Abstract
This study evaluated the microbiota of root canals undergoing retreatment. The most prevalent taxa detected by checkerboard included Propionibacterium species, Fusobacterium nucleatum, streptococci, and Pseudoramibacter alactolyticus. Quantitative real-time PCR detected Enterococcus faecalis and streptococci in 38% and 41% of the cases, comprising 9.76% and 65.78% of the total bacterial counts, respectively. The findings call into question the status of E. faecalis as the main pathogen and suggest that other species can be candidate pathogens associated with persistent/secondary endodontic infections.
TEXT
Posttreatment apical periodontitis is an inflammatory disease caused by a persistent/secondary infection of the dental root canal (8, 11, 13, 15, 26, 30). Enterococcus faecalis has been the most frequently detected species in root canal-treated teeth as revealed by both culture and molecular studies (5, 8, 11, 12, 16, 23, 24, 26, 30, 31), but recent studies have called into question its etiologic role in posttreatment disease (7, 15, 20, 31). Moreover, taxa related to several other genera, including Streptococcus, Dialister, Fusobacterium, Filifactor, Parvimonas, Prevotella, Propionibacterium, and Pyramidobacter, have also been detected in treated teeth (5, 8, 11, 12, 15, 20, 22, 26, 27, 29, 30).
The purpose of this molecular study was 2-fold. First, the samples from root canal-treated teeth with posttreatment apical periodontitis undergoing retreatment were surveyed for the presence of 28 bacterial taxa using the reverse capture-checkerboard DNA-DNA hybridization approach. Then, the total bacterial counts and the presence, levels, and proportions of E. faecalis and streptococci were determined by using a quantitative real-time PCR (qPCR) assay.
Forty-two teeth were selected from patients (30 females and 12 males aged 16 to 70 years; mean age, 41 years) who had been referred for root canal retreatment to the Department of Endodontics, Estácio de Sá University. All the root canal-treated teeth were asymptomatic, showed radiographic evidence of apical periodontitis, and had had endodontic therapy completed more than 2 years earlier. All teeth were coronally restored and no direct exposure of the root canal filling material to the oral cavity was evident. The termini of the root canal fillings ranged from 0 to 4 mm short of the radiographic apex. No case was overfilled. The selected teeth showed an absence of periodontal pockets >4 mm in size. Approval for the study protocol was obtained from the Ethics Committee of the Estácio de Sá University.
The procedures for disinfecting the operative field and taking samples from treated root canals were as described previously (22, 26). Eight participants (out of 50 patients that started the experiment) were excluded because control samples from the operative field were positive for bacterial DNA as determined by broad-range PCR. DNA was extracted from clinical samples using the QIAamp DNA minikit (Qiagen, Valencia, CA), following the protocol recommended by the manufacturer. To maximize DNA extraction, a step of preincubation with lysozyme for 30 min was added. DNA from a panel of several oral bacterial species was also prepared to serve as controls (27).
The reverse-capture checkerboard assay was conducted to determine the presence and levels of 28 bacterial taxa as described previously (9, 14, 19, 25). Probes were based on 16S rRNA gene sequences of the target bacteria and were described and validated elsewhere (1, 9, 12, 14). A semiquantitative analysis of the checkerboard findings was performed as reported previously (14, 17).
To quantify the total bacterial load and the prevalence and levels of E. faecalis and streptococci, 16S rRNA gene-targeted qPCR was performed with Power SYBR green PCR master mix (Applied Biosystems, Foster City, CA) on an ABI 7500 real-time PCR instrument (Applied Biosystems) in a total reaction mixture volume of 20 μl. The primers and the respective annealing temperatures are shown in Table 1. Primers in a concentration of 0.5 μM each and DNA extract volume of 2 μl were added to the PCR master mix in MicroAmp optical 96-well reaction plates. Plates were sealed, centrifuged, and then subjected to amplification. Cycling conditions for the qPCR included 95°C for 10 min and 40 repeats of the following steps: 95°C for 1 min, annealing for 1 min (specific temperatures are shown in Table 1), and 72°C for 1 min. Double-stranded DNA product was measured at 78°C. At each cycle, the accumulation of PCR products was detected by monitoring the increase in fluorescence of the reporter dye. All measurements were done in triplicate for samples and standards. In all experiments, triplicates of appropriate negative controls containing no template DNA were subjected to the same procedures. Following amplification, melting curve analysis was performed to determine the specificity of the amplified products. The melting curve was obtained from 60°C to 95°C, with continuous fluorescence measurements taken at every 1% increase in temperature. Data acquisition and analysis were performed using the ABI 7500 software, version 2.0.4 (Applied Biosystems).
