produce outer membrane vesicles (OMVs) that are important in virulence factor secretion, immune modulation, biofilm formation, and QS. Previously, Marvin Whiteley's group at the University of Texas—Austin demonstrated that the Pseudomonas quinolone signal (PQS) is not only packaged into OMVs, it can contribute to OMV formation (38). At the conference, Jeffrey Schertzer (J. W. Schertzer and M. Whiteley, CCCB-11, abstr. S2:2) described the development of a system to further analyze the interaction of the PQS with membranes by taking advantage of the red blood cell (RBC) model to separate curvature from QS effects. It was hypothesized that PQS accumulation in the outer leaflet of the membrane leads to outer leaflet expansion and thereby induces the curvature necessary for OMV formation. The RBC system was successfully used to demonstrate that the PQS can indeed insert into the model membranes and induce crenation (bulges). This process specifically required the alkyl chain and 3-hydroxyl substituents of the PQS and was reversible by addition of chlorpromazine, a compound known to accumulate in the inner leaflet to cause invagination/cup formation of the RBCs. These studies have provided further insight into the role of the PQS in OMV formation in Pseudomonas spp. but also may have implications for better understanding OMV trafficking in a broader sense.
Additional insights into the complexities of more “traditional” acyl-HSL (AHL) and peptide signal transduction pathways were provided by the groups of Clay Fuqua at Indiana University and Gary Dunny at the University of Minnesota, respectively. The Fuqua lab has used biosensors to survey bacterial symbionts of sponges (phylum Porifera) for their ability to produce AHLs (J. Zan et al., CCCB-11, abstr. 27). Sponges form close associations with their symbiotic microbes, which account for up to 40% of the sponge biomass. Between 40 and 50% of the cultivated organisms from two marine sponges were found to produce AHLs. One particular isolate, Ruegeria sp. strain KLH11, was studied in more detail to ascertain the molecular basis of its ability to produce AHLs (J. Zan et al., CCCB-11, abstr. S2:3). Two genetic loci, designated symbiont loci A and B (designated ssa and ssb), were found to encode the LuxR/I homologues SsaR/I and SsbR/I, respectively. SsaI directs synthesis of long-chain AHLs with 3-oxo substitutions, while SsbI directs synthesis of long-chain AHLs with 3-hydroxy substitutions. SsaI is necessary for the production of AHLs by SsbI, but SsbI, in turn, modulates the levels of SsaI-produced AHLs, suggesting a complex regulatory circuitry with feedback mechanisms. SsaR/I are necessary for swimming motility and production of flagella. Thus, this QS system was hypothesized to play an important role in controlling dispersal of the bacteria within and perhaps even between host sponges. Interestingly, there also appears to be an orphan LuxI homologue that will undoubtedly add additional levels of complexity to the Ruegeria QS signal transduction pathway.
On the Gram-positive side, Laura Cook from the Dunny lab (L.C. Cook et al., CCCB-11, abstr. 85) presented a new model for how two different signaling peptides, chromosomally encoded cCF10 and plasmid-encoded iCF10I, influence the conjugative transfer of antibiotic resistance (pCF10) in Enterococcus faecalis. It has been known for some time that cCF10 activates conjugation while iCF10 inhibits the process. However, the new data show that iCF10 also acts as a QS signal to reduce conjugation by plasmid-containing donors at high cell densities. Thus, Gram-positive peptide signals may have dual roles.
An example of the importance of second messenger signals in QS signal transduction cascades was provided by Max Dow from University College Cork, Ireland (M. Dow et al., CCCB-11, abstr. S3:3), who described his group's latest findings about the importance of the HD-GYP and GGDEF domains in mediating the virulence of the plant pathogen Xanthomonas campestris. The primary QS signal in this system, diffusible signal factor (DSF), is the unsaturated fatty acid cis-11-methyl-dodecenoic acid (6, 71). It is sensed by the sensor kinase RpfC and the response regulator RpfG. RpfG has a CheY-like receiver domain attached to a HD-GYP domain that functions to degrade the second messenger cyclic di-GMP. Using a yeast two-hybrid system and fluorescence resonance energy transfer (FRET) analysis, physical interaction of RpfG with two proteins containing diguanylate cyclase (GGDEF) domains was found to occur. Altering the GYP motif at key residues or deleting the two GGDEF domains reduces motility in the organism but does not impact production of extracellular enzymes important to virulence or biofilm formation. Thus, it was proposed that the primary DSF QS system controls a downstream signal transduction cascade involving RpfC/G that influences motility through the localized expression of cyclic di-GMP.
Finally, several examples were presented of how metabolic substrates, intermediates, and end products can play important roles in cell-cell signaling. Linking QS pathways with other metabolic processes in the cell using a systems biology approach is emerging as one of the future challenges for the field. Brian Hammer's group at Georgia Tech (E. S. Antonova and B. K. Hammer, CCCB-11, abstr. 5) has demonstrated that both QS signals and the substrate chitin (an abundant marine polymer associated with environmental zooplankton molts) are important in coordinating horizontal gene transfer in the human pathogen Vibrio cholerae by promoting competence for genetic transformation (3). Chitin induces production of the TfoX transcription factor, which indirectly permits the QS signals CAI-1 [(S)-3-hydroxytridecan-4-one] and autoinducer-2 (AI-2) to induce the comEA competence gene and DNA uptake through HapR. Alecia Septer from Eric Stabb's group at the University of Georgia (A. N. Septer and E. V. Stabb, CCCB-11, abstr. S6:3 and abstr. 92) presented findings that in Vibrio fischeri, the tricarboxylic acid (TCA) cycle intermediate citrate can promote luminescence, a known QS-controlled phenotype. Proposed mechanisms suggest that the effects are perhaps due to decreasing free iron and/or activating the Gac/Csr regulatory cascade.
Antibiotics are key secondary metabolites that Julian Davies from the University of British Columbia, Canada (J. E. Davies, CCCB-11, abstr. S7:6), argues are actually interkingdom signals modulating transcription at subinhibitory concentrations. Davies explained his new conceptual theory of the “parvome” (complementary to the genome, proteome, etc.), consisting of all the bioactive small molecules produced by living organisms (16). Focusing on the components of the parvome with antibiotic properties, Davies pointed out that these compounds are not made at high enough concentrations in nature to kill bacteria, and thus there is almost nothing known about their natural biological function. Data from Davies's group demonstrate that antibiotics at physiologically relevant concentrations may actually induce transcriptional changes, mediating microbial behaviors similarly to QS signals.
