Abstract
Bacillus subtilis spores that germinated poorly with saturating levels of nutrient germinants, termed superdormant spores, were separated from the great majority of dormant spore populations that germinated more rapidly. These purified superdormant spores (1.5 to 3% of spore populations) germinated extremely poorly with the germinants used to isolate them but better with germinants targeting germinant receptors not activated in superdormant spore isolation although not as well as the initial dormant spores. The level of β-galactosidase from a gerA-lacZ fusion in superdormant spores isolated by germination via the GerA germinant receptor was identical to that in the initial dormant spores. Levels of the germination proteins GerD and SpoVAD were also identical in dormant and superdormant spores. However, levels of subunits of a germinant receptor or germinant receptors activated in superdormant spore isolation were 6- to 10-fold lower than those in dormant spores, while levels of subunits of germinant receptors not activated in superdormant spore isolation were only ≤2-fold lower. These results indicate that (i) levels of β-galactosidase from lacZ fusions to operons encoding germinant receptors may not be an accurate reflection of actual germinant receptor levels in spores and (ii) a low level of a specific germinant receptor or germinant receptors is a major cause of spore superdormancy.
INTRODUCTION
Spores of Bacillus and Clostridium species are formed in sporulation and are metabolically dormant and resistant to a variety of environmental factors, including heat and toxic chemicals (28). These spores can remain dormant for long periods but can return to life in the process of spore germination followed by outgrowth (10, 27, 28). Spore germination is generally triggered by the addition of specific nutrients called germinants that are recognized by specific germinant receptors (GRs) located in the spore's inner membrane. In addition to having intrinsic interest, there is also applied interest in spore germination, as spores of some Bacillus and Clostridium species cause food spoilage, food-borne disease, and several other diseases (28). When spores germinate, they lose their resistance and hence can be killed easily, for example, by a relatively mild heat treatment. Consequently, the triggering of germination in some fashion could facilitate spore inactivation. This might be particularly advantageous for the food industry, in that a food could be rendered free of spores without significantly compromising the food's texture and taste, since germinated spores can be killed at relatively low temperatures (28).
Unfortunately, the simple strategy described above cannot generally be applied, since the germination of populations of spores of both Bacillus and Clostridium species is quite heterogeneous (7, 8, 10, 13, 14, 28, 30, 36, 37, 40, 41). Thus, while the great majority of a spore population may germinate within 30 to 60 min after the addition of nutrient germinants, others may not germinate for many hours or even days. These slow-germinating spores are often referred to as superdormant (SD) spores, and they also have higher levels of wet heat resistance and require higher temperatures for heat activation prior to germination than dormant spores (9). This phenomenon of spore superdormancy appears to be a classic example of “bet hedging” in bacteria, as it will protect a spore population against extinction because of an entire population's decision to germinate rapidly, if conditions then rapidly change and become deleterious to the germinated spores and growing cells (29, 32). SD spores are of major concern not only for the food industry but also in making decisions as to how long a person exposed to Bacillus anthracis spores should be given antibiotics, since some of these spores can be SD and potentially come back to life and cause disease long after antibiotic use is discontinued (2, 12).
Clearly, a major question about spore superdormancy is what causes SD spores to germinate so much slower than the great majority of spore populations. One simple explanation is that SD spores have much lower GR levels than the majority of individuals in spore populations. This simple hypothesis is consistent with a number of observations, including (i) the relatively low average numbers of GRs per spore, making GR levels in individual spores particularly subject to stochastic variations (22, 27; K.-A. V. Stewart and P. Setlow, unpublished data); (ii) the finding that GR numbers in individual spores in populations vary over a rather wide range (11); (iii) the marked decrease in yields of SD spores from spore populations in which GRs have been overexpressed (7); (iv) the significant increases in rates of spore germination with germinants that target specific GRs when these GRs are overexpressed (3); (v) the essentially complete absence of nutrient germination in spores that lack GRs (21); (vi) the lower rates of germination with various GRs when spores contain lower levels of these GRs (24); and (vii) the normal rates of germination of SD spores of Bacillus species with germinants such as dodecylamine or the 1:1 chelation of Ca2+ and dipicolinic acid (DPA), which trigger germination without GR involvement (7, 8). Based on these observations, it has been suggested that SD spores have low levels of specific GRs compared to levels in the initial dormant spore population.
Recently, methods have been developed to isolate significant levels of SD spores of several Bacillus species (7, 8) and to determine the levels of a number of germination proteins, including GR subunits, in Bacillus subtilis spores (15, 25, 34). We have therefore used these methods to determine the levels of various germination proteins, including GR subunits, in dormant and SD B. subtilis spore populations, with SD spores being prepared with a variety of nutrient germinants. The results of these analyses indicate that at least one cause of spore superdormancy is low levels of specific GRs.
