Abstract
Vancomycin-resistant enterococci (VRE) are one of the leading causes of nosocomial infections in health care facilities around the globe. In particular, infections caused by vancomycin-resistant Enterococcus faecium are becoming increasingly common. Comparative and functional genomic studies of E. faecium isolates have so far been limited owing to the lack of a fully assembled E. faecium genome sequence. Here we address this issue and report the complete 3.0-Mb genome sequence of the multilocus sequence type 17 vancomycin-resistant Enterococcus faecium strain Aus0004, isolated from the bloodstream of a patient in Melbourne, Australia, in 1998. The genome comprises a 2.9-Mb circular chromosome and three circular plasmids. The chromosome harbors putative E. faecium virulence factors such as enterococcal surface protein, hemolysin, and collagen-binding adhesin. Aus0004 has a very large accessory genome (38%) that includes three prophage and two genomic islands absent among 22 other E. faecium genomes. One of the prophage was present as inverted 50-kb repeats that appear to have facilitated a 683-kb chromosomal inversion across the replication terminus, resulting in a striking replichore imbalance. Other distinctive features include 76 insertion sequence elements and a single chromosomal copy of Tn1549 containing the vanB vancomycin resistance element. A complete E. faecium genome will be a useful resource to assist our understanding of this emerging nosocomial pathogen.
INTRODUCTION
Enterococcus faecium is a commensal bacterium of the human gastrointestinal tract and an important nosocomial pathogen, particularly through its ability to develop resistance to multiple antibiotics, including vancomycin (9, 18). Vancomycin-resistant enterococci (VRE) were first reported in hospitals in the 1980s and have since been reported in health care settings worldwide. Resistance to vancomycin is typically mediated by acquisition of the vanA or vanB gene cluster (5, 58). Rates of acquired antibiotic resistance are significantly higher in E. faecium than in other enterococcal species, and together with observations of increasing E. faecium infections, they suggest that there is a strong correlation between antimicrobial resistance acquisition and the infectious nature of the pathogen (30).
Multilocus sequence typing (MLST) is frequently employed in molecular epidemiological analyses of E. faecium strains, and analysis by eBURST suggests the emergence of a lineage, termed clonal complex 17 (CC17), that appears to represent a hospital-adapted subpopulation of E. faecium strains, as they have been associated with clinical infections and E. faecium outbreaks on five continents (54, 58). Strains belonging to CC17 are proposed to possess particular traits for enhancing persistence in the health care environment, including acquiring ampicillin and quinolone resistance, and a pathogenicity island that commonly harbors the esp gene encoding the putative virulence factor enterococcal surface protein (6, 11, 53). A recent comparative study, aligning 100 orthologs from 21 publicly available E. faecium genomes, identified 5,932 single nucleotide polymorphisms (SNPs) and was used to infer a phylogeny that showed a distinct separation between hospital- and community-acquired E. faecium (20).
VRE first emerged in the United Kingdom and Europe in the late 1980s, 15 years after glycopeptide antibiotic use was first introduced to clinics and hospitals (39). Following the first reported emergence of VRE, incidence rates have continued to increase (17). In the Australian context, VRE was first isolated in 1994 at the Austin Hospital in Victoria from a liver transplant recipient (33). This isolate was an E. faecium strain with vanA-type glycopeptide resistance. In contrast, the majority of VRE isolates in Australia today, in particular, E. faecium, possess vanB-type resistance (8, 43). Following the first Australian case in 1994, VRE was soon reported in other Australian health care facilities, with outbreaks involving VRE colonization of the hospital environment. In the past decade, the rates of VRE and vancomycin-susceptible E. faecium (VSE) colonization and, to a lesser extent, infection have increased dramatically Australia-wide (32, 33, 43). A recent molecular epidemiological analysis of E. faecium bacteremia isolates at one Australian institution demonstrated that ST17 was the predominant MLST clone prior to 2005; however, this was subsequently replaced by a new clone, ST203, which was associated with a significant increase in episodes of bacteremia (32). The bacterial and genomic factors associated with the emergence of ST203 VRE, the manner in which its genome and phenotypic behavior contrast with those of the former predominant ST17 clones, and the increase in E. faecium bacteremia overall are not understood, and this is in part hampered by the lack of any publicly available complete E. faecium genome sequence.
The first partially assembled, draft genome sequence for E. faecium strain TX0016 (also referred to as strain DO), isolated from an endocarditis patient in 1992, was published in 2000 (19). At least 23 additional E. faecium strains have since been sequenced and partially assembled. In these studies, the genome data have been used to analyze the evolutionary relationships and genomic diversity between strains isolated from different sources. Recent genome-based studies have revealed significant differences (up to 12%) between the gene contents of E. faecium strains and have highlighted the frequency and ease with which this species acquires and loses mobile genetic elements (55, 56). The genomic plasticity observed in E. faecium strains is presumed to be largely responsible for the different properties exhibited by commensal and clinical isolates (36). Thus, while there are several published draft genome sequences available for E. faecium, no one has yet produced a fully assembled reference for this species (19, 55).
Here, we have addressed this issue by completing the genome sequence of a representative vanB-positive, vancomycin-resistant E. faecium strain (Aus0004), isolated from the bloodstream of a patient at the Austin Hospital, in Melbourne, Australia, in 1998. This strain belongs to MLST group ST17, a member of clonal complex 17, which also represents the majority of E. faecium strains isolated from hospitals globally (28, 58).
MATERIALS AND METHODS
Bacterial sequences and strains.
The E. faecium isolates and genome sequences used in this study are listed in Table 1.