Table 1.
PCR primers used for bacterial quantification in samples from root canal-treated teeth with posttreatment apical periodontitis
| Target | Primer sequence | Annealing temp (°C) | Fragment length (bp) | Reference |
|---|---|---|---|---|
| Enterococcus faecalis | 5′-GTT TAT GCC GCA TGG CAT AAG AG-3′ | 60 | 310 | 30 |
| 5′-CCG TCA GGG GAC GTT CAG-3′ | ||||
| Streptococcus species | 5′-AGA GTT TGA TYM TGG CTC AG-3′a | 58 | 502 | 12 |
| 5′-TTA GCC GTC CCT TTC TGG T-3′ | ||||
| Universal 16S rRNA gene | 5′-GAT TAG ATA CCC TGG TAG TCC AC-3′ | 52 | 733 | 1 |
| 5′-TAC CTT GTT ACG ACT T-3′ |
Universal forward primer.
Bacterial levels were inferred for each sample based on the standard curves obtained. Standard curves for E. faecalis and streptococci were constructed using DNA extracted from known concentrations of E. faecalis ATCC 29212 and Streptococcus mutans ATCC 25175 grown in pure culture. E. faecalis was also used to quantify total bacteria using the pair of universal primers. DNA was isolated from fresh pure cultures of these strains using the QIAamp DNA minikit and quantified using a BioPhotometer (Eppendorf, Hamburg, Germany). Knowing the genome size of both E. faecalis (3.2 Mb) and S. mutans (2 Mb) and the average molecular mass of one base pair (660 Da), the measured DNA value could then be converted into target genomic copy levels per microliter using the formula m = n[1 mol/6 × 1023 (bp)][660 (g)/mole] = n[1.096 × 10−21 (g/bp)], where m is the genomic mass of a single cell and n the genome size. Genome copy levels were considered numerically equivalent to bacterial cell levels. The standards were then 10-fold diluted from 107 to 102 cells in Tris-EDTA buffer and used for standard curve construction. The levels of total bacteria could not be precisely calculated because of the differences in numbers of rrn operons. Therefore, a pure culture of E. faecalis, which contains 4 copies of the gene, was used, with 4 being considered the average copy number in the range of most known oral bacteria (http://www.cbs.dtu.dk/services/GenomeAtlas-3.0). Relative amounts were calculated as the percentages of E. faecalis and streptococci in the total bacterial load.
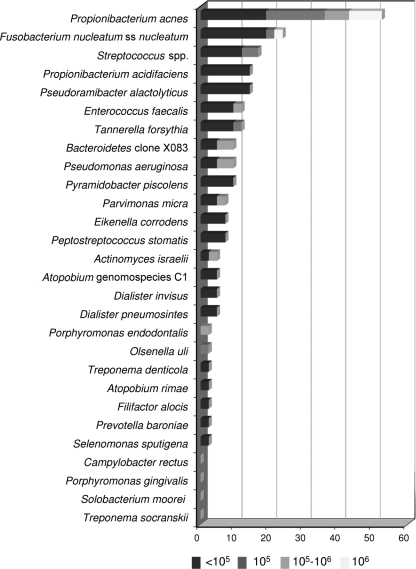
All clinical samples were positive for the presence of bacteria in both assays, confirming the primary infectious etiology of posttreatment apical periodontitis. The checkerboard results revealed that 24 of the 28 taxon-specific probes tested were reactive with one or more samples. The number of selected bacterial taxa detected per sample ranged from 1 to 12. Only 5 cases harbored more than 5 target taxa. The taxa detected more frequently included Propionibacterium acnes (22/42 cases [52%]), Fusobacterium nucleatum subspecies nucleatum (10/42 [24%]), Streptococcus species (7/42 [17%]), Propionibacterium acidifaciens (6/42 [14%]), Pseudoramibacter alactolyticus (6/42 [14%]), Enterococcus faecalis (5/42 [12%]), and Tannerella forsythia (5/42 [12%]) (Fig. 1). Semiquantitative analysis showed that no taxon was found at >106 and only 8 taxa were present at levels of >105. Of these, only 3 occurred in more than one sample: P. acnes (7 cases), Bacteroidetes clone X083 (2 cases), and Pseudomonas aeruginosa (2 cases) (Fig. 1).