MATERIALS AND METHODS
B. subtilis strains used and spore preparation.
The strains used in this work are isogenic derivatives of strain PS832, a laboratory derivative of strain 168, and are (i) PS533 (wild-type) (26), which carries plasmid pUB110, encoding resistance to kanamycin (10 μg/ml); (ii) FB10 (gerBB*) (20), carrying a specific mutation in the gerBB cistron of the gerB operon encoding the GerB GR such that the GerB variant, termed GerB*, triggers spore germination in response to l-asparagine alone; and (iii) PS767 (gerA-lacZ) (6), obtained by transforming strain PS832 to resistance to erythromycin (1 μg/ml) and lincomycin (25 μg/ml) (macrolide-lincosamide-streptogramin resistance [MLSr]) with plasmid pAAM81 (43), giving a strain with a transcriptional fusion of the gerA promoter to a promoterless lacZ gene inserted upstream of the intact gerA operon encoding the GerA GR (the spores of this strain germinate normally with l-alanine or l-valine). Spores of these strains were prepared at 37°C on 2× Schaeffer's glucose (SG) medium agar plates without antibiotics and were harvested and purified as described previously (18, 19). All spores used in this work were >98% free of sporulating cells, germinated spores, or cell debris, as determined by phase-contrast microscopic examination.
Preparation of SD spores.
SD spores were isolated following germination with saturating concentrations of (i) l-valine (10 mM) in 25 mM Tris-HCl buffer (pH 7.4) for PS533 or PS767 spores, (ii) AGFK (12 mM l-asparagine–13 mM d-glucose–13 mM D-fructose–12.5 mM KPO4 buffer [pH 7.4]) for PS533 spores, or (iii) l-asparagine (6 mM) in 25 mM KPO4 buffer (pH 7.4) for FB10 spores, as follows. Dormant spores at an optical density at 600 nm (OD600) of ∼10 in water were heat activated at 75°C for 30 min and cooled on ice for at least 15 min. Shortly afterwards, the spores were germinated at 37°C to an OD600 of ∼1 for 5 h with one of the germinants described above. Spores that had not germinated in 5 h were then isolated essentially as described previously (7–9), taking advantage of the fact that dormant spores have a higher core wet density than germinated spores, primarily because of the large CaDPA depot in the dormant spore core, which is released and replaced by water early during spore germination. Hence, spores that remained dormant after 5 h were isolated by buoyant density gradient centrifugation in a solution of 50% Nycodenz. These potential SD spores were again germinated for 5 h as described above, and spores that still had not germinated were again isolated by buoyant density gradient centrifugation, washed with water, and stored at −80°C. Routinely, ∼1 g (dry weight) of initial dormant spores was germinated for each SD preparation, and yields of final SD spores were ∼1.5 to 3% of the starting dormant spores, as generally found previously (7, 8).
Measurement of spore germination.
Heat-activated spores were germinated, as described above, in 1 ml, and germination was routinely monitored by measuring the drop in the OD600 of the spore suspension caused by the release of CaDPA and its replacement by water as well as the hydrolysis of the spores' peptidoglycan cortex, followed by core swelling and further water uptake (7, 8, 27). The level of spore germination in these experiments was also assessed by phase-contrast microscopy, as dormant spores appear phase bright, while germinated spores appear phase dark. Routinely, ∼100 spores were examined per field, and 3 fields were examined for each experiment.
β-Galactosidase assay.
Extracts for assays of β-galactosidase from the gerA-lacZ fusion were prepared from dormant and SD PS767 (gerA-lacZ) spores and from dormant PS533 (wild-type) spores by decoating, lysing, and sonicating the lysate briefly to reduce its viscosity and, finally, by centrifugation in a microcentrifuge as described previously (6, 25). β-Galactosidase in the resultant supernatant fluid was assayed at 37°C by using methyl-umbelliferyl-β-d-galactoside (MUG) as the substrate and measuring MUG hydrolysis fluorometrically by the production of methyl-umbelliferone as described previously (6, 25). The β-galactosidase activities in extracts of dormant and SD PS533 (wild-type) spores that lacked a lacZ fusion were <15% of that in the PS767 spores (see Results). All β-galactosidase spore activities reported in this work are averages of data from at least duplicate assays on extracts from at least two dormant and SD spore preparations. The β-galactosidase specific activities were calculated relative to the spores' dry weights determined from the OD600 of the purified spores, as 1 mg dry B. subtilis spores suspended in 1 ml water has an OD600 of 8.0.
Determination of levels of GR subunits, GerD, and SpoVAD in dormant and SD spores.