Table 1.
E. faecium genome sequences used in this study
| E. faecium strain | Accession no.a | Comments | Reference |
|---|---|---|---|
| C68 | NZ_ACJQ00000000.1 | Clinical isolate, ST16, CC17; closely related to ST17; represents most prevalent VRE clone in 11 Cleveland hospitals in 1996 | Broad Institute, unpublished data; strain first described in reference 12 |
| TC 6 | NZ_ACOB00000000.1 | Colonizing (mouse model) transconjugant strain resulting from the mating between C68 and D344SRF | Broad Institute, unpublished data; strain first described in reference 47 |
| D344SRF | NZ_ACZZ00000000.1 | Noncolonizing (mouse model) recipient strain | Broad Institute, unpublished data |
| E1039 | NZ_ACOS00000000.1 | Fecal isolate from community surveillance program (nonhospitalized person), ST42; isolated in the Netherlands, 1998; no antimicrobial resistance | 55 |
| E1162 | NZ_ABQJ00000000.1 | Clinical isolate, bloodstream infection, ST17, CC17; isolated in France, 1997; high resistance to ampicillin | 55 |
| E1071 | NZ_ABQI00000000.1 | Fecal isolate from hospital surveillance program, hospitalized patient; ST32, not CC17; isolated in the Netherlands, 2000; vanA VRE | 55 |
| E1636 | NZ_ABRY00000000.1 | Clinical isolate, bloodstream infection. ST106; isolated in the Netherlands, 1961; low-level resistance to ampicillin | 55 |
| E1679 | NZ_ABSC00000000.1 | Clinical isolate, from a vascular catheter; ST114; isolated in Brazil, 1998; vanA VRE. high resistance to ampicillin; resistant to ciprofloxacin | 55 |
| U0317 | NZ_ABSW00000000.1 | Clinical isolate, urinary tract infection; ST78, CC17; isolated in the Netherlands, 2005; high resistance to ampicillin; resistance to ciprofloxacin | 55 |
| DO | NZ_ACIY00000000.1 | Obtained from the University of Texas Medical School, ST18 | 19; strain first described in reference 2 |
| 1,230,933 | NZ_ACAS00000000.1 | Obtained from a clinical isolate repository (Eurofins Medinet), ST18 | 44 |
| 1,231,502 | NZ_ACAX00000000.1 | Obtained from a clinical isolate repository (Eurofins Medinet), ST203 | 44 |
| 1,231,501 | NZ_ACAY00000000.1 | Obtained from a clinical isolate repository (Eurofins Medinet), ST52 | 44 |
| 1,231,410 | NZ_ACBA00000000.1 | Obtained from a clinical isolate repository (Eurofins Medinet), ST17 | 44 |
| 1,231,408 | NZ_ACBB00000000.1 | Obtained from a clinical isolate repository (Eurofins Medinet), ST582 | 44 |
| TX0082 | NZ_AEBU00000000.1 | Clinical isolate, endocarditis/bloodstream infection, ST17; isolated in Texas, 1999; resistant to vancomycin and ampicillin | Unpublished data; strain first described in reference 41 |
| TX0133A | NZ_AECH00000000.1 | Clinical isolate, bloodstream infection; isolated in Texas, 28 March 2006; resistant to vancomycin, vanA, ST17 | Strain first described in reference 4 |
| TX0133B | NZ_AECI00000000.1 | Clinical isolate, bloodstream infection; isolated in Texas, 9 May 2006; susceptible to vancomycin | Strain first described in reference 4 |
| TX0133a01 | NZ_AECJ00000000.1 | Isolate obtained from inhibition zone surrounding vancomycin Etest strip; resistant to vancomycin | Strain first described in reference 4 |
| TX0133a04 | NZ_AEBC00000000.1 | Isolate obtained from outside the inhibition zone surrounding vancomycin Etest strip; susceptible to vancomycin | Strain first described in reference 4 |
| TX0133C | NZ_AEBG00000000.1 | Clinical isolate, bloodstream infection; isolated in Texas, 26 May 2006; resistant to vancomycin, vanA | Strain first described in reference 4 |
| AUS0085 | SRX014391 | Clinical isolate, bloodstream infection; ST203, CC17 | 32 |
All accession numbers are from GenBank, except SRX014391, which is from SRA.
Genome sequencing.
The complete genome sequence of E. faecium Aus0004 was determined using 454 GS FLX 3-kbp paired-end sequencing (Roche Diagostics, Basel, Switzerland), yielding 48.7 Mbp from 120,556 reads and assembled with Newbler (v2.6). Finishing was facilitated using primer walking and managed with the genome assembly program Gap4 (v1.7.1b) (10). Read mapping of our previously published Illumina paired-end reads (accession no. SRX014390) was used to correct errors in the final assembly as described previously (32). An additional 25- to 40-kb-insert-size bacterial artificial chromosome (BAC) library was also prepared to resolve chromosomal duplications (51).
Genome analysis.