(ii) Signal receptors.
During 2011, the crystal structures of two LuxR homologues, CviR (12) and QscR (36), were published, providing further evidence that there is not a one-size-fits-all model with regard to recognition of AHL signals. QscR is an example of an “orphan” LuxR homologue, a LuxR homologue with no obvious LuxI partner, and Vittorio Venturi's group at the International Centre for Genetic Engineering and Biotechnology (ICGEB) in Italy (D. Passos et al., CCCB-11, abstr. S6:4, and J. F. Gonzalez et al., CCCB-11, abstr. 7), has demonstrated the widespread distribution of these types of receptors across a number of plant-associated bacteria. It now appears that these receptors may play a critical role not only in microbe-microbe interactions but also in microbe-plant communication (see Part 3, Interspecies and Interkingdom Communication, below). They appear to cluster together phylogenetically, as does another subfamily of LuxR homologues represented by EsaR from the plant pathogen Pantoea stewartii (66).
The EsaR subfamily is unique in that its members are biologically active in the absence rather than in the presence of their cognate AHLs. Thus, at low cell density/low AHL levels, they have the capacity to function as either repressors or activators of transcription depending on the position of their binding site within target promoters. It is the former activity, that of repression, that Cynthia Collins's group at Rensselaer Polytechnic Institute is interested in utilizing to develop EsaR as a tool for synthetic biology applications (C. H. Collins, CCCB-11, abstr. S5:5). Using a repressor in this capacity has the potential to enable the design of systems that can rapidly respond to changes in AHL concentration and those with increased robustness in response to fluctuations. The limitation of the wild-type EsaR protein comes from the high level of AHL that is naturally needed in the system to fully inhibit the activity of EsaR; micromolar amounts are required, whereas nanomolar levels of ligand stimulate most LuxR activators. Collins has developed a genetic screen to identify variant forms of EsaR with mutations in the AHL- and DNA-binding domains that now respond to nanomolar levels of AHL. The system is being engineered further by modifying the location and number of esa boxes in synthetic promoters. In a complementary approach, Ann Stevens's lab at Virginia Tech has focused on better understanding the interactions between wild-type EsaR and its native ligand. They have found that in the presence of AHL, EsaR is not rapidly degraded by proteases and its apparent multimeric state does not change (J. S. Geissinger et al., CCCB-11, abstr. 80) (60). Further, they have isolated variant forms of EsaR that no longer respond to AHL, a process which has helped to define the AHL binding pocket in the protein and has given some clues to the interdomain signaling that occurs. The regulatory mechanism of control by EsaR enables differential gene control at both low and high cell densities. The total regulon controlled by EsaR is being examined by proteomic approaches in the Stevens lab (R. Ramachandran et al., CCCB-11, abstr. 77), and the total regulon controlled by the AHL-hindered transcription factor VirR of Pectobacterium atrosepticum was ascertained using a chromatin immunoprecipitation approach by George Salmond's group at the University of Cambridge, United Kingdom (R. E. Monson et al., CCCB-11, abstr. 71). Collectively, these efforts are providing key insights into the global activities under the control of the EsaR subfamily of transcription factors for the first time.
The interactions between the protein receptor CqsS and its ligand CAI-1 from Vibrio cholerae were the focus of work presented by Lark Perez from the Semmelhack/Bassler group at Princeton (L. J. Perez et al., CCCB-11, abstr. S1:3). This group has worked out a unique, multistep enzymatic process for the biosynthetic assembly of CAI-1 (72) and synthesized some of its analogues (10). They have used these compounds to understand features of the signals and the membrane-bound histidine kinase receptor, CqsS, that are critical for ligand binding, permitting analysis of signal molecule recognition and fidelity in the system (45, 46). This type of approach is at the heart of efforts to design better agonists and antagonists of QS systems.
Michael Meijler's lab at Ben-Gurion University of the Negev, Israel (M. M. Meijler et al., CCCB-11, abstr. S2:7), has developed a set of electrophilic probes that covalently bind to LasR, one of the AHL receptors in Pseudomonas aeruginosa, leading to inhibition of its activity (2). This approach has now been extended to enable the visualization of the LasR ligand recognition event within live cells. The approach involved covalent bonding of a reactive mimic of 3-oxo-C12-HSL to LasR, followed by aniline-catalyzed oxime formation between this complex and a fluorescent BODIPY dye derivative (54). Results indicate that LasR is concentrated at the poles of the cells, which may provide a mechanism that permits for a reduction in noise generated by AHL secretion. This approach may be utilized to examine QS receptor localization in other bacteria as well.
(iii) Signal transduction modulation/interference.
Exogenous addition of QS receptor agonists and antagonists is just one mechanism whereby the QS response may be modulated. Quenching of QS was discussed in three talks given by Wim Quax from the University of Groningen, Netherlands (G. Koch et al., CCCB-11, abstr. S2:5), Steven Christiaen from Tom Coenye's laboratory at Ghent University, Belgium (S. E. Christiaen et al., CCCB-11, abstr. S2:6), and Jiro Nakayama from Kysahu University, Japan (J. Nakayama, CCCB-11, abstr. S5:2). Quax's talk focused on the activity of acylases from the N-terminal nucleophile (Ntn) hydrolase family that are capable of cleaving the amide bond between the acyl side chains and homoserine lactone rings of AHLs. The Pseudomonas aeruginosa genome encodes at least four of these acylases. The dual activity of one of them, PvdQ, has been examined with regard to how it binds the AHL substrate (8) and plays a role in hydrolyzing a precursor for the siderophore pyoverdine (18). A complete understanding of the physiological role of these acylases in an organism that itself synthesizes AHLs remains to be determined. However, efforts to evolve these acylases into effective quorum quenchers for AHLs produced by other bacteria, such as Burkholderia species, is under way. Christiaen's talk described high-throughput methods to identify AHL-degrading bacteria from environmental samples. Isolates were obtained from plating samples of minimal medium containing AHLs as the sole C and N sources. In this manner, 41 isolates that appear to have a variety of mechanisms for quorum quenching have been obtained. Some, but not all, mechanisms are heat sensitive; proteinase K destroyed the activity of some but not others, and size exclusion chromatography indicated that small and large quorum-sensing inhibitory molecules were being utilized. Nakayama has worked to identify inhibitors that target the cyclic-peptide-mediated QS systems utilized by some Gram-positive bacteria. A large-scale screening of microbial secondary metabolites from extracts of fungi and actinomycetes as well as a library of natural and synthetic compounds has led to the identification of several candidates that can inhibit either the staphylococci agr or the enterococci fsr QS system or both (42, 43) (unpublished data). A second approach was used to identify antagonists of the gelatinase biosynthesis-activating pheromone (GBAP) lactone peptide of E. faecalis via reverse alanine-scanning mutagenesis (48).
