Abstract
Hereditary hemorrhagic telangiectasia (HHT) is a disease characterized by arteriovenous malformations (AVMs). Brain abscess is a complication of HHT with AVMs. Literature provides evidence that Enterococcus faecalis can cause endodontic infections. We present the case of an HHT patient who developed brain abscess due to E. faecalis after a dental procedure.
CASE REPORT
A28-year-old Caucasian woman was presented to our hospital with a 5-day history of visual disturbances. She complained of loss of some part of her normal visual field and especially a defect in her peripheral vision. Her past medical history was significant on account of a brain abscess, surgically treated 6 years previously (in 2005), on the occasion of which the diagnosis of hereditary hemorrhagic telangiectasia (HHT) was made and two small arteriovenous malformations (AVMs) in the lungs were detected.
On admission, the patient was alert, oriented, and afebrile. The neurological examination revealed left homonymous hemianopsia, without decrease in visual acuity. There was no neck stiffness, and the fundoscopy result was normal. The remainder of the physical examination was remarkable on account of hypoxemia (oxygen saturation[SatO2] = 90%), mild cyanosis, and digital clubbing as well as sparse telangiectasias on the mucosal surface of her lower lip. No cardiac or extracardiac murmurs were present, and there was no tachypnea or complaint of dyspnea.
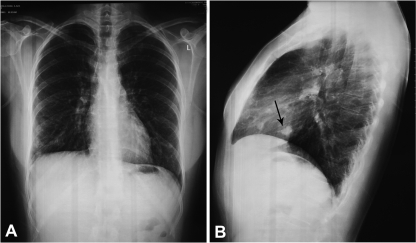
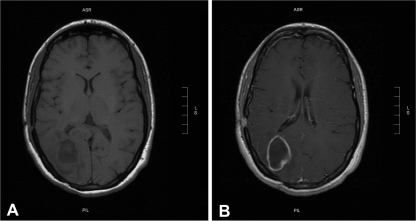
The whole-blood count, C-reactive protein level, and urinalysis result were normal except for the hemoglobin level (16.9 g/dl). Chest X-ray lateral films showed a 10-mm-diameter nodular lesion corresponding to one of the patient's known AVMs (Fig. 1). Magnetic resonance investigation (MRI) revealed a hypodense lesion in the right occipital lobe with ring enhancement after contrast administration, exerting mild pressure on the occipital horn of the ipsilateral lateral ventricle (Fig. 2). Based on the patient's history, a brain abscess was strongly suspected, and a computed tomography (CT)-guided stereotactic aspiration was carried out. Culture of the pus drained yielded Enterococcus faecalis, while blood cultures were sterile. Cardiac and abdominal ultrasonography results showed no site of potential microbial entry. However, additional clinical history elicited on admission disclosed a dental procedure 10 days before the onset of symptoms. The patient was initially treated with ceftriaxone and metronidazole intravenously, followed by moxifloxacin intravenously for 2 weeks and then orally (p.o.) for 5 more weeks. The patient's course was uneventful, and she was discharged asymptomatic 20 days after admission.
Fig 1.
Frontal (A) and lateral (B) chest radiographs of the patient. The lateral film demonstrates a nodular lesion corresponding to one of the patient's known arteriovenous malformations (arrow).
Fig 2.
Magnetic resonance investigation (MRI) revealed a hypodense lesion in the right occipital lobe (A) with ring enhancement after contrast administration, exerting mild pressure on the occipital horn of the ipsilateral lateral ventricle (B).
Hereditary hemorrhagic telangiectasia (HHT), also known as Rendu-Osler-Weber syndrome, is an inherited autosomal dominant disorder affecting approximately 1 in 5,000 individuals (13). HHT was first described as a familial disease characterized by episodes of spontaneous and recurrent epistaxis, cutaneous and mucosal visible dilated blood vessels (telangiectasias), and visceral arteriovenous malformations (AVMs) usually involving the pulmonary, hepatic, cerebral, and spinal and other circulation pathways (10, 13). These features are the Curacao criteria, which remain the mainstay of diagnosis (10). An individual is considered to have “definite” HHT if three out of four criteria are satisfied and “suspected” HHT if two are present, and HHT is considered “unlikely” if only one is present (10, 13). Screening programs in asymptomatic individuals indicate that pulmonary AVMs affect almost 50% of HHT patients (3). The development of brain abscess is a potential complication of HHT with pulmonary AVMs and can be the initial presentation of it (5). Brain abscess is believed to occur in about 5 to 10% of patients with HHT (9). Bacteremias, especially of dental origin, are considered to play a role in the pathogenesis of brain abscess in patients with HHT (11). Moreover, there is evidence that these patients might also exhibit mild immunodeficiency (2).
Brain abscess usually develops from a contiguous focus of infection, most often from infections in the middle ear, mastoid cells, or paranasal sinuses and less often from dental infection and trauma or hematogenous dissemination (7). HHT is a predisposing factor that leads to hematogenously acquired brain abscess, which is almost always observed in patients with coexisting pulmonary arteriovenous malformations, perhaps by allowing septic emboli to cross the pulmonary circulation without capillary filtration or by bacterial seeding of an ischemic portion of the brain after paradoxical sterile emboli have formed (13). Cerebral hypoxia and polycythemia due to a right-to-left shunt are considered to enhance bacterial growth (13). HHT patients are expected to experience prolonged bacteremic periods, since the shunt provided by AMVs abolishes the exposure to the capillary bed reticuloendothelial cell system (12).