Genome annotation was managed using in-house tools Prokka and Wasabi (http://www.bioinformatics.net.au). Regions containing prophage or other foreign DNA were predicted using Phast and Alien Hunter (57, 59). CRISPR loci were detected by CRISPRfinder (23). For comparative genomics, a read-mapping approach was developed to define an E. faecium core genome and the accessory genome for a given strain. Sequence reads from each E. faecium strain (Table 1) were aligned separately with the completed Aus0004 reference genome using SHRiMP 2.0 (48). Those positions in Aus0004 that were covered by at least three reads from each and every genome defined an E. faecium core genome. Reads from Aus0004 that did not map to any E. faecium genome were used to define Aus0004 regions of difference. Reads from any strain that did not map to the core genome represented that strain's accessory genome. SNPs were identified using Nesoni v0.35 (http://www.bioinformatics.net.au), which used the sequence reads for each genome aligned with the above-defined core genome to construct a tally of putative differences at each nucleotide position, including substitutions, insertions, and deletions. This tally was then employed in a Bayesian model to decide whether a base (or deletion) could be called for the position, and if so, whether it differed from the reference sequence. A similar procedure was used to determine the presence or absence of insertions between positions in the reference. NCBI BLASTN+, Artemis, BRIG, and Easyfig were also used for intra- and inter-Aus0004 genome comparisons (1, 13, 52). Phylogenetic analyses were performed using a distance method, based on pairwise nucleotide sequence alignments for the E. faecium core genome among all strains. Phylogeny was inferred by both neighbor-joining and split decomposition analyses, using uncorrected p distances with bootstrapping as implemented in SplitsTree4 v4.11.3 (29). Tree drawing was managed with FigTree (46).
Nucleotide sequence accession numbers.
The complete genome sequence for E. faecium Aus0004 has been deposited in GenBank under accession numbers CP003351 to CP003354.
RESULTS AND DISCUSSION
Genome summary.
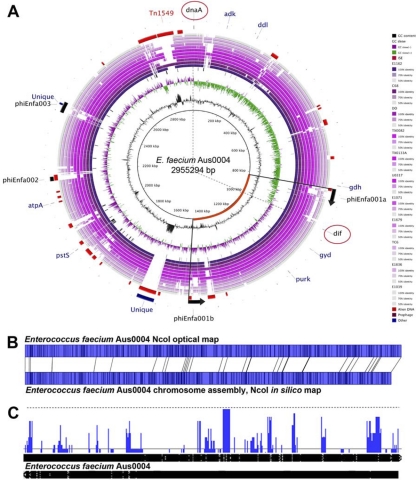
The genome of E. faecium strain Aus0004 comprises a single circular chromosome of 2,955,294 bp (GC content, 38%) and three circular plasmids, Aus0004_p1, Aus0004_p2, and Aus0004_p3, with lengths of 56,520 bp, 4,119 bp, and 3,847 bp and GC contents of 35%, 37%, and 39%, respectively (Fig. 1A). An asymmetric chromosomal GC skew pattern was observed, indicating a recent, large DNA inversion (see later discussion) (Fig. 1A). Comparison of an in silico AUS0004 chromosome NcoI map against an Aus0004 NcoI optical map, generated in a previous study (32), confirmed the integrity of our final assembly (Fig. 1B). A total of 2,860 protein-coding DNA sequences (CDS), 47 tRNA genes, and 6 rRNA operons were predicted in the Aus0004 chromosome.
Fig 1.
Analysis of the Enterococcus faecium AUS0004 complete genome. (A) Circular map of AUS0004 by comparative BLASTN analysis against the contigs from the partially assembled genomes of 11 E. faecium strains, showing the locations of a large chromosomal inversion and Aus0004 accessory genome elements. Track identification, moving outwards, is as follows: G+C content, GC skew (G-C/G+C), IS elements, E. faecium isolates (next 11 tracks, as listed here and in Table 1), followed by regions (red arcs) potentially acquired by HGT revealed by AlienHunter, location of prophage, Aus004 unique regions revealed by read mapping against 23 E. faecium genomes (blue arcs), and finally the location of the eight MLST genes. Dotted lines indicate likely replication origin (dnaA) and terminus (dif) and highlight a replichore imbalance, caused by a phiEnfa001-mediated chromosomal inversion. (B) NcoI optical map of E. faecium AUS0004 compared with in silico-derived NcoI map demonstrating correct chromosome assembly. (C) Artemis linear view of Aus0004 chromosome and plasmids (appended), with vertical blue bars identifying the positions of accessory genome elements as determined by read mapping against 23 publicly available partially assembled genome sequences. Increasing height of vertical blue lines on this map indicates increasing specificity for Aus0004.
Comparisons of Aus0004 with other E. faecium strains.
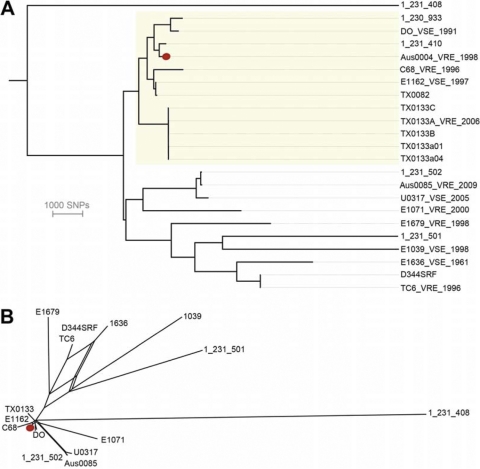
The availability of a complete E. faecium reference sequence allowed us to define a core E. faecium genome and an accessory Aus0004 genome and to produce a high-resolution E. faecium phylogeny. The DNA sequence reads from 22 other publicly available E. faecium strains of predominantly clinical origin (Table 1) were mapped to the Aus0004 chromosome and revealed that 1,866,631 of 3,019,780 bp of Aus0004 (62%), comprising 456 segments at least 200 bases long, were conserved among all 23 strains. That is, 38% of Aus0004 can be considered accessory (Fig. 1C). This is a higher percentage than that observed from comparisons of conserved CDS in E. faecalis with accessory genomes of around 30% (38, 49). Pairwise alignment of the 1,866,631-bp core among all 23 E. faecium strains uncovered 54,250 variable nucleotide positions among these strains (data not shown). These comparisons were used to construct a distance matrix, and a high-resolution phylogeny was inferred by neighbor joining (Fig. 2A). Aus0004 is most closely related to 1_231_410, an ST17 clinical isolate (Table 1) (44). A phylogeny was also inferred with the same core genome data using split decomposition (29) (Fig. 2B). While the same relationships between strains were displayed, parallel edges with high bootstrap support linking TC6, E1636 (ST106), and E1039 (ST42) are suggestive of recombination shaping the evolution of these strains. The topologies of our trees were mostly congruent with that reported from a comparison among 100 orthologous E. faecium CDS using essentially the same genome sequence data (20). However, our whole-genome approach increased resolution 10-fold (54,250 versus 5,932 variable sites) and therefore offered additional discrimination among the ST17 and ST16 isolates (Fig. 2).