It was shown previously that a number of different germination proteins, including GR subunits, GerD, and SpoVAD, are located in the spore's inner membrane (22, 24, 27, 34). Consequently, levels of these proteins were determined by Western blot analysis of equal amounts of the inner membrane protein from dormant and SD spores. The preparation of rabbit antisera against the different GR subunits and the GerD and SpoVAD proteins and the sources of secondary antisera were described previously (15, 25, 34). Chemiluminescence due to the activity of horseradish peroxidase coupled to goat anti-rabbit IgG was used to detect the binding of the primary rabbit antisera to proteins on Western blot membranes, and X-ray film was used to detect chemiluminescence. In most cases, the same blot was stripped and then reprobed with a different antiserum, as described previously (25).
The isolation of the spore inner membrane is a multistep process involving several centrifugations and washings, as described previously (22, 24, 25, 34), and the recoveries of the inner membrane from different spore preparations can vary. Hence, a series of serial 2-fold dilutions of inner membrane fractions from dormant and SD spores were first run on denaturing polyacrylamide gels and stained with Coomassie blue. A subsequent visual examination of the stained gels allowed the determination of the amounts of inner membrane fractions that needed to be used to allow a comparison of equal amounts of dormant and SD spore inner membrane proteins for Western blot analysis. In order to precisely quantify differences in levels of germination proteins in dormant and SD spores, the Western blot intensities of bands given by serial 2-fold dilutions of the SD spore inner membrane protein were visually compared to the intensities of bands given by the same amounts of the dormant spore inner membrane protein on the same Western blot membrane, with visual estimates of superdormant spore protein levels that fell between the various dilutions of the dormant spore protein. All Western blot analyses of germination protein levels in spores were performed in duplicate on inner membrane fractions from two independent spore preparations, with essentially identical results (<±15%), such that four different values for each germination protein were obtained and averaged. Differences in GR subunit levels between dormant and SD spores were analyzed for their significance by a two-tailed Student t test. While levels of germination proteins were initially determined by a visual inspection of Western blots as described above, the intensities of various bands were also analyzed densitometrically using ImageJ, with essentially identical results.
RESULTS
Nutrient germination of dormant and SD spores.
B. subtilis spores contain three major GRs, GerA, GerB, and GerK (27, 28). l-Valine or l-alanine triggers spore germination via the GerA GR, while the GerB and GerK GRs are both required for spore germination with the AGFK mixture. As expected, the triggering of the GerA GR alone or both the GerB and GerK GRs gave a >90% germination of dormant spores in ≤2 h (Table 1). B. subtilis SD spores can also be prepared by using either l-valine or AGFK as the initial germinant (7) (Table 1). As expected (7–9, 40), the SD spores prepared by initial germination with either l-valine or AGFK germinated extremely poorly with the germinants used to isolate these SD spores. In contrast, SD spores prepared by an initial germination with l-valine germinated significantly better with AGFK, while SD spores prepared by an initial germination with AGFK germinated significantly better with l-valine. However, with all nutrient germinants tested, the germination of the SD spores was neither as efficient nor as fast as that of the initial dormant spores, as described previously (7–9) (Table 1 and data not shown).
Table 1.
Germination of dormant and SD spores isolated using different nutrient germinantsa
| Germinant | Spore germination in 2 h (%) |
|||
|---|---|---|---|---|
| Dormant | Superdormant, with nutrient used for SD spore isolation |
|||
| l-Valineb | AGFKb | l-Asparaginec | ||
| l-Valine | 95 | 3 | 25 | 18 |
| AGFK | 93 | 22 | 7 | NT |
| l-Asparagine | 95 | NT | NT | 5 |
Dormant and SD PS533 (wild-type) and FB10 (gerBB*) spores were isolated as described in Materials and Methods, with SD spores being prepared by germination with l-valine or AGFK (PS533 spores) or l-asparagine (FB10 spores). The dormant and SD spores were germinated with l-valine, AGFK, or l-asparagine, and the percentage of spores that germinated in 2 h was determined by phase-contrast microscopy as described in Materials and Methods. NT, not tested.
PS533 spores.
FB10 spores.
Levels of GerA-LacZ expression in dormant and SD spores prepared by germination with l-valine.
While there could be a number of reasons why spores are SD for germination with a particular nutrient germinant, an obvious one is that the SD spores lack or have very low levels of the appropriate GR. One possible reason for the low levels of GRs in some individual spores in populations could be altered levels of transcription factors involved in GR operon transcription, such as the SpoVT protein or the forespore-specific sigma factor for RNA polymerase, σG (1, 27, 38). To test this possibility, we measured the levels of β-galactosidase in dormant spores of a strain carrying a gerA-lacZ transcriptional fusion as well as in SD spores of this strain isolated by germination with l-valine, which triggers germination via the GerA GR (28). Strikingly, the β-galactosidase specific activities obtained from the dormant and SD spores were essentially identical (Table 2).
Table 2.