Fig 1.
Stacked bar chart of frequencies of detection and levels of bacterial species/phylotypes in root canal samples of treated teeth with posttreatment apical periodontitis from 42 individuals. Total length of each bar stack indicates percentage of positive samples; i.e., prevalence of bacterial species/phylotypes. Different shades within each bar indicate the percentage of samples containing different levels of the species. ss, subspecies.
Samples from 29 root canal-treated teeth were available for qPCR analysis. The correlation coefficient (r2), amplification efficiency (E), and y-intercept values of the standard curves for each qPCR assay, respectively, were as follows: 0.993, 101%, and 41.4 for total bacteria; 0.995, 117%, and 40.7 for E. faecalis; and 0.992, 109%, and 43 for Streptococcus species. The accuracy of the amplification was confirmed by melting curve analysis.
Analysis using universal primers revealed a mean bacterial load of 3.2 × 105 (range, 1.27 × 103 to 7.25 × 106). These values fit into the range of 103 to 107 bacterial cell equivalents revealed by previous culture and molecular studies for treated teeth (2, 10, 24).
E. faecalis was detected by qPCR in 11/29 (38%) cases, with a mean number of cells of 1.28 × 103. In the samples positive for E. faecalis, this species comprised from 0.3% to 91% of total bacterial counts (median, 1.1%; mean, 9.76%) (Table 2). In 6 cases, it corresponded to >1% of the population, and in only 1 case did this species comprise >10% of the community (91%). The overall findings for E. faecalis are very similar to those reported by Sedgley et al. (24), who also used qPCR and reported that E. faecalis comprised from 0.14% to 100% of the total counts (median, 0.98%; mean, 9.84%).
Table 2.
Quantitative real-time PCR data for total bacteria, Enterococcus faecalis, and streptococci in root canal-treated teeth with apical periodontitis
| Statistic | Cell no. of total bacteria | % of total bacteria that were: |
|
|---|---|---|---|
| Enterococcus faecalis | Streptococcus species | ||
| Mean | 3.2 × 105 | 9.76 | 65.78 |
| Median | 1.18 × 104 | 1.1 | 75.5 |
| Range | 1.27 × 103–7.25 × 106 | 0.3–91 | 9–99 |
Our findings are in line with recent studies that have questioned the status of E. faecalis as the main pathogen in posttreatment apical periodontitis (7, 15, 22, 31). In the checkerboard assay, only 5 of the 42 cases were positive for E. faecalis, and in none of these was this species the most dominant taxon. In the qPCR experiment, which is more sensitive than the checkerboard, E. faecalis was detected at a higher frequency, but in terms of proportion, in only one case was it the main component of the community.
Analysis by qPCR revealed streptococci in 12/29 (41%) cases, with a mean number of 2.45 × 105 cells. These bacteria comprised from 9% to 99% of the total bacterial counts (median, 75.5%; mean, 65.78%) (Table 2). In 11 cases, streptococci corresponded to >10% of the overall community, and in 8 cases, they comprised >50% of the community.