Another mechanism of QS control began to emerge as a recurring theme at the conference, the important role of regulatory RNAs. Gary Dunny (G. M. Dunny et al., CCCB-11, abstr. S1:1) described evidence from the Enterococcus faecalis sex pheromone system demonstrating that antisense transcription impacts gene expression by direct RNA-RNA interactions of Anti-Q and Qs (29) and by interference of elongating RNA polymerase complexes with convergently transcribing complexes on the opposite strand of the DNA in the prgQ operon (11). Hidetada Hirakawa from the Harwood/Greenberg labs has identified the presence of an antisense RNA for the gene encoding RpaR, the receptor for p-coumaroyl-homoserine lactone in Rhodopseudomonas palustris (H. Hirakawa et al., CCCB-11, abstr. 50). Interestingly, RpaR controls expression of its cis-RNA, which in turn regulates RpaR levels and, thereby, rpaI expression and pC-HSL production. It appears that the cis-RNA functions by base pairing with rpaR transcripts and thus inhibits translation. Stephen Winans's lab from Cornell presented evidence that two small RNAs (sRNAs) involved in QS exist in Yersinia enterocolitica (C.-S. Tsai and S. C. Winans, CCCB-11, abstr. 96). One, called YenS, binds to a region that overlaps the YenI start codon (67). A second sRNA gene, yenT, was found directly downstream of yenS and antagonizes YenS function, probably by RNA-RNA hybridization. In addition, yenR and yenI are convergently transcribed with 3′ overlapping ends, as are the genes encoding all EsaR/I homologues (66). Thus, the possibility for transcriptional interference exists in these systems as well. It is becoming clear that antisense transcription is much more widespread in microbes than previously recognized. This adds an additional layer of control to the regulation of and by QS systems that must be considered in the future.
(iv) New technologies/approaches.
The latest technologies were used in the work described in presentations throughout the conference. However, there were two in particular that are pushing the envelope and giving us powerful approaches for better understanding microbial cell-cell communications in particular. Dipankar Koley from the University of Texas—Austin (D. Koley et al., CCCB-11, abstr. S5:4) demonstrated how the real-time distribution of local metabolite concentrations may be determined for live cells using scanning electrochemical microscopy (SECM). In addition, the redox state of electroactive metabolites may be determined. In particular, Koley showed that a reduced layer of pyocyanin exists for 500 μm above a Pseudomonas biofilm, even in the presence of oxygen. This may be critical to the organism's ability to scavenge iron (32). The SECM approach enables quantitative measurements of redox metabolite production in real time and in three dimensions (3D) that will undoubtedly facilitate a better understanding of how they aid bacterial survival and growth.
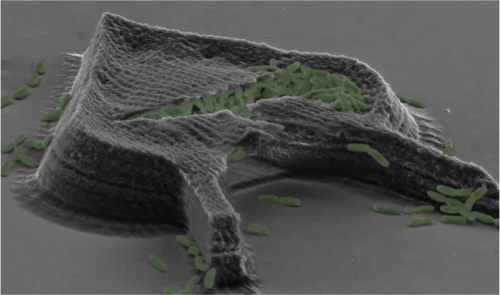
Perhaps some of the most memorable images of the conferences are those of the bovine serum albumin microfabricated chambers/traps synthesized by the laboratory of Jason Shear at the University of Texas—Austin (J. L. Connell et al., CCCB-11, abstr. S5:1) (Fig. 4). The laser-based microfabrication strategy used to produce these three-dimensional picoliter-scale microchambers to study microbial populations has been published (30, 47). They permit relatively low numbers of cells to be maintained at a high cell density with 3D confinement, rapid exchange of materials (below a certain size) with the environment, and variable flow rates. In collaboration with Marvin Whiteley's group, Shear's lab has used these microchambers to study the molecular processes controlling antibiotic resistance and QS-mediated virulence factor production at cell densities relevant to the natural transmission of pathogens (13). Future applications include the possibility of using avidin to enable the chambers to be “decorated” with biotin conjugated to enzymes or antibodies and the transcription analysis of cells collected from the traps under growth conditions of low cell numbers at high cell densities, further pushing the boundaries of our understanding of bacterial cell-cell communication in a physiologically relevant context.
Fig 4.
Scanning electron microscopy (SEM) image of a heart-shaped bacterial “lobster trap” filled with Pseudomonas aeruginosa. The tear in the roof occurred during SEM preparation. The bacteria are shown in green (false color). Reprinted from reference 13 with permission.
PART 3. INTERSPECIES AND INTERKINGDOM COMMUNICATION
A common interwoven theme of this year's conference was an emphasis on exploring how QS contributes to interspecies and bacterium-host relationships. It appears that as our knowledge of the inner workings of the QS systems of individual species expands, many investigators are broadening their scope and are trying to understand how interspecies and interkingdom signaling (ISS and IKS, respectively) contribute to symbiosis and pathogenesis. ISS and IKS have been emerging fields of research for a number of years, and it has been speculated that the ability of bacteria to directly communicate with host cells via QSS may impact processes ranging from host development to disease (64). It is well established that many bacterial QSS directly affect neighboring cells, whether they be prokaryotic or eukaryotic, but important questions remain. For example, how do neighboring cells perceive and respond to QSS? Is there an evolutionary advantage for bacteria to directly communicate with other species or their host via QSS or vice versa? And, of course, should these interactions be considered signaling, or are neighboring cells simply responding to environmental cues? Several presentations at this year's CCCB conference addressed these important gaps in knowledge.