Levels of β-galactosidase from gerA-lacZ in dormant and SD sporesa
| Spore type analyzed | Avg β-galactosidase sp act (RFU/mg dry spores) ± SDb |
|---|---|
| PS533 (wild type) | 266 ± 50 |
| PS767 (gerA-lacZ) (dormant) | 2,189 ± 160 |
| PS767 (gerA-lacZ) (SD) | 2,224 ± 150 |
PS533 and PS767 spores were prepared and purified and SD spores were prepared by germination with l-valine as described in Materials Methods. Purified spores were decoated and lysed, extracts were assayed for β-galactosidase, and specific activities were calculated as described in Materials and Methods.
β-Galactosidase specific activities were determined in duplicate for two independent dormant and SD spore preparations, and values are given as relative fluorescence units (RFU). Values shown are the averages ± variations between the values for the independent spore preparations.
Levels of germination proteins, including GR subunits, in dormant and SD spores.
The similar levels of gerA-lacZ-generated β-galactosidase in both dormant and SD spores indicated that levels of gerA-lacZ transcription and, perhaps, the intact gerA operon's transcription in forespores that became SD spores were similar to those in the whole forespore population. However, there are several reasons why actual GR levels might differ in dormant and SD spores despite similar levels of gerA-lacZ transcription, including the regulation of gerA expression at the translational level as well as stochastic variations in the levels of gerA-lacZ and gerA operon transcription and the translation of the resultant mRNAs. Consequently, levels of β-galactosidase from gerA-lacZ may not be an accurate reflection of the actual levels of the GerA GR and even more likely may not reflect the levels of the GerB and GerK GRs as well as other germination proteins in these SD spores.
Given the concerns raised above, we turned to the use of Western blot analyses with a variety of specific antisera to directly determine the levels of various germination proteins in dormant and SD spores. The antisera that we used were prepared against the A and C subunits of the B. subtilis GerA GR (GerAA and GerAC), the C subunit of the GerB GR (GerBC), and the A subunit of the GerK GR (GerKA); each of these GR subunits is essential for their GR's function (27). These antisera are specific for their respective antigens, as they show no detectable cross-reaction with the analogous subunits of other GRs, and the various antigens detected migrate at the expected positions for their predicted molecular weights (15, 25; data not shown). We also used an antiserum raised against the Geobacillus stearothermophilus GerD protein, which was shown previously to react specifically with B. subtilis GerD (25). The GerD protein is essential for rapid spore germination via any of the GRs (23). The final antiserum was used against the B. subtilis SpoVAD protein (34). This protein is encoded by one of the seven cistrons in the spoVA operon that is transcribed in the developing forespore just prior to the developing spore's accumulation of DPA. There is much evidence that at least six of the SpoVA proteins, including SpoVAD, are essential for DPA uptake into the developing forespore, and there is some evidence that these proteins are essential for DPA release during spore germination (5, 16, 27, 33, 35).
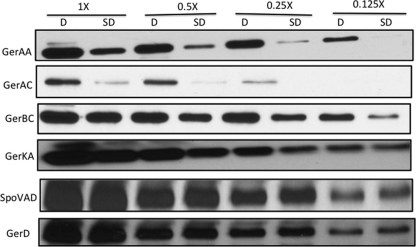
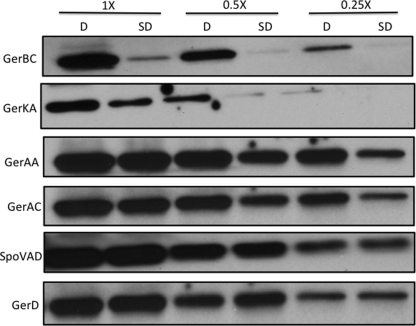
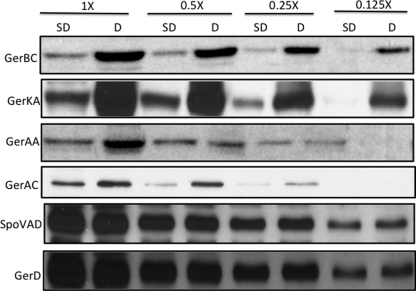
All the germination proteins described above to which we had antisera have been reported to be largely if not completely present in the spore's inner membrane (22, 24, 25, 27, 34). Consequently, we compared the levels of the GR subunits, GerD, and SpoVAD in the inner membrane fractions of dormant and SD spores, with the SD spores being prepared with several different germinants (Fig. 1 to 3 and Table 3). Strikingly, levels of the GR or GRs that were activated in order to isolate the SD spores were 6- to >10-fold lower than those in the initial dormant spores, even if both the GerB and GerK GRs needed to be activated for SD spore isolation. It was also notable that the levels of the GerAA and GerAC GerA GR subunits in SD PS767 (gerA-lacZ) spores isolated by l-valine germination via the GerA GR were 6- to 8-fold lower than those in the dormant spores, since the level of GerA-LacZ expression in these SD spores was identical to that in the starting PS767 dormant spores. In contrast to the much lower SD spore levels of subunits of GRs activated for SD spore isolation, levels of GRs not activated for SD spore isolation were generally ∼2-fold lower than those in the dormant spores, and even these smaller differences in GR subunit levels were highly significant (Table 3). This smaller difference in GR subunit levels in SD spores was particularly notable when SD FB10 spores were isolated by initial germination with l-asparagine alone via the GerB* GR, since in these SD spores, the GerKA level was only ∼2-fold lower than that in dormant spores and, thus, ∼4-fold higher than that in SD PS533 spores isolated by germination with AGFK, which requires both the GerB and GerK GRs.