Fig 2.
(A) Neighbor-joining tree showing relationship of AUS0004 compared to 22 other publicly available E. faecium genome sequences. Phylogeny was inferred by alignment of the 54,250 variable nucleotide positions (gaps removed) among all 23 E. faecium strains. Strains belonging to ST17 are highlighted. Red dot indicates Aus0004. E. faecium 1_231_408 was used as an outgroup to root the tree. (B) Network structure revealed among the same strains by split decomposition analysis of the same data set. All nodes in both trees had greater than 90% bootstrap support (1,000 replicates).
Aus0004 accessory genome.
To explore accessory genome content differences among strains compared to Aus0004, we next mapped the contigs from 21 partially assembled, publicly available E. faecium genomes (Fig. 1A). Contig and read mapping in conjunction with DNA composition analysis (Alien Hunter) were used to identify DNA segments potentially acquired by horizontal gene transfer (HGT). In addition to detecting the Tn1549-like conjugative transposon harboring the vancomycin resistance-encoding vanB locus (nucleotide positions 2835604 to 2869174), we identified several pathogenicity islands and prophage (Fig. 1A). Hot spots for insertion of the Tn1549-like vanB transposon have previously been proposed, but the insertion site of Tn1549 in Aus0004 does not match any reported in that study (34). However, comparison of Aus0004 with strain C68, also a VRE strain, suggests that Tn1549 has been inserted into the same chromosomal site (Fig. 1A). Given the similar insertion sites, there may be several preferred target insertion sites. Adjacent to Tn1549 in E. faecium Aus0004 is a 13.8-kb pathogenicity island that spans nucleotide positions 2808879 to 2822670. This region carries the enterococcal surface protein-encoding esp gene, which is a putative virulence factor commonly present in clinical enterococcal isolates. This pathogenicity island was first described in E. faecium strain E300 (35), and alignment of this element with the same element from strain E300 revealed 97% overall sequence identity. An additional 60-kb genomic island, spanning nucleotides 1607500 to 1667500, was also identified. This region is not present in the other E. faecium strains (Fig. 1A and C), suggesting that it was recently acquired by Aus0004. The CDS within this region encode hypothetical proteins, putative transcriptional regulators, and various phage-related genes. Some of the phage elements are identical to phage components in E. faecalis D6 and E. faecalis ATCC 4200.
Three distinct prophages were present in AUS0004. Their positions are indicated in Fig. 1A, and we named them phiEnfa001a/b (duplicated), phiEnfa002, and phiEnfa003. Notably, these phages are present in all ST17 genomes, but they appear variously present in other E. faecium isolates (Fig. 1A). They share a high degree of similarity to phages found not only in enterococci but also in species of other genera, including Clostridium, Listeria, Lactobacillus, and Staphylococcus. The frequency and diverse origins of the mobile genetic elements seen in Aus0004 suggest that the barriers to foreign DNA acquisition are low.
Clustered, regularly interspaced short palindromic repeats (CRISPR) are observed in up to 45% of bacterial genomes and together with cas genes serve as a sequence-specific defense mechanism against the integration of exogenous foreign DNA into the bacterial genome (7, 50). Clinical E. faecium isolates, in particular CC17 strains are known to lack CRISPR loci, and this is thought to explain their increased propensity to acquire exogenous DNA compared with that of E. faecium strains from nonclinical environments (55). A CRISPR-cas system was not detected in E. faecium Aus0004. However, two paralogous CDS (EFAU004_00823 and EFAU004_01436) encoding a protein homologous to a CRISPR-associated protein (encoded by cas2) were identified within phiEnfa001and phiEnfa002. However, without the other required cas genes, spacers, and repeat sequences that comprise the CRISPR-cas system, the two paralogous CDS most likely bear no functional significance.
Phage are major contributors to genome plasticity and have contributed to substantial chromosome remodeling in Aus0004. Prophage phiEnfa001a and phiEnfa001b are present as 50-kb inverted repeats (Fig. 1A). Their locations align with an asymmetrical GC skew pattern that suggests they have facilitated a 683-kb chromosomal inversion that crosses the predicted replication terminus, resulting in a significant replichore imbalance (Fig. 1A). We confirmed the likely location of the replication terminus in Aus0004 by identifying a single dif sequence (ACTTTGTATAATATATATTATGTAAACT, positions 964426 to 964453) that aligns with the shift in GC skew (Fig. 1A). The dif motif is strongly associated with replication termini (27). It is not known how widespread this rearrangement is among E. faecium isolates, because chromosomes from other isolates have not been sufficiently well assembled, as the inversion occurs around a large duplication that is unlikely to be resolved by automated assembly processes. However, a normal GC skew pattern, with balanced replichores, is found in E. faecalis V583. Replichore imbalances have been proposed to cause a fitness cost (37). Simple growth curve analysis of Aus0004 in the absence of competition shows no growth defect (data not shown).