(i) Chemical communication in symbiotic relationships.
The relationship between Vibrio fischeri and Euprymna scolopes (the bobtail squid) is perhaps the quintessential example of the role of QS in symbiosis. Academic lore tells us that while Woody Hastings and his graduate student were trying to isolate and characterize luciferase, the enzyme responsible for the bioluminescence of V. fischeri, they noticed that luminescence increased dramatically during the mid- to late exponential phase of bacterial growth (25). A few years later, Hastings, along with Ken Nealson and Terry Platt, was able to conclude that bacteria produced diffusible “autoinducer” compounds that accumulated in the medium during growth and initiated light product (44), and thus, a new field of study was born. This burgeoning field would not mature or even get the name “quorum sensing” for another 24 years. And now, more than 40 years later, the squid-Vibrio relationship still serves as a model for new discoveries, several of which were discussed at this year's conference in session 6, Symbiosis, Mutualism, and Microbe-Microbe Communication.
Spencer Nyholm from the University of Connecticut discussed how the symbiotic relationship between E. scolopes and V. fischeri is maintained by a “molecular dialogue,” which leads to the selection of V. fischeri, above all other nonsymbionts, for colonization of the squid's light organ (S. V. Nyholm, CCCB-11, abstr. S6:2). Nyholm's group utilized high-throughput proteomics to identify both host and symbiont proteins present in the adult squid's light organ which are potentially important for symbiosis (59). Many of the squid proteins identified were predicted to be involved with the innate immune system, including pattern recognition receptors (PPRs) that respond to microbe-associated molecular patterns. In addition, both host and symbiont proteins involved in responding to oxidative stress were identified, a result which is significant because E. scolopes is thought to use reactive oxygen species to prevent colonization by nonsymbiotic bacteria. Further data from the Nyholm group demonstrated that the primary innate immune cells in E. scolopes, macrophage-like hemocytes, respond differently to symbiotic and nonsymbiotic bacteria and that this differential response may facilitate immune tolerance to V. fischeri (49). The group is currently employing transcriptomics and proteomics to understand how squid hemocytes respond differently to V. fischeri than to nonsymbiotic bacteria, with a focus on PRRs and signaling pathways in the host and outer membrane proteins of V. fischeri that may be involved in adhesion to host hemocytes.
Once Vibrio has successfully colonized the light organ of the squid, different environmental cues may dramatically affect the subsequent production of light. For example, Alecia Septer from Eric Stabb's group at the University of Georgia presented data indicating that high citrate levels, potentially influenced by the amount of reactive oxygen species in the host, may serve as a signal sensed by the V. fischeri GacS/GacA two-component system, which, in turn, results in higher activation of luminescence through inactivation of aconitase, a TCA cycle enzyme that converts citrate to isocitrate (A. N. Septer et al., CCCB-11, abstr. S6:3). In addition to environmental cues, differential light production may also have genetic explanations. Tim Miyashiro from the University of Wisconsin—Madison School of Medicine and Public Health described a new plasmid-associated gene cluster in V. fischeri that may also have important roles in regulating light production (T. Miyashiro et al., CCCB-11, abstr. S2:4). This gene cluster regulates expression of the small regulatory RNA Qrr1, which posttranscriptionally regulates the global regulator LitR. Overexpression of the plasmid-associated genes resulted in reduced colonization and luminescence by V. fischeri in vivo. There was heterogeneity in the expression levels of these genes among isogenic bacterial populations in individual crypts of the light organ, suggesting that a subpopulation of cells produces low levels of bioluminescence, as had previously been proposed by modeling studies (75). Taken together, these presentations not only provided important new insights into the squid-Vibrio relationship but also illustrated the complexity of this well-studied model system.
Another fascinating symbiotic relationship exists between the vertically transmitted gammaproteobacterium Buchnera aphidicola and its insect host, the pea aphid. In this relationship, communication is facilitated by metabolite exchange, and Angela Douglas's group from Cornell University has demonstrated by metabolic modeling that 33 metabolites are absolutely required by B. aphidicola and that, in turn, 25 are released (A. E. Douglas, CCCB-11, abstr. S6:1) (50). Douglas presented quantitative proteomics data indicating that metabolite exchange between bacteria and the insect bacteriocyte cells plays a key role in maintaining this symbiotic relationship. Interestingly, peptidoglycan shedding by B. aphidicola is thought to function as a colonization signal to the host, but the insect enzymes required for the proper processing of peptidoglycan, which elicits a favorable immune response, were acquired laterally from bacteria other than Buchnera (37, 77). Therefore, this symbiotic relationship is an excellent example of how a long coevolutionary history of metabolic networks can result in efficient metabolite exchanges, which double as communication signals.
(ii) Chemical communication in pathogenesis.
Historically, while there have been a few excellent model systems employed to study beneficial signaling between bacteria and their hosts, many more studies have focused on determining how QS facilitates pathogenesis. This year's conference provided an extraordinary array of model systems and new insights into the role of bacterial communication in infection. In fact, an entire session was devoted to this topic (session 4, Host-Pathogen Signaling).
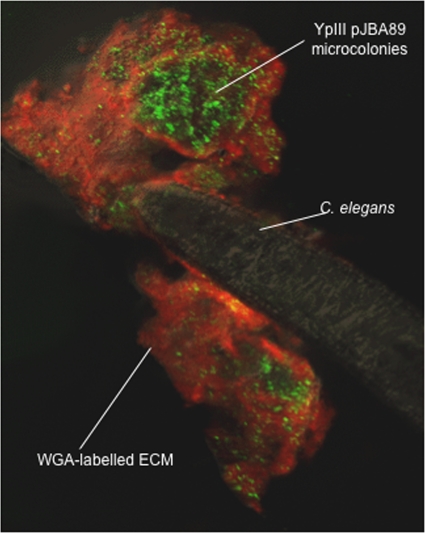
Paul Williams from the University of Nottingham described his group's recent investigations into the involvement of QS for Yersinia pseudotuberculosis pathogenesis (P. Williams, CCCB-11, abstr. S1:2). Utilizing a nematode model of infection, Williams's group demonstrated that Y. pseudotuberculosis forms biofilms around the mouth of Caenorhabditis elegans, blocking feeding and resulting in worm death (5) (Fig. 5). Their studies have implicated the production of AHLs as a prerequisite for biofilm formation, indicating that QS is required for pathogenesis. However, while the Y. pseudotuberculosis QS system, which involves two pairs of LuxRI orthologues (YpsR/I and YtbR/I) and multiple AHLs, controls swimming motility via regulation of the master motility regulator FlhDC and the flagellum-specific sigma factor FliA (4), biofilm development was shown to be independent of flagellum-mediated motility. Interestingly, the biofilm formation defect of an flhDC mutant may be alleviated by manipulating the type III secretion system, which is repressed by QS in Y. pseudotuberculosis. Taken together, these data indicate a role for the type III needle in modulating biofilm formation on C. elegans and switching the bacteria from a planktonic to a biofilm-associated lifestyle.