Fig 1.
Levels of germination proteins in dormant (D) and SD spores isolated using l-valine germination. Dormant PS533 (wild-type) spores were prepared and purified, SD spores were isolated by l-valine germination via the GerA GR, and inner membranes were isolated as described in Materials and Methods. Aliquots of equal amounts of inner membrane proteins from the dormant and SD spores were then used for Western blot analysis using different antisera, as described in Materials and Methods. Values above the lanes refer to the amounts of inner membrane protein run in the lanes, with 1× being the protein from approximately 1 mg (dry weight) of spores. In this experiment, the same blot was stripped and reprobed for GerAA, GerAC, GerBC, GerKA, and SpoVAD, while the GerD strip was from a separate blot.
Fig 3.
Levels of germination proteins in dormant and SD spores isolated using l-asparagine germination. Dormant FB10 (gerBB*) spores were prepared and purified, SD spores were isolated by l-asparagine germination via the GerB* GR, and inner membranes were isolated as described in Materials and Methods. Aliquots of equal amounts of inner membrane proteins were then used for Western blot analysis using different antisera, as described in Materials and Methods. Values above the lanes refer to the amounts of inner membrane protein run in the lane, with 1× being the protein from approximately 1 mg (dry weight) of spores. In this experiment, the same blot was stripped and reprobed for GerAA, GerAC, GerBC, GerKA, SpoVAD, and GerD.
Table 3.
Relative levels of germination proteins in dormant and SD sporesa
| Germination protein | Level in SD spores/level in dormant spores with GRs triggered to prepare SD sporesb |
||
|---|---|---|---|
| GerAc | GerB + GerK | GerB* | |
| GerAA | 0.13d (0.16) | 0.5e (0.5) | 0.5e (0.47) |
| GerAC | 0.17d (0.18) | 0.5e (0.64) | 0.4e (0.33) |
| GerBC | 0.5d (0.63) | <0.1d,f (0.08) | <0.17d,f (0.12) |
| GerKA | 0.6g (0.6) | 0.13d (0.11) | 0.5g (0.4) |
| SpoVAD | 1.0 (1.1) | 1.0 (0.99) | 1.0 (0.96) |
| GerD | 1.0 (1.0) | 1.0 (0.8) | 1.0 (0.92) |
Dormant and SD spores of strains PS533 (wild-type) (l-valine germination via the GerA GR and AGFK germination via the GerB and GerK GRs) and FB10 (gerBB*) (l-asparagine germination via the GerB* GR) were prepared, inner membrane fractions were isolated, and levels of germination proteins were determined as described in Materials and Methods and as shown in Fig. 1 to 3. Values shown are averages of results from two independent spore preparations, and these differed by ≤±15%.
Values were determined by visual inspection or by densitometric analysis using Image J (values in parentheses).
Essentially identical results were obtained with PS767 dormant and SD spores, with the SD spores being prepared by germination with l-valine via the GerA GR (data not shown).
P < 0.001.
P < 0.005.
These numbers were estimated by visual inspection and extrapolation from the intensity of the band given by the lowest level of dormant spore inner membrane protein analyzed in parallel.
P < 0.1.
Fig 2.
Levels of germination proteins in dormant and SD spores isolated using AGFK germination. Dormant PS533 (wild-type) spores were prepared and purified, SD spores were isolated by AGFK germination via the GerB and GerK GRs, and inner membranes were isolated as described in Materials and Methods. Aliquots of equal amounts of inner membrane proteins from the dormant and SD spores were then used for Western blot analysis using different antisera, as described in Materials and Methods. Values above the lanes refer to the amounts of inner membrane protein run in the lanes, with 1× being the protein from approximately 1 mg (dry weight) of spores. In this experiment, the same blot was stripped and reprobed for GerAA, GerAC, GerBC, GerKA, and SpoVAD, while the GerD strip was from a separate blot.
In sharp contrast to the lower levels of GR subunits in SD spores, in particular subunits of GRs activated for SD spore isolation, the levels of the GerD and SpoVAD proteins were identical in dormant and SD spores (Fig. 1 to 3 and Table 3). This was the case not only for PS533 (wild-type) spores but also for FB10 (gerBB*) spores and was the case regardless of the germinant used for SD spore isolation.