CDS associated with virulence.
Genes encoding a number of putative enterococcal virulence factors were identified, including hemolysin (EFAU004_01337). Hemolysin is expressed in many E. faecium bacteremia isolates, and its cytolytic properties permit the lysis of a wide range of cells at the primary site of infection in the human host and subsequently enable the release of the isolate into the bloodstream (15). Also present were the genes for enterococcal surface protein (esp; EFAU004_02750) and collagen-binding adhesin (acm; EFAU004_02292). Enterococcal surface protein and collagen-binding adhesin reportedly promote colonization and hence the establishment of infection, although a number of recent studies have demonstrated that enterococcal surface protein is not essential for infection in murine infection models (26, 42, 45). Genes encoding other putative enterococcal virulence factors commonly associated with E. faecalis or E. faecium blood culture isolates, such as gelatinase, aggregation substance, lipase, and hemagglutinin (16), are absent in E. faecium strain Aus0004. As previously reported, Aus0004 does not contain hyaluronidase (hyl) (32).
Insertion sequence elements.
The Aus0004 genome is rich with repetitive DNA, highlighted by the presence of multiple insertion sequence elements (ISEs). At least 21 different ISEs were detected, ranging in copy number from 1 to 13, representing 76 distinct copies and distributed around the chromosome (Fig. 1A) and plasmids (Table 2). The most frequently observed ISE types were from the ISL3 and ISEf1 families. Notably, ISEf1 is also common in E. faecalis strains and was first identified as a predominant ISE type in the sequenced E. faecalis strain V583. Interestingly, none of the ISEs directly disrupted any CDS, although impacts on gene expression through ISE promoter adjacency may be occurring.
Table 2.
Insertion sequences identified in E. faecium Aus0004
| Insertion sequence | No. of copies | Locus tag(s) in Aus0004a |
|---|---|---|
| ISEnfa3 | 4 | EFAU004_00019+00020, EFAU004_01902+01903+01904, EFAU004_01898+01899+01900, EFAU004_00210 |
| ISEfa11 | 8 | EFAU004_00021, EFAU004_00594+00595, EFAU004_00659, EFAU004_01043+01044, EFAU004_01114, EFAU004_01490, EFAU004_01891, EFAU004_01919 |
| ISEf1 | 13 | EFAU004_00043, EFAU004_00157+00158, EFAU004_00187, EFAU004_00318, EFAU004_01145, EFAU004_01263, EFAU004_01527+01528+01529, EFAU004_01764+01765, EFAU004_01797, EFAU004_01901, EFAU004_01984, EFAU004_02700, EFAU004_02709 |
| ISEfa10 | 1 | EFAU004_00477 |
| ISEfa7 | 5 | EFAU004_00580, EFAU004_01762, EFAU004_02708, EFAU004_01780, EFAU004_01781 |
| ISEfa8 | 3 | EFAU004_00848+00849+00850, EFAU004_01409+01410+01411, EFAU004_01840+01841 |
| ISEfm1 | 6 | EFAU004_00289, EFAU004_02446, EFAU004_00867, EFAU004_00300, EFAU004_01982, EFAU004_01779 |
| IS16 | 3 | EFAU004_01890, EFAU004_02358, EFAU004_02683 |
| IS1380 | 1 | EFAU004_01897 |
| IS1476 | 1 | EFAU004_01884 |
| IS1485 | 2 | EFAU004_02218+02219, EFAU004_02710+02711 |
| IS6770 | 2 | EFAU004_01022, EFAU004_01956 |
| ISL3 family | 1 | EFAU004_01362 |
| IS4 family | 1 | EFAU004_01844 |
| IS66 family | 6 | EFAU004_00014, EFAU004_01985, EFAU004_01825, EFAU004_01957, EFAU004_01958, EFAU004_01589+01590 |
| IS116 family | 6 | EFAU004_01259, EFAU004_01518, EFAU004_01893, EFAU004_01894, EFAU004_02174, EFAU004_02661 |
| IS1251-like | 8 | EFAU004_00913, EFAU004_01298, EFAU004_01364, EFAU004_01404, EFAU004_01760, EFAU004_01885, EFAU004_02480, EFAU004_02481 |
| IS200/IS605 family | 1 | EFAU004_02276 |
| Transposase-like | 1 | EFAU004_02756 |
| Transposase-like | 2 | EFAU004_02757, EFAU004_02758 |
| Transposase-like | 1 | EFAU004_02811 |
Locus tags linked by “+” represent multiple transposases belonging to a single IS.