Fig 5.
Confocal microscope image showing a Yersinia pseudotuberculosis biofilm around the head of the nematode worm Caenorhabditis elegans. Y. pseudotuberculosis YpIII transformed with the AHL reporter pJBA89 fluoresces green in response to AHLs produced within the biofilm. The biofilm extracellular matrix has been stained red using a wheat germ agglutinin (WGA) rhodamine conjugate. Figure reproduced from reference 5 with permission of Paul Williams, University of Nottingham, United Kingdom.
Vanessa Sperandio's group at the University of Texas Southwestern Medical Center has long studied how mammalian hormonal signals can influence QS in some bacteria. For example, both the quorum signal AI-3 and the mammalian hormones epinephrine and norepinephrine can modulate QS in Escherichia coli O157:H7 (enterohemorrhagic E. coli [EHEC]), activating flagellar and virulence-associated genes and culminating in the production of attaching and effacing lesions on the host's intestinal epithelial cells and eventually diarrhea (62). New studies from her laboratory revealed a new two-component response regulator located on a pathogenicity island found only in EHEC (V. Sperandio, CCCB-11, abstr. S4:3). This new two-component regulator, FusK/FusR, can repress many of EHEC's virulence factors, effectively reigning in its pathogenicity. In fact, infection with a fusKR mutant resulted in dramatically more attaching and effacing lesions on cultured epithelial cells. As FusK/FusR is modulated by fucose utilization, the group postulates that it may represent a mechanism for EHEC to gauge its location within the gut by sensing the amount of fucose present. Therefore, if EHEC were to sense high levels of fucose, as would be expected in the gut lumen, FusK/R would effectively repress virulence genes. Presumably, this allows the bacteria to attach and create lesions only in close proximity to the intestinal epithelia.
The Gram-positive pathogens were also represented, as Alex Horswill from the University of Iowa discussed his group's latest findings investigating the involvement of QS with the dispersal of biofilms by S. aureus (A. Horswill, CCCB-11, abstr. S4:1). Noting that S. aureus mutants that had mutations in the accessory gene regulator (agr) QS system actually formed better biofilms, the group previously demonstrated that adding autoinducing peptide (AIP) to wild-type S. aureus cultures and clinical isolates caused biofilm dispersal (9, 34). Subsequent investigations revealed several QS-controlled proteases that were responsible for biofilm degradation, including two cysteine proteases or “staphopains.” Purified staphopains were able to prevent biofilm formation by S. aureus, and studies are ongoing to further delineate the role of QS in biofilm dispersal.
(iii) Interspecies interactions important for pathogenesis.
Many, if not most, bacterial infections are polymicrobial; however, very little is understood about how signaling between pathogens or host flora influences the course of infection. Several presentations at this year's CCCB addressed interspecies interactions, with a focus on ISS. In addition to the well-characterized AHL signaling products of QS, the DSF signaling molecules (cis-2-unsaturated fatty acids) made by several bacterial species may also have a role as an interspecies signal that affects pathogenesis. Robert Ryan from the University of Cork explained how DSF might increase the antimicrobial resistance and persistence of P. aeruginosa in lung infections (K. B. Twomey et al., CCCB-11, abstr. S4:5). DSF is made by Burkholderia cenocepacia and Stenotrophomonas maltophilia (among others), two species that frequently accompany P. aeruginosa in the lung infections of cystic fibrosis (CF) patients. Although P. aeruginosa does not make DSF itself, previous work from the group demonstrated that DSF altered P. aeruginosa biofilm formation and led to increased antimicrobial tolerance (56). The group's new findings demonstrate that DSF is present in CF lung infections and that the presence of DSF in sputum correlated with S. maltophilia and/or B. cenocepacia colonization. P. aeruginosa clinical isolates displayed increased antimicrobial tolerance when treated with DSF, and DSF also increased P. aeruginosa persistence in a mouse lung infection model (68). Taken together, these data strongly indicate that DSF contributes to the refractory nature of polymicrobial lung infections.
QSS may also help with the coordinated accumulation of the multiple species that make up dental plaque. Richard Lamont from the University of Louisville described how LuxS/AI-2 and other signals are crucial to the interspecies balancing act and how shifts in this population distribution can greatly affect the pathogenesis of the community (R. J. Lamont, CCCB-11, abstr. S4:6). One potential new signal modulating the composition of the oral microbial community was discussed by Carla Cugini (The Forsyth Institute) (C. Cugini et al., CCCB-11, abstr. S4:7). Her group has identified an arginine deiminase (ADI), which modulates Porphyromonas gingivalis biofilm formation, specifically through the downregulation of gene expression of the major and minor fimbriae. Interestingly, this protein, which is normally intracellular, appears to have traditional enzymatic roles in both nutrient acquisition and pH homoeostasis but is also secreted and serves as a signal to modulate biofilm development.