DISCUSSION
The results of the current work show clearly that SD B. subtilis spores have quite low levels of the GR or GRs activated in SD spore isolation. It is known that (i) an elevation of GR levels results in increased germination via that GR (3), (ii) the overexpression of one GR results in lower yields of SD spores when germination via the overexpressed GR is used to isolate the SD spores (7), (iii) decreases in GR levels are associated with poorer germination via that GR or GRs (21, 25), and (iv) the absence of a GR or GRs results in large increases in levels of SD spores when germination via the absent GR or GRs is used to isolate the SD spores (21). Consequently, the most significant conclusion from the current work is that a major cause of B. subtilis spore superdormancy is almost certainly extremely low levels of specific GRs. In addition, since SD spores of several other Bacillus species exhibit properties very similar to those of SD B. subtilis spores (7–9), it seems most likely that especially low levels of specific GRs are also a major cause of superdormancy in spores of all Bacillus species. This could also be the case for spores of those Clostridium species in which GRs trigger spore germination. The situation in SD B. subtilis spores, which have especially low levels of one or two specific GRs, is markedly different from the situation identified recently by comparing B. subtilis spores prepared in rich sporulation medium to those prepared in poor sporulation medium (25). Spores prepared in the poor medium germinated more slowly with all nutrient germinants and had 2- to 3-fold-lower levels of all GRs as well as GerD, although spores in both rich and poor media had identical SpoVAD levels (25). However, the mechanisms causing the lower levels of GerD and all GRs in spores prepared in poor medium are not known.
While particularly low levels of specific GRs appear likely to cause a significant amount of spore superdormancy, the reasons behind a number of other observations about SD B. subtilis spores made in the current work are unclear. Thus, the level of β-galactosidase from a gerA-lacZ fusion in SD spores prepared by germination via the GerA GR was identical to the level in the initial dormant spore population, while levels of the GerAA and GerAC proteins were ∼7-fold lower in the SD spores. This certainly indicates the potential danger in interpreting the β-galactosidase level from a lacZ gene fusion as a direct reflection of the level of the protein product of the gene to which lacZ is fused. However, why there is such a discrepancy between GerA-LacZ and GerA GR subunit levels is not clear. Possible explanations include the regulation of gerA mRNA translation or stochastic variation in the transcription of gerA-lacZ and the intact gerA operon or the translation of gerA-lacZ and gerA operon mRNA in individual developing forespores. Indeed, stochastic variation could be extremely important in the heterogeneity of the levels of the GerA GR, which is most likely expressed at levels of only tens of molecules per spore (4, 17, 22, 27, 31, 32; Stewart and Setlow, unpublished). Clearly, further work will be required to decide between these different possibilities.
Another unexplained result is that levels of GRs not selected against in SD spore isolation were lower in SD spores albeit only ∼2-fold lower. However, the lower level of the GRs not selected against in SD spore isolation was certainly consistent with the poorer germination of SD spores with germinants recognized by GRs not activated in SD spore isolation. It was somewhat surprising that a ∼2-fold decrease in average GR levels resulted in such a large decrease in spore germination efficiency. However, there is some evidence that GRs can act cooperatively (11, 39), so perhaps a linear relationship between GR levels and rates of spore germination is not to be expected. It is also notable that different SD spore isolates have been found to give rather different quantitative results when germination via GRs not selected against in SD spore isolation is measured (7, 41; this work). These results have varied from large decreases in germination via unselected GRs to only small decreases, even though all these SD spores germinated extremely poorly via the GRs selected against in SD spore isolation. The reason for the significant variation in the germination of SD spores via GRs not selected against in SD spore isolation is not clear. However, it appears most likely that dormant spore populations are extremely heterogeneous mixtures of individuals with widely different levels of various GRs (11), such that slight alterations in dormant spore preparations or the precise conditions for the selection of SD spores may alter the properties of the spores selected as SD. It is also possible that there is some cotranscriptional regulation of operons encoding GRs, for example, by the SpoVT protein. This protein represses the transcription of the gerA, gerB, and gerK operons but has no effect on gerD transcription and stimulates spoVA operon transcription only ∼1.5-fold (1, 38). Thus, variations in SpoVT levels in individual developing forespores could cause a concerted ∼2-fold decrease in levels of all GRs, with stochastic variability then allowing selection for much lower levels of the specific GR or GRs selected against in SD spore isolation. However, the identical expression levels of GerA-LacZ in dormant spores and SD spores prepared by germination via the GerA GR appear to make a general concerted transcriptional regulation of all GR-encoding operons unlikely to be of primary importance in causing spore superdormancy.