Plasmids Enterococcus species have been reported to contain several plasmids that often mediate resistance to antimicrobials and particular heavy metals and contribute to enhanced virulence and/or DNA repair mechanisms (3, 21, 22, 24). The plasmid copy number per cell was estimated by observing Illumina sequence read coverage distribution and indicated 1 copy of Aus0004_p1, 24 copies of Aus0004_p2, and 86 copies of Aus0004_p3. Comparison of their repA genes indicates that Aus0004_p1 belongs to the rep17 family while Aus0004_p2 and Aus0004_p3 belong to the rep14 family (31). Plasmid Aus0004_p1 harbors genes that encode a toxin-antitoxin system, a conjugation system, and several proteins with no known function. Toxin-antitoxin systems are frequently reported in E. faecium strains (40), and the Aus0004 chromosome additionally harbors at least five such systems. Their presence is thought to ensure the maintenance of the plasmid in the bacterial population (25, 40). Plasmids Aus0004_p2 and Aus0004_p3 each have shared segments of high DNA sequence similarity, 88% and 97%, respectively, with previously described 6,038-bp cryptic plasmid pRI1 identified in isolates of E. faecium from various animals and human origin (21). Excluding those of the replication initiation protein and plasmid recombination enzyme, the functions of the proteins encoded by the other CDS present on the plasmids are unknown. Although an apparent biological role appears to be lacking, filter-mating experiments have demonstrated the cotransfer of plasmid pRI1 with antimicrobial resistance-encoding plasmids, suggesting that cryptic plasmids may play some role in the transfer of antimicrobial resistance between bacterial strains (21).
MLST gene distribution.
We plotted the locations of the seven genes used for MLST analysis of E. faecium. Their distribution around the chromosome is shown in Fig. 1A and summarized in Table 3. We observed variations in the sequence of published primer sequences versus Aus0004 target sequences for gyd and gdh, as well as differences in the predicted PCR product sizes for various loci (Table 3).
Table 3.
Analysis of MLST loci in E. faecium Aus0004
| Gene name | Locus tag in Aus0004 | MLST PCR producta |
||||
|---|---|---|---|---|---|---|
| Locus start | Locus end | Length in Aus0004 (bp) | Published length (bp) (28) | Oligonucleotide primer sequence differences | ||
| adk | EFAU004_00116 | 108013 | 108542 | 529 | 437 | None |
| atpA | EFAU004_02059 | 2086115 | 2086768 | 653 | 556 | None |
| ddl | EFAU004_00216 | 210951 | 211494 | 543 | 465 | None |
| gdh | EFAU004_00777 | 810740 | 811399 | 659 | 530 | gdh1: GGCGCACT-AAAGATATGGT |
| Aus004: GGCGCACTTAAAGACATGGT | ||||||
| gdh2: CCAAGATTGGGCAACTTCGTCCCA | ||||||
| Aus004: CCAAGATTGAGCGACTTCTTCCCA | ||||||
| gyd | EFAU004_01020 | 1042017 | 1042396 | 379 | 395 | gyd1: CAAACTGCTTAGCTCCAATGGC |
| Aus004: CAAACTGTTTAGCACCTATGGC | ||||||
| gyd2: CATTTCGTTGTCATACCAAGC | ||||||
| Aus004: GATTTGACGGTCATCATACCC | ||||||
| purK | EFAU004_01116 | 1145293 | 1145951 | 658 | 492 | None |
| pstS | EFAU004_01886 | 1910865 | 1911495 | 630 | 583 | None |
Underlining indicates sequence differences between the oligonucleotide primers used for MLST and the genome sequence of Aus0004.
Conclusion.
While partially assembled draft genomes have provided some important insights into the diversity and evolution of E. faecium, complete and closed genomes will further exploit the capacity of genomics to understand this important human pathogen. The complete E. faecium Aus0004 genome has enabled us to examine the architecture of the genome and more precisely describe the genomic features of this strain. Using these data we have concisely defined an E. faecium core genome, and we also describe the genomic relationships between all the publicly available partially assembled E. faecium genomes. We have highlighted an abundance of mobile genetic elements, including multiple prophage and insertion sequence elements, plasmids, two large genomic islands, and the location of the Tn1549-like vancomycin resistance-encoding transposon, in our sequenced strain. As with E. faecalis, a significant portion of Aus0004 is comprised of mobile DNA that has led to the accumulation of a relatively large accessory genome (38%). In comparison, Staphylococcus aureus has an accessory genome of 11 to 18% among diverse strains (14). Further comparative and functional genomic research is required to pinpoint specific genetic features that explain the emergence of hospital epidemic and endemic E. faecium strains such as Aus0004. The complete E. faecium Aus0004 genome sequence described here represents an important resource in our efforts to understand this species and control the emergence of yet another hospital superbug.
ACKNOWLEDGMENTS
We thank Elizabeth Grabsch for assistance with characterization of the strain.
The National Health and Medical Research Council (NHMRC) of Australia supported this work.