Infection does not always rely solely on the pathogen. An interesting relationship between a pathogen and commensal was described by Vittorio Venturi from the International Centre for Genetic Engineering and Biotechnology (D. Passos et al., CCCB-11, abstr. S6:4). Utilizing the olive tree as a host, Venturi's group showed that Pseudomonas savastanoi pv. savastanoi can cause tumors (or knot disease) on the tree, but particularly aggressive infections were seen when P. savastanoi pv. savastanoi was associated with the plant endophyte Erwinia toletana (27). Interestingly, P. savastanoi pv. savastanoi and E. toletana produce the same type of AHL signal and appear to colocalize in the infected nodule. Data thus far suggest that these two species have evolved to share an environmental niche. Further examples of studies focused on characterizing interspecies relationships were found among the poster presentations. For example, Brian Ahmer's team at The Ohio State University presented posters (M. M. Ali et al., CCCB-11, abstr. 34 and 72) on how they are using a genetic screening tool called transposon site hybridization (TraSH) to uncover new interspecies relationships critical to infection. The team used TraSH to screen Salmonella mutant libraries for defects in microbial interactions. Their strategy was to change just one member of the flora between two groups of animals so that all other variables remained the same. For example, in one screen, the group identified all Salmonella genes required for fitness in germfree mice and in germfree mice monoassociated with Enterobacter cloacae. In another study, the team identified all Salmonella genes required for fitness in conventional pigs and in conventional pigs infected with Yersinia enterocolitica. Interestingly, in the second study, srgE was the only Salmonella enterica gene that met the most-stringent criteria for being required for fitness in Y. enterocolitica in infected pigs and this gene is known to be upregulated by AHLs. These and other preliminary data presented by the group demonstrate the promise of this technique in unraveling the complex interactions of microbes in vivo.
SUMMARY AND PERSPECTIVE
The 4th ASM Conference on Cell-Cell Communication in Bacteria in Miami, FL, continued a tradition of excellent, thought-provoking scientific discussion and interaction between experts from around the world that work in the field of QS. The three key themes emphasized in this review, sociomicrobiology, signal transduction mechanisms, and interspecies communication, have been used to represent some, but not all, of the cutting edge research that was presented. The field of QS has clearly become progressively more complex and interdisciplinary in nature. A cross-disciplinary approach is necessary to address some of the most challenging questions still facing us. Will we ever obtain the ability to fully understand the complex communication systems that exist in natural multispecies environments? Will we eventually become successful in achieving a level of understanding that enables us to manipulate QS-controlled systems to the benefit of society? Can what we learn about microbial societies give us further insights into evolutionary processes and pressures? By bringing together individuals committed to working for the common good, this conference has facilitated person-to-person dialogues that will undoubtedly help continue to push the field forward to address these questions and others still awaiting us in the future.
ACKNOWLEDGMENTS
We thank all of the participants, especially those that gave talks or poster presentations and the many individuals who stepped up to the microphone to engage in lively discussions. We give credit to Heidi Kaplan, Marvin Whiteley, and the other conference organizers for putting together the program. We give special thanks to those who contributed figures and provided editorial comments for the review.
Cell-cell communication research in the authors' laboratories is supported by grants from the National Science Foundation, MCB-0919984 to A.M.S. and MCB-0843102 to M.S., and the CH Foundation (to K.P.R.).
Footnotes
Published ahead of print 2 March 2012
REFERENCES
- 1. Ahlgren NA, Harwood CS, Schaefer AL, Giraud E, Greenberg EP. 2011. Aryl-homoserine lactone quorum sensing in stem-nodulating photosynthetic bradyrhizobia. Proc. Natl. Acad. Sci. U. S. A. 108:7183–7188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amara N, et al. 2009. Covalent inhibition of bacterial quorum sensing. J. Am. Chem. Soc. 131:10610–10619 [DOI] [PubMed] [Google Scholar]
- 3. Antonova ES, Hammer BK. 2011. Quorum-sensing autoinducer molecules produced by members of a multispecies biofilm promote horizontal gene transfer to Vibrio cholerae. FEMS Microbiol. Lett. 322:68–76 [DOI] [PubMed] [Google Scholar]
- 4. Atkinson S, et al. 2008. Functional interplay between the Yersinia pseudotuberculosis YpsRI and YtbRI quorum sensing systems modulates swimming motility by controlling expression of flhDC and fliA. Mol. Microbiol. 69:137–151 [DOI] [PubMed] [Google Scholar]
- 5. Atkinson S, et al. 2011. Biofilm development on Caenorhabditis elegans by Yersinia is facilitated by quorum sensing-dependent repression of type III secretion. PLoS Pathog. 7:e1001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barber CE, et al. 1997. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol. Microbiol. 24:555–566 [DOI] [PubMed] [Google Scholar]
- 7. Berleman JE, Chumley T, Cheung P, Kirby JR. 2006. Rippling is a predatory behavior in Myxococcus xanthus. J. Bacteriol. 188:5888–5895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bokhove M, Nadal Jimenez P, Quax WJ, Dijkstra BW. 2010. The quorum-quenching N-acyl homoserine lactone acylase PvdQ is an Ntn-hydrolase with an unusual substrate-binding pocket. Proc. Natl. Acad. Sci. U. S. A. 107:686–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boles BR, Horswill AR. 2008. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 4:e1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bolitho ME, et al. 2011. Small molecule probes of the receptor binding site in the Vibrio cholerae CAI-1 quorum sensing circuit. Bioorg. Med. Chem. 19:6906–6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chatterjee A, et al. 2011. Convergent transcription confers a bistable switch in Enterococcus faecalis conjugation. Proc. Natl. Acad. Sci. U. S. A. 108:9721–9726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen G, et al. 2011. A strategy for antagonizing quorum sensing. Mol. Cell 42:199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Connell JL, et al. 2010. Probing prokaryotic social behaviors with bacterial “lobster traps”. mBio 4:e00202–10 doi:10.1128/mbio.00202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coulthurst SJ, Barnard AM, Salmond GP. 2005. Regulation and biosynthesis of carbapenem antibiotics in bacteria. Nat. Rev. Microbiol. 3:295–306 [DOI] [PubMed] [Google Scholar]