Work with spores of several Bacillus species as well as Clostridium perfringens spores has shown that the major variable in the kinetics of spore germination with nutrients is the time between the mixing of the spores and germinant and the initiation of a rapid release of CaDPA, termed Tlag (14, 25, 36, 37, 40, 41). Values of Tlag can vary between a few minutes to many hours for individuals in the same spore population. Not surprisingly, increased average GR levels decrease the Tlag, and lower average GR levels are associated with a longer Tlag, with SD B. subtilis spores having Tlags of hours (25, 40). The latter effect of GR levels on the Tlag is certainly consistent with the low levels of GRs activated by the germinant used for SD spore isolation being responsible for these SD spores' extremely slow germination with this germinant and the extremely long Tlag for such a germination (41). However, there appear to be variables, in addition to GR levels, that play roles in germination heterogeneity and, thus, presumably spore superdormancy. Thus, the germination of spores by molecules such as dodecylamine and CaDPA, both of which trigger germination independent of GRs, is also extremely heterogeneous, with highly variable Tlags for individual spores (14, 37, 41). However, the reason for this heterogeneity in GR-independent spore germination is not known. Similarly, it was shown recently that the average structure of the spores' peptidoglycan cortex can also have significant effects on Tlag in both GR-dependent and GR-independent spore germination (42), although again, the reason for this effect is not known. Consequently, while it is most likely that low GR levels are a major factor in causing the heterogeneity and, thus, superdormancy in nutrient germination, it appears likely that other factors such as the precise cortex structure and undoubtedly other unknown factors play significant roles as well.
ACKNOWLEDGMENTS
This work was supported by a Department of Defense Multi-Disciplinary University Research Initiative through the U.S. Army Research Laboratory and the U.S. Army Research Office under contract number W911NF-09-1-0286.
We are grateful to George Korza and Kerry-Ann V. Stewart for advice on Western blot analysis of spore germination proteins.
Footnotes
Published ahead of print 17 February 2012
REFERENCES
- 1. Bagyan I, Hobot J, Cutting SM. 1996. A compartmentalized regulator of developmental gene expression in Bacillus subtilis. J. Bacteriol. 178:4500–4507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brookmeyer R, Johnson E, Bollinger R. 2003. Modeling the optimum duration of antibiotic prophylaxis in an anthrax outbreak. Proc. Natl. Acad. Sci. U. S. A. 100:10129–10132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cabrera-Martinez RM, Tovar-Rojo F, Vepachedu VR, Setlow P. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J. Bacteriol. 95:167–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Elowitz MB, Levine AJ, Siggia ED, Swain PS. 2002. Stochastic gene expression in a single cell. Science 297:1183–1186 [DOI] [PubMed] [Google Scholar]
- 5. Errington J. 1993. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol. Rev. 57:1–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feavers IM, et al. 1990. The regulation of transcription of the gerA spore germination operon of Bacillus subtilis. Mol. Microbiol. 4:275–282 [DOI] [PubMed] [Google Scholar]
- 7. Ghosh S, Setlow P. 2009. Isolation and characterization of superdormant spores of Bacillus species. J. Bacteriol. 191:1787–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghosh S, Setlow P. 2010. The preparation, germination properties and stability of superdormant spores of Bacillus cereus. J. Appl. Microbiol. 108:582–590 [DOI] [PubMed] [Google Scholar]
- 9. Ghosh S, Zhang P, Li Y, Setlow P. 2009. Superdormant spores of Bacillus species have elevated wet heat resistance and temperature requirements for heat activation. J. Bacteriol. 191:5584–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gould GW. 1970. Germination and the problem of dormancy. J. Appl. Bacteriol. 33:34–49 [DOI] [PubMed] [Google Scholar]
- 11. Griffiths KK, Zhang J, Cowan AE, Yu J, Setlow PP. 2011. Germination proteins in the inner membrane of dormant Bacillus subtilis spores colocalize in a discrete cluster. Mol. Microbiol. 81:1061–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heine HS, et al. 2007. Determination of antibiotic efficacy against Bacillus anthracis in a mouse aerosol challenge model. Antimicrob. Agents Chemother. 51:1373–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Indest KJ, Buchholz WG, Faeder JR, Setlow P. 2009. Workshop report: modeling the molecular mechanism of bacterial spore germination and elucidating reasons for germination heterogeneity. J. Food Sci. 74:R73–R78 [DOI] [PubMed] [Google Scholar]
- 14. Kong L, Zhang P, Setlow P, Li Y. 2010. Characterization of bacterial spore germination using integrated phase contrast microscopy, Raman spectroscopy and optical tweezers. Anal. Chem. 82:3840–3847 [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Catta P, Stewart Dufner K-AM, Setlow P, Hao B. 2011. Structure-based functional studies of the effects of amino acid substitutions in GerBC, the C subunit of the Bacillus subtilis GerB spore germinant receptor. J. Bacteriol. 193:4143–4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, et al. 2012. Role of a SpoVA protein in dipicolinic acid uptake into developing spores of Bacillus subtilis. J. Bacteriol. 194:1875–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naoki H, Sakumura Y, Ishii S. 2008. Stochastic control of spontaneous signal generation for gradient sensing in chemotaxis. J. Theor. Biol. 255:259–266 [DOI] [PubMed] [Google Scholar]