Streptococcus species have commonly been detected in samples taken immediately after endodontic treatment procedures (3, 4, 18, 19, 21, 28) and in root canal-treated teeth undergoing retreatment (6, 8, 11, 12, 26, 30). This suggests that these bacteria may be involved with persistent infections that result in persistent disease. Because most studies show no specific species involved with posttreatment disease, we decided to use primers and probes to detect Streptococcus as a group. Our findings indicate that streptococci may be dominant in the bacterial community associated with many cases of posttreatment disease. Further studies are warranted to elaborate on the specific Streptococcus species participating in the process.
Over the last decade, the knowledge of the bacterial diversity associated with posttreatment apical periodontitis has been substantially refined and redefined by molecular microbiology methods. The present findings contribute to this knowledge by calling into question the status of E. faecalis as the main pathogen in posttreatment apical periodontitis, reinforcing the role of streptococci in persistent/secondary infections, and allowing the inclusion of some new species in the set of candidate pathogens associated with posttreatment disease.
ACKNOWLEDGMENTS
This study was supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazilian Governmental Institutions.
The authors deny any conflicts of interest.
Footnotes
Published ahead of print 7 March 2012
REFERENCES
- 1. Becker MR, et al. 2002. Molecular analysis of bacterial species associated with childhood caries. J. Clin. Microbiol. 40:1001–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blome B, Braun A, Sobarzo V, Jepsen S. 2008. Molecular identification and quantification of bacteria from endodontic infections using real-time polymerase chain reaction. Oral Microbiol. Immunol. 23:384–390 [DOI] [PubMed] [Google Scholar]
- 3. Byström A, Sundqvist G. 1985. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int. Endod. J. 18:35–40 [DOI] [PubMed] [Google Scholar]
- 4. Chavez de Paz L, Svensater G, Dahlen G, Bergenholtz G. 2005. Streptococci from root canals in teeth with apical periodontitis receiving endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 100:232–241 [DOI] [PubMed] [Google Scholar]
- 5. Gomes BP, et al. 2008. Microbial analysis of canals of root-filled teeth with periapical lesions using polymerase chain reaction. J. Endod. 34:537–540 [DOI] [PubMed] [Google Scholar]
- 6. Hancock HH, III, Sigurdsson A, Trope M, Moiseiwitsch J. 2001. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 91:579–586 [DOI] [PubMed] [Google Scholar]
- 7. Kaufman B, Spangberg L, Barry J, Fouad AF. 2005. Enterococcus spp. in endodontically treated teeth with and without periradicular lesions. J. Endod. 31:851–856 [DOI] [PubMed] [Google Scholar]
- 8. Molander A, Reit C, Dahlen G, Kvist T. 1998. Microbiological status of root-filled teeth with apical periodontitis. Int. Endod. J. 31:1–7 [PubMed] [Google Scholar]
- 9. Paster BJ, Bartoszyk IM, Dewhirst FE. 1998. Identification of oral streptococci using PCR-based, reverse-capture, checkerboard hybridization. Methods Cell Sci. 20:223–231 [Google Scholar]
- 10. Peciuliene V, Reynaud AH, Balciuniene I, Haapasalo M. 2001. Isolation of yeasts and enteric bacteria in root-filled teeth with chronic apical periodontitis. Int. Endod. J. 34:429–434 [DOI] [PubMed] [Google Scholar]
- 11. Pinheiro ET, et al. 2003. Microorganisms from canals of root-filled teeth with periapical lesions. Int. Endod. J. 36:1–11 [DOI] [PubMed] [Google Scholar]
- 12. Rôças IN, Hulsmann M, Siqueira JF., Jr 2008. Microorganisms in root canal-treated teeth from a German population. J. Endod. 34:926–931 [DOI] [PubMed] [Google Scholar]
- 13. Rôças IN, Jung IY, Lee CY, Siqueira JF., Jr 2004. Polymerase chain reaction identification of microorganisms in previously root-filled teeth in a South Korean population. J. Endod. 30:504–508 [PubMed] [Google Scholar]