Footnotes
Published ahead of print 24 February 2012
REFERENCES
- 1. Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. 2011. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arduino RC, Murray BE, Rakita RM. 1994. Roles of antibodies and complement in phagocytic killing of enterococci. Infect. Immun. 62:987–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arias CA, Panesso D, Singh KV, Rice LB, Murray BE. 2009. Cotransfer of antibiotic resistance genes and a hylEfm-containing virulence plasmid in Enterococcus faecium. Antimicrob. Agents Chemother. 53:4240–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arias CA, et al. 2007. Failure of daptomycin monotherapy for endocarditis caused by an Enterococcus faecium strain with vancomycin-resistant and vancomycin-susceptible subpopulations and evidence of in vivo loss of the vanA gene cluster. Clin. Infect. Dis. 45:1343–1346 [DOI] [PubMed] [Google Scholar]
- 5. Arthur M, Reynolds P, Courvalin P. 1996. Glycopeptide resistance in enterococci. Trends Microbiol. 4:401–407 [DOI] [PubMed] [Google Scholar]
- 6. Baldassarri L, Bertuccini L, Ammendolia MG, Gherardi G, Creti R. 2001. Variant esp gene in vancomycin-sensitive Enterococcus faecium. Lancet 357:1802. [DOI] [PubMed] [Google Scholar]
- 7. Barrangou R, et al. 2007. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709–1712 [DOI] [PubMed] [Google Scholar]
- 8. Bell JM, Paton JC, Turnidge J. 1998. Emergence of vancomycin-resistant enterococci in Australia: phenotypic and genotypic characteristics of isolates. J. Clin. Microbiol. 36:2187–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Billstrom H, Lund B, Sullivan A, Nord CE. 2008. Virulence and antimicrobial resistance in clinical Enterococcus faecium. Int. J. Antimicrob. Agents 32:374–377 [DOI] [PubMed] [Google Scholar]
- 10. Bonfield JK, Smith K, Staden R. 1995. A new DNA sequence assembly program. Nucleic Acids Res. 23:4992–4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonten MJ, Willems R, Weinstein RA. 2001. Vancomycin-resistant enterococci: why are they here, and where do they come from? Lancet Infect. Dis. 1:314–325 [DOI] [PubMed] [Google Scholar]
- 12. Carias LL, Rudin SD, Donskey CJ, Rice LB. 1998. Genetic linkage and cotransfer of a novel, vanB-containing transposon (Tn5382) and a low-affinity penicillin-binding protein 5 gene in a clinical vancomycin-resistant Enterococcus faecium isolate. J. Bacteriol. 180:4426–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carver T, Harris SR, Berriman M, Parkhill J, McQuillan JA. 2012. Artemis: an integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics 28:464–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chua KY, et al. 2011. The dominant Australian community-acquired methicillin-resistant Staphylococcus aureus clone ST93-IV [2B] is highly virulent and genetically distinct. PLoS One 6:e25887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cox CR, Coburn PS, Gilmore MS. 2005. Enterococcal cytolysin: a novel two component peptide system that serves as a bacterial defense against eukaryotic and prokaryotic cells. Curr. Protein Pept. Sci. 6:77–84 [DOI] [PubMed] [Google Scholar]
- 16. Elsner HA, et al. 2000. Virulence factors of Enterococcus faecalis and Enterococcus faecium blood culture isolates. Eur. J. Clin. Microbiol. Infect. Dis. 19:39–42 [DOI] [PubMed] [Google Scholar]
- 17. Endtz HP, van den Braak N, Verbrugh HA, van Belkum A. 1999. Vancomycin resistance: status quo and quo vadis. Eur. J. Clin. Microbiol. Infect. Dis. 18:683–690 [DOI] [PubMed] [Google Scholar]
- 18. Fisher K, Phillips C. 2009. The ecology, epidemiology and virulence of Enterococcus. Microbiology 155:1749–1757 [DOI] [PubMed] [Google Scholar]
- 19. Fox J. 2000. DOE Joint Genome Institute feat: one-day sequencing of E. faecium. ASM News 66:388 [Google Scholar]
- 20. Galloway-Pena J, Roh JH, Latorre M, Qin X, Murray BE. 2012. Genomic and SNP analyses demonstrate a distant separation of the hospital and community-associated clades of Enterococcus faecium. PLoS One 7:e30187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garcia-Migura L, Hasman H, Jensen LB. 2009. Presence of pRI1: a small cryptic mobilizable plasmid isolated from Enterococcus faecium of human and animal origin. Curr. Microbiol. 58:95–100 [DOI] [PubMed] [Google Scholar]
- 22. Garcia-Migura L, Liebana E, Jensen LB. 2007. Transposon characterization of vancomycin-resistant Enterococcus faecium (VREF) and dissemination of resistance associated with transferable plasmids. J. Antimicrob. Chemother. 60:263–268 [DOI] [PubMed] [Google Scholar]
- 23. Grissa I, Vergnaud G, Pourcel C. 2007. CRISPRFinder: a web tool to identify clustered regularly interspaced short palindromic repeats. Nucleic Acids Res. 35:W52–W57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hasman H, et al. 2006. Copper resistance in Enterococcus faecium, mediated by the tcrB gene, is selected by supplementation of pig feed with copper sulfate. Appl. Environ. Microbiol. 72:5784–5789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hayes F. 2003. Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest. Science 301:1496–1499 [DOI] [PubMed] [Google Scholar]
- 26. Heikens E, et al. 2009. Enterococcal surface protein Esp is not essential for cell adhesion and intestinal colonization of Enterococcus faecium in mice. BMC Microbiol. 9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hendrickson H, Lawrence JG. 2007. Mutational bias suggests that replication termination occurs near the dif site, not at Ter sites. Mol. Microbiol. 64:42–56 [DOI] [PubMed] [Google Scholar]
- 28. Homan WL, et al. 2002. Multilocus sequence typing scheme for Enterococcus faecium. J. Clin. Microbiol. 40:1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23:254–267 [DOI] [PubMed] [Google Scholar]