- 18. Nicholson WL, Setlow P. 1990. Sporulation, germination and outgrowth, p 391–450 In Harwood CR, Cutting SM. (ed), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom [Google Scholar]
- 19. Paidhungat M, Setlow B, Driks A, Setlow P. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505–5512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paidhungat M, Setlow P. 1999. Isolation and characterization of mutations in Bacillus subtilis that allow spore germination in the novel germinant D-alanine. J. Bacteriol. 181:3341–3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paidhungat M, Setlow P. 2000. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 182:2513–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paidhungat M, Setlow P. 2001. Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J. Bacteriol. 183:3982–3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pelczar PL, Igarashi T, Setlow B, Setlow P. 2007. The role of GerD in the germination of Bacillus subtilis spores. J. Bacteriol. 189:1090–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pelczar PL, Setlow P. 2008. Localization of the germination protein GerD to the inner membrane in Bacillus subtilis spores. J. Bacteriol. 190:5635–5641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramirez-Peralta A, Zhang P, Li Y, Setlow P. 2012. Effects of sporulation conditions on the germination and germination protein levels of Bacillus subtilis spores. Appl. Environ. Microbiol. 78:2689–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Setlow P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550–556 [DOI] [PubMed] [Google Scholar]
- 28. Setlow P, Johnson EA. 2012. Spores and their significance. In Doyle MP, Buchanan R. (ed), Food microbiology: fundamentals and frontiers, 4th ed, in press ASM Press, Washington, DC [Google Scholar]
- 29. Setlow P, Liu J, Faeder JR. 2012. Heterogeneity in bacterial spore populations, p 201–216 In Abel-Santos E. (ed), Bacterial spores: current research and applications. Horizon Scientific Press, Norwich, United Kingdom [Google Scholar]
- 30. Stringer SC, Webb MD, George SM, Pin C, Peck MW. 2005. Heterogeneity of times required for germination and outgrowth from single spores of Clostridium botulinum. Appl. Environ. Microbiol. 71:4998–5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taniguchi Y, et al. 2010. Quantifying E. coli proteome and transcriptome with single molecule sensitivity. Science 329:533–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Veening J-W, Klaas Smits W, Kuipers OP. 2008. Bistability, epigenetics, and bet-hedging in bacteria. Annu. Rev. Microbiol. 62:193–210 [DOI] [PubMed] [Google Scholar]
- 33. Vepachedu VR, Setlow P. 2004. Analysis of the germination of spores of Bacillus subtilis with temperature sensitive spo mutations in the spoVA operon. FEMS Microbiol. Lett. 239:71–77 [DOI] [PubMed] [Google Scholar]
- 34. Vepachedu VR, Setlow P. 2005. Localization of SpoVAD to the inner membrane of spores of Bacillus subtilis. J. Bacteriol. 187:5677–5682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vepachedu VR, Setlow P. 2007. Role of SpoVA proteins in the release of dipicolinic acid during germination of Bacillus subtilis spores triggered by dodecylamine or lysozyme. J. Bacteriol. 189:1565–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang G, Yi X, Li Y, Setlow P. 2011. Germination of individual Bacillus subtilis spores with alterations in the GerD and SpoVA proteins. J. Bacteriol. 193:2301–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang G, et al. 2011. Analysis of the germination of individual Clostridium perfringens spores and its heterogeneity. J. Appl. Microbiol. 111:1212–1223 [DOI] [PubMed] [Google Scholar]
- 38. Wang ST, et al. 2006. The forespore line of gene expression in Bacillus subtilis. J. Mol. Biol. 358:16–37 [DOI] [PubMed] [Google Scholar]
- 39. Yi X, Liu J, Faeder JR, Setlow P. 2011. Synergism between different germinant receptors in the germination of Bacillus subtilis spores. J. Bacteriol. 193:4664–4671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang P, et al. 2010. Factors affecting variability in time between addition of nutrient germinants and rapid dipicolinic acid release during germination of spores of Bacillus species. J. Bacteriol. 192:3608–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang P, et al. 2012. Analysis of the slow germination of multiple individual superdormant Bacillus subtilis spores using multifocus Raman microspectroscopy and DIC microscopy. J. Appl. Microbiol. 112:526–536 doi:10.1111/j.1365-2672.2011.05230.x [DOI] [PubMed] [Google Scholar]
- 42. Zhang P, Thomas S, Li Y, Setlow P. 2012. Effects of cortex peptidoglycan structure and cortex hydrolysis on the kinetics of Ca2+-dipicolinic acid release during Bacillus subtilis spore germination. J. Bacteriol. 194:646–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zuberi AR, Moir A, Feavers IM. 1987. The nucleotide sequence and gene organization of the gerA spore germination operon of Bacillus subtilis 168. Gene 51:1–11 [DOI] [PubMed] [Google Scholar]