- 15. Darwin C. 1859. On the origin of species by means of natural selection, or the preservation of favored races in the struggle for life. Murray, London, United Kingdom [Google Scholar]
- 16. Davies J, Ryan KS. 2012. Introducing the parvome: bioactive compounds in the microbial world. ACS Chem. Biol. 7:252–259 [DOI] [PubMed] [Google Scholar]
- 17. Diggle SP, Griffin AS, Campbell GS, West SA. 2007. Cooperation and conflict in quorum-sensing bacterial populations. Nature 450:411–414 [DOI] [PubMed] [Google Scholar]
- 18. Drake EJ, Gulick AM. 2011. Structural characterization and high-throughput screening of inhibitors of PvdQ, an NTN hydrolase involved in pyoverdine synthesis. ACS Chem. Biol. 6:1277–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eldar A. 2011. Social conflict drives the evolutionary divergence of quorum sensing. Proc. Natl. Acad. Sci. U. S. A. 108:13635–13640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher RA. 1958. The genetical theory of natural selection, 2nd ed Dover Publications, Mineola, NY. [Google Scholar]
- 21. Foster KR, Shaulsky G, Strassmann JE, Queller DC, Thompson CR. 2004. Pleiotropy as a mechanism to stabilize cooperation. Nature 431:693–696 [DOI] [PubMed] [Google Scholar]
- 22. Gardner A, West SA, Wild G. 2011. The genetical theory of kin selection. J. Evol. Biol. 24:1020–1043 [DOI] [PubMed] [Google Scholar]
- 23. Griffin AS, West SA, Buckling A. 2004. Cooperation and competition in pathogenic bacteria. Nature 430:1024–1027 [DOI] [PubMed] [Google Scholar]
- 24. Hamilton WD. 1964. The genetical evolution of social behaviour. I. J. Theor. Biol. 7:1–16 [DOI] [PubMed] [Google Scholar]
- 25. Hastings JW. 2010. Bioluminescence insights and quorum sensibilities. Microbe 5:212–214 [Google Scholar]
- 26. Hense BA, et al. 2007. Does efficiency sensing unify diffusion and quorum sensing? Nat. Rev. Microbiol. 5:230–239 [DOI] [PubMed] [Google Scholar]
- 27. Hosni T, et al. 2011. Sharing of quorum-sensing signals and role of interspecies communities in a bacterial plant disease. ISME J. 5:1857–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ji G, Beavis R, Novick RP. 1997. Bacterial interference caused by autoinducing peptide variants. Science 276:2027–2030 [DOI] [PubMed] [Google Scholar]
- 29. Johnson CM, et al. 2010. Direct evidence for control of the pheromone-inducible prgQ operon of Enterococcus faecalis plasmid pCF10 by a countertranscript-driven attenuation mechanism. J. Bacteriol. 192:1634–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaehr B, Shear JB. 2009. High-throughput design of microfluidics based on directed bacterial motility. Lab Chip 9:2632–2637 [DOI] [PubMed] [Google Scholar]
- 31. Keller L, Surette MG. 2006. Communication in bacteria: an ecological and evolutionary perspective. Nat. Rev. Microbiol. 4:249–258 [DOI] [PubMed] [Google Scholar]
- 32. Koley D, Ramsey MM, Bard AJ, Whiteley M. 2011. Discovery of a biofilm electrocline using real-time 3D metabolite analysis. Proc. Natl. Acad. Sci. U. S. A. 108:19996–20001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Konovalova A, Petters T, Sogaard-Andersen L. 2010. Extracellular biology of Myxococcus xanthus. FEMS Microbiol. Rev. 34:89–106 [DOI] [PubMed] [Google Scholar]
- 34. Lauderdale KJ, Malone CL, Boles BR, Morcuende J, Horswill AR. 2010. Biofilm dispersal of community-associated methicillin-resistant Staphylococcus aureus on orthopedic implant material. J. Orthop. Res. 28:55–61 [DOI] [PubMed] [Google Scholar]
- 35. Lindemann A, et al. 2011. Isovaleryl-homoserine lactone, an unusual branched-chain quorum-sensing signal from the soybean symbiont Bradyrhizobium japonicum. Proc. Natl. Acad. Sci. U. S. A. 108:16765–16770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lintz MJ, Oinuma K, Wysoczynski CL, Greenberg EP, Churchill ME. 2011. Crystal structure of QscR, a Pseudomonas aeruginosa quorum sensing signal receptor. Proc. Natl. Acad. Sci. U. S. A. 108:15763–15768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. MacDonald SJ, Thomas GH, Douglas AE. 2011. Genetic and metabolic determinants of nutritional phenotype in an insect-bacterial symbiosis. Mol. Ecol. 20:2073–2084 [DOI] [PubMed] [Google Scholar]
- 38. Mashburn LM, Whiteley M. 2005. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437:422–425 [DOI] [PubMed] [Google Scholar]
- 39. McCormick JR, Flardh K. 2012. Signals and regulators that govern Streptomyces development. FEMS Microbiol. Rev. 36:206–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mellbye B, Schuster M. 2011. The sociomicrobiology of antivirulence drug resistance: a proof of concept. mBio 5:e00131–11 doi:10.1128/mBio.00131-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mittal S, Kroos L. 2009. A combination of unusual transcription factors binds cooperatively to control Myxococcus xanthus developmental gene expression. Proc. Natl. Acad. Sci. U. S. A. 106:1965–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakayama J, et al. 2007. Siamycin attenuates fsr quorum sensing mediated by a gelatinase biosynthesis-activating pheromone in Enterococcus faecalis. J. Bacteriol. 189:1358–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakayama J, et al. 2009. Ambuic acid inhibits the biosynthesis of cyclic peptide quormones in gram-positive bacteria. Antimicrob. Agents Chemother. 53:580–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nealson KH, Platt T, Hastings JW. 1970. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 104:313–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ng WL, et al. 2011. Signal production and detection specificity in Vibrio CqsA/CqsS quorum-sensing systems. Mol. Microbiol. 79:1407–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ng WL, et al. 2010. Probing bacterial transmembrane histidine kinase receptor-ligand interactions with natural and synthetic molecules. Proc. Natl. Acad. Sci. U. S. A. 107:5575–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nielson R, Kaehr B, Shear JB. 2009. Microreplication and design of biological architectures using dynamic-mask multiphoton lithography. Small 5:120–125 [DOI] [PubMed] [Google Scholar]
