Abstract
The usefulness of 5-cyano-2,3-ditolyl-tetrazolium chloride (CTC) staining to determine the respiratory activity of Acanthamoeba was evaluated in this study. Acanthamoeba trophozoites and cysts have a red fluorescence after staining with CTC. To determine the effectiveness of CTC staining as a CTC biocidal assay for Acanthamoeba, the trophozoites and cysts of Acanthamoeba castellanii (ATCC 5037) were treated with serial concentrations of disinfectant solutions, namely, polyhexamethylene biguanide (PHMB) and commercial soft contact lens (SCL) disinfectant solutions. The treated Acanthamoeba organisms were stained with CTC, and their respiratory activity was determined by the intensity of fluorescence in a fluorescence microplate reader. The survival rates of the same samples were determined by a culture-dependent biocidal assay using the Spearman-Karber method. Our results showed that the respiratory activities determined by the CTC biocidal assay and the survival rates determined by the culture-dependent biocidal assay for Acanthamoeba