- 14. Rôças IN, Siqueira JF., Jr 2008. Root canal microbiota of teeth with chronic apical periodontitis. J. Clin. Microbiol. 46:3599–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rôças IN, Siqueira JF, Jr, Aboim MC, Rosado AS. 2004. Denaturing gradient gel electrophoresis analysis of bacterial communities associated with failed endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 98:741–749 [DOI] [PubMed] [Google Scholar]
- 16. Rôças IN, Siqueira JF, Jr, Santos KR. 2004. Association of Enterococcus faecalis with different forms of periradicular diseases. J. Endod. 30:315–320 [DOI] [PubMed] [Google Scholar]
- 17. Rôças IN, Siqueira JF., Jr 2011. Comparison of the in vivo antimicrobial effectiveness of sodium hypochlorite and chlorhexidine used as root canal irrigants: a molecular microbiology study. J. Endod. 37:143–150 [DOI] [PubMed] [Google Scholar]
- 18. Rôças IN, Siqueira JF., Jr 2010. Identification of bacteria enduring endodontic treatment procedures by a combined reverse transcriptase-polymerase chain reaction and reverse-capture checkerboard approach. J. Endod. 36:45–52 [DOI] [PubMed] [Google Scholar]
- 19. Rôças IN, Siqueira JF., Jr 2011. In vivo antimicrobial effects of endodontic treatment procedures as assessed by molecular microbiologic techniques. J. Endod. 37:304–310 [DOI] [PubMed] [Google Scholar]
- 20. Rolph HJ, et al. 2001. Molecular identification of microorganisms from endodontic infections. J. Clin. Microbiol. 39:3282–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakamoto M, Siqueira JF, Jr, Rôças IN, Benno Y. 2007. Bacterial reduction and persistence after endodontic treatment procedures. Oral Microbiol. Immunol. 22:19–23 [DOI] [PubMed] [Google Scholar]
- 22. Sakamoto M, Siqueira JF, Jr, Rôças IN, Benno Y. 2008. Molecular analysis of the root canal microbiota associated with endodontic treatment failures. Oral Microbiol. Immunol. 23:275–281 [DOI] [PubMed] [Google Scholar]
- 23. Schirrmeister JF, et al. 2009. New bacterial compositions in root-filled teeth with periradicular lesions. J. Endod. 35:169–174 [DOI] [PubMed] [Google Scholar]
- 24. Sedgley C, Nagel A, Dahlen G, Reit C, Molander A. 2006. Real-time quantitative polymerase chain reaction and culture analyses of Enterococcus faecalis in root canals. J. Endod. 32:173–177 [DOI] [PubMed] [Google Scholar]
- 25. Siqueira JF, Jr, Rôças IN. 2009. The microbiota of acute apical abscesses. J. Dent. Res. 88:61–65 [DOI] [PubMed] [Google Scholar]
- 26. Siqueira JF, Jr, Rôças IN. 2004. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 97:85–94 [DOI] [PubMed] [Google Scholar]
- 27. Siqueira JF, Jr, Rôças IN. 2005. Uncultivated phylotypes and newly named species associated with primary and persistent endodontic infections. J. Clin. Microbiol. 43:3314–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siqueira JF, Jr, et al. 2007. Bacteriologic investigation of the effects of sodium hypochlorite and chlorhexidine during the endodontic treatment of teeth with apical periodontitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 104:122–130 [DOI] [PubMed] [Google Scholar]
- 29. Sunde PT, Tronstad L, Eribe ER, Lind PO, Olsen I. 2000. Assessment of periradicular microbiota by DNA-DNA hybridization. Endod. Dent. Traumatol. 16:191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sundqvist G, Figdor D, Persson S, Sjogren U. 1998. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative retreatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 85:86–93 [DOI] [PubMed] [Google Scholar]
- 31. Zoletti GO, Siqueira JF, Jr, Santos KR. 2006. Identification of Enterococcus faecalis in root-filled teeth with or without periradicular lesions by culture-dependent and -independent approaches. J. Endod. 32:722–726 [DOI] [PubMed] [Google Scholar]