Although dental infection is considered to be an uncommon source of hematogenous dissemination, resulting in the formation of brain abscess in the general population (7), many cases in the literature report the isolation of components of the normal oral flora from the brain abscess of HHT patients. In a recent study, Shovlin et al. confirmed that the majority of organisms isolated from brain abscess aspirates of HHT patients were microaerophilic and anaerobic bacteria commonly found in endo- and periodontal infections. Moreover, many of the HHT patients seemed to have experienced identifiable events known to be associated with bacteremia in the weeks preceding their abscess, particularly dental work, further confirming the pathogenic link between oral bacteria and HHT-associated brain abscess (11). For the above-mentioned reasons, and despite the controversies concerning the use of prophylactic antibiotics, even for the prevention of bacterial endocarditis, antibiotic prophylaxis is still suggested for HHT patients with AVMs prior to dental procedures (12).
E. faecalis is an infrequent causative agent of brain abscess. This organism is usually a nosocomial pathogen, and its presence in the central nervous system (CNS), rarely if ever as a cause of meningitis, has been associated with anatomic defects, prior neurosurgery and trauma, or high-grade bacteremia and immunodeficiency (8). Common portals of entry for enterococcal bacteremia are the urinary tract and sites of intra-abdominal and pelvic sepsis (4, 8). However, recently, literature provided evidence that due to its ability to produce biofilms, E. faecalis is often involved in endodontic infections (4), rendering sites of dental work a potential portal of enterococcal bacteremia.
Our patient's brain abscess could have been secondary to a dental procedure for the following reasons. The patient had untreated AMVs. She was subjected to an interventional dental procedure 10 days before admission without having received antibiotic prophylaxis. E. faecalis, which is considered to be a causative agent of endodontic infections, was isolated from the abscess pus while no other site of enterococcal entry was apparent. A literature review revealed three cases of brain abscess involving pus from which E. faecalis was isolated (1, 6, 14). However, in each case, a predisposing factor, such as prior neurosurgery (1), enterococcal bacteremia due to aspiration pneumonia (6), or urinary tract infection (14), was present. To the best of our knowledge, this is the first report of a brain abscess due to E. faecalis in an HHT patient without any site of enterococcal entry apart from that of a dental procedure.
In conclusion, our patient's case is both unique and instructive. It identifies interventional dental work as a potential source of E. faecalis seeding, highlighting the fundamental role of E. faecalis as a cause of endodontal infections. Also, it demonstrates that antibiotic prophylaxis seems to be necessary for all bacteremia-causing procedures in patients with HHT and AMVs.
Footnotes
Published ahead of print 15 February 2012
REFERENCES
- 1. Cano P, Horseman MA, Surani S. 2002. Rhinocerebral mucormycosis complicated by bacterial brain abscess. Am. J. Med. Sci. 34:507–510 [DOI] [PubMed] [Google Scholar]
- 2. Cirulli A, et al. 2006. Patients with hereditary hemorrhagic telangectasia (HHT) exhibit a deficit of poly-morphonuclear cell and monocyte oxidative burst and phago-cytosis: a possible correlation with altered adaptive immune responsiveness in HHT. Curr. Pharm. Des. 12:1209–1215 [DOI] [PubMed] [Google Scholar]
- 3. Cottin V, et al. 2004. Pulmonary arteriovenous malformations in patients with hereditary haemorrhagic telangiectasia. Am. J. Respir. Crit. Care Med. 169:994–1000 [DOI] [PubMed] [Google Scholar]
- 4. Fisher K, Phillips C. 2009. The ecology, epidemiology and virulence of Enterococcus. Microbiology 155:1749–1757 [DOI] [PubMed] [Google Scholar]
- 5. Hall WA. 1994. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu Disease) presenting with polymicrobial brain abscess. J. Neurosurg. 81:294–296 [DOI] [PubMed] [Google Scholar]
- 6. Inamasu J, et al. 2002. Brain abscess developing at the site of preceding intracerebral hemorrhage. J. Neurol. 249:221–223 [DOI] [PubMed] [Google Scholar]
- 7. Mathisen GE, Johnson JP. 1997. Brain abscess. State-of-the-art. Clinical article. Clin. Infect. Dis. 25:763–781 [DOI] [PubMed] [Google Scholar]
- 8. Moellering RC., Jr 2010. Enterococcus species, Enterocuccus bovis and Leuconstoc species, p 2411–2420 In Mandel GL, Bennett JE, Dolin R. (ed), Principles and practice of infectious diseases, 7th ed Elsevier, Philadelphia, PA [Google Scholar]
- 9. Sell B, Evans J, Horn D. 2008. Brain abscess and hereditary hemorrhagic telangiectasia. South Med. J. 101:618–625 [DOI] [PubMed] [Google Scholar]
- 10. Shovlin CL, et al. 2000. Diagnostic criteria for hereditary haemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am. J. Med. Genet. 91:66–67 [DOI] [PubMed] [Google Scholar]
- 11. Shovlin CL, et al. 2008. Primary determinants of ischaemic stroke/ brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax 63:259–260 [DOI] [PubMed] [Google Scholar]
- 12. Shovlin CL, Bamford K, Wray D. 2008. Post-NICE 2008: antibiotic prophylaxis prior to dental procedures for patients with pulmonary arteriovenous malformations (PAMVs) and hereditary haemorrhagic telangiectasia. Br. Dent. J. 205:531–533 [DOI] [PubMed] [Google Scholar]
- 13. Shovlin CL. 2010. Hereditary haemorrhagic telangiectasia: pathophysiology, diagnosis and treatment. Blood Rev. 24:203–219 [DOI] [PubMed] [Google Scholar]
- 14. Siatouni A, et al. 2007. Brain abscess following intracerebral haemorrhage. J. Clin. Neurosci. 14:986–989 [DOI] [PubMed] [Google Scholar]