- 30. Iwen PC, et al. 1997. Change in prevalence and antibiotic resistance of Enterococcus species isolated from blood cultures over an 8-year period. Antimicrob. Agents Chemother. 41:494–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jensen LB, et al. 2010. A classification system for plasmids from enterococci and other Gram-positive bacteria. J. Microbiol. Methods 80:25–43 [DOI] [PubMed] [Google Scholar]
- 32. Johnson PD, et al. 2010. A sustained hospital outbreak of vancomycin-resistant Enterococcus faecium bacteremia due to emergence of vanB E. faecium sequence type 203. J. Infect. Dis. 202:1278–1286 [DOI] [PubMed] [Google Scholar]
- 33. Kamarulzaman A, Tosolini FA, Boquest J, Geddes JE, Richards MJ. 1995. Vancomycin-resistant Enterococcus faecium in a liver transplant recipient. ANZ J. Med. 25:560 [Google Scholar]
- 34. Launay A, Ballard SA, Johnson PD, Grayson ML, Lambert T. 2006. Transfer of vancomycin resistance transposon Tn1549 from Clostridium symbiosum to Enterococcus spp. in the gut of gnotobiotic mice. Antimicrob. Agents Chemother. 50:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leavis H, et al. 2004. A novel putative enterococcal pathogenicity island linked to the esp virulence gene of Enterococcus faecium and associated with epidemicity. J. Bacteriol. 186:672–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leavis HL, et al. 2007. Insertion sequence-driven diversification creates a globally dispersed emerging multiresistant subspecies of E. faecium. PLoS Pathog. 3:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matthews TD, Maloy S. 2010. Fitness effects of replichore imbalance in Salmonella enterica. J. Bacteriol. 192:6086–6088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McBride SM, Fischetti VA, Leblanc DJ, Moellering RC, Jr, Gilmore MS. 2007. Genetic diversity among Enterococcus faecalis. PLoS One 2:e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Metan G, Zarakolu P, Unal S. 2005. Rapid detection of antibacterial resistance in emerging Gram-positive cocci. J. Hosp. Infect. 61:93–99 [DOI] [PubMed] [Google Scholar]
- 40. Moritz EM, Hergenrother PJ. 2007. Toxin-antitoxin systems are ubiquitous and plasmid-encoded in vancomycin-resistant enterococci. Proc. Natl. Acad. Sci. U. S. A. 104:311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nallapareddy SR, Singh KV, Murray BE. 2006. Construction of improved temperature-sensitive and mobilizable vectors and their use for constructing mutations in the adhesin-encoding acm gene of poorly transformable clinical Enterococcus faecium strains. Appl. Environ. Microbiol. 72:334–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nallapareddy SR, Weinstock GM, Murray BE. 2003. Clinical isolates of Enterococcus faecium exhibit strain-specific collagen binding mediated by Acm, a new member of the MSCRAMM family. Mol. Microbiol. 47:1733–1747 [DOI] [PubMed] [Google Scholar]
- 43. Padiglione AA, et al. 2003. Risk factors for new detection of vancomycin-resistant enterococci in acute-care hospitals that employ strict infection control procedures. Antimicrob. Agents Chemother. 47:2492–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Palmer KL, et al. 2010. High-quality draft genome sequences of 28 Enterococcus sp. isolates. J. Bacteriol. 192:2469–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pultz NJ, Shankar N, Baghdayan AS, Donskey CJ. 2005. Enterococcal surface protein Esp does not facilitate intestinal colonization or translocation of Enterococcus faecalis in clindamycin-treated mice. FEMS Microbiol. Lett. 242:217–219 [DOI] [PubMed] [Google Scholar]
- 46. Rambaut A. 2009. FigTree, tree figure drawing tool, v. 1.3.1.
- 47. Rice LB, et al. 2009. Transferable capacity for gastrointestinal colonization in Enterococcus faecium in a mouse model. J. Infect. Dis. 199:342–349 [DOI] [PubMed] [Google Scholar]
- 48. Rumble SM, et al. 2009. SHRiMP: accurate mapping of short color-space reads. PLoS Comput. Biol. 5:e1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Solheim M, Aakra A, Snipen LG, Brede DA, Nes IF. 2009. Comparative genomics of Enterococcus faecalis from healthy Norwegian infants. BMC Genomics 10:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sorek R, Kunin V, Hugenholtz P. 2008. CRISPR—a widespread system that provides acquired resistance against phages in bacteria and archaea. Nat. Rev. Microbiol. 6:181–186 [DOI] [PubMed] [Google Scholar]
- 51. Stinear TP, et al. 2004. Giant plasmid-encoded polyketide synthases produce the macrolide toxin of Mycobacterium ulcerans. Proc. Natl. Acad. Sci. U. S. A. 101:1345–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sullivan MJ, Petty NK, Beatson SA. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Top J, Willems R, Bonten M. 2008. Emergence of CC17 Enterococcus faecium: from commensal to hospital-adapted pathogen. FEMS Immunol. Med. Microbiol. 52:297–308 [DOI] [PubMed] [Google Scholar]
- 54. Top J, Willems R, van der Velden S, Asbroek M, Bonten M. 2008. Emergence of clonal complex 17 Enterococcus faecium in the Netherlands. J. Clin. Microbiol. 46:214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Schaik W, et al. 2010. Pyrosequencing-based comparative genome analysis of the nosocomial pathogen Enterococcus faecium and identification of a large transferable pathogenicity island. BMC Genomics 11:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van Schaik W, Willems RJL. 2010. Genome-based insights into the evolution of enterococci. Clin. Microbiol. Infect. 16:527–532 [DOI] [PubMed] [Google Scholar]
- 57. Vernikos GS, Parkhill J. 2006. Interpolated variable order motifs for identification of horizontally acquired DNA: revisiting the Salmonella pathogenicity islands. Bioinformatics 22:2196–2203 [DOI] [PubMed] [Google Scholar]
- 58. Willems RJ, et al. 2005. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg. Infect. Dis. 11:821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhou Y, Liang Y, Lynch KH, Dennis JJ, Wishart DS. 2011. PHAST: a fast phage search tool. Nucleic Acids Res. 39:W347–W352 [DOI] [PMC free article] [PubMed] [Google Scholar]