- 48. Nishiguchi K, Nagata K, Tanokura M, Sonomoto K, Nakayama J. 2009. Structure-activity relationship of gelatinase biosynthesis-activating pheromone of Enterococcus faecalis. J. Bacteriol. 191:641–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nyholm SV, Stewart JJ, Ruby EG, McFall-Ngai MJ. 2009. Recognition between symbiotic Vibrio fischeri and the haemocytes of Euprymna scolopes. Environ. Microbiol. 11:483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poliakov A, et al. 2011. Large-scale label-free quantitative proteomics of the pea aphid-Buchnera symbiosis. Mol. Cell Proteomics 10:M110.007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Potrykus K, Cashel M. 2008. (p)ppGpp: still magical? Annu. Rev. Microbiol. 62:35–51 [DOI] [PubMed] [Google Scholar]
- 52. Price GR. 1970. Selection and covariance. Nature 227:520–521 [DOI] [PubMed] [Google Scholar]
- 53. Ramsay JP, Williamson NR, Spring DR, Salmond GP. 2011. A quorum-sensing molecule acts as a morphogen controlling gas vesicle organelle biogenesis and adaptive flotation in an enterobacterium. Proc. Natl. Acad. Sci. U. S. A. 108:14932–14937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rayo J, Amara N, Krief P, Meijler MM. 2011. Live cell labeling of native intracellular bacterial receptors using aniline-catalyzed oxime ligation. J. Am. Chem. Soc. 133:7469–7475 [DOI] [PubMed] [Google Scholar]
- 55. Redfield RJ. 2002. Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 10:365–370 [DOI] [PubMed] [Google Scholar]
- 56. Ryan RP, et al. 2008. Interspecies signalling via the Stenotrophomonas maltophilia diffusible signal factor influences biofilm formation and polymyxin tolerance in Pseudomonas aeruginosa. Mol. Microbiol. 68:75–86 [DOI] [PubMed] [Google Scholar]
- 57. Sandoz K, Mitzimberg S, Schuster M. 2007. Social cheating in Pseudomonas aeruginosa quorum sensing. Proc. Natl. Acad. Sci. U. S. A. 104:15876–15881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schaefer AL, et al. 2008. A new class of homoserine lactone quorum-sensing signals. Nature 454:595–599 [DOI] [PubMed] [Google Scholar]
- 59. Schleicher TR, Nyholm SV. 2011. Characterizing the host and symbiont proteomes in the association between the bobtail squid, Euprymna scolopes, and the bacterium, Vibrio fischeri. PLoS One 6:e25649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schu DJ, Ramachandran R, Geissinger JS, Stevens AM. 2011. Probing the impact of ligand binding on the acyl-homoserine lactone-hindered transcription factor EsaR of Pantoea stewartii subsp. stewartii. J. Bacteriol. 193:6315–6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schuster M, Lohstroh CP, Ogi T, Greenberg EP. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB. 2003. Bacteria-host communication: the language of hormones. Proc. Natl. Acad. Sci. U. S. A. 100:8951–8956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stefanic P, Mandic-Mulec I. 2009. Social interactions and distribution of Bacillus subtilis pherotypes at microscale. J. Bacteriol. 191:1756–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Teplitski M, Mathesius U, Rumbaugh KP. 2011. Perception and degradation of N-acyl homoserine lactone quorum sensing signals by mammalian and plant cells. Chem. Rev. 111:100–116 [DOI] [PubMed] [Google Scholar]
- 65. Tran LS, Nagai T, Itoh Y. 2000. Divergent structure of the ComQXPA quorum-sensing components: molecular basis of strain-specific communication mechanism in Bacillus subtilis. Mol. Microbiol. 37:1159–1171 [DOI] [PubMed] [Google Scholar]
- 66. Tsai CS, Winans SC. 2010. LuxR-type quorum-sensing regulators that are detached from common scents. Mol. Microbiol. 77:1072–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tsai CS, Winans SC. 2011. The quorum-hindered transcription factor YenR of Yersinia enterocolitica inhibits pheromone production and promotes motility via a small non-coding RNA. Mol. Microbiol. 80:556–571 [DOI] [PubMed] [Google Scholar]
- 68. Twomey KB, et al. 1 December 2011, posting date Bacterial cis-2-unsaturated fatty acids found in the cystic fibrosis airway modulate virulence and persistence of Pseudomonas aeruginosa. ISME J. doi:10.1038/ismej.2011.167. [DOI] [PMC free article] [PubMed]
- 69. Velicer GJ, Kroos L, Lenski RE. 2000. Developmental cheating in the social bacterium Myxococcus xanthus. Nature 404:598–601 [DOI] [PubMed] [Google Scholar]
- 70. Velicer GJ, Vos M. 2009. Sociobiology of the myxobacteria. Annu. Rev. Microbiol. 63:599–623 [DOI] [PubMed] [Google Scholar]
- 71. Wang LH, et al. 2004. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol. Microbiol. 51:903–912 [DOI] [PubMed] [Google Scholar]
- 72. Wei Y, Perez LJ, Ng WL, Semmelhack MF, Bassler BL. 2011. Mechanism of Vibrio cholerae autoinducer-1 biosynthesis. ACS Chem. Biol. 6:356–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. West SA, Griffin AS, Gardner A, Diggle SP. 2006. Social evolution theory for microorganisms. Nat. Rev. Microbiol. 4:597–607 [DOI] [PubMed] [Google Scholar]
- 74. Wilder CN, Diggle SP, Schuster M. 2011. Cooperation and cheating in Pseudomonas aeruginosa: the roles of the las, rhl and pqs quorum-sensing systems. ISME J. 5:1332–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Williams JW, Cui X, Levchenko A, Stevens AM. 2008. Robust and sensitive control of a quorum-sensing circuit by two interlocked feedback loops. Mol. Syst. Biol. 4:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Williamson NR, Fineran PC, Leeper FJ, Salmond GP. 2006. The biosynthesis and regulation of bacterial prodiginines. Nat. Rev. Microbiol. 4:887–899 [DOI] [PubMed] [Google Scholar]
- 77. Wilson AC, et al. 2010. Genomic insight into the amino acid relations of the pea aphid, Acyrthosiphon pisum, with its symbiotic bacterium Buchnera aphidicola. Insect Mol. Biol. 19(Suppl 2):249–258 [DOI] [PubMed] [Google Scholar]
- 78. Yates EA, et al. 2002. N-acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect. Immun. 70:5635–5646 [DOI] [PMC free article] [PubMed] [Google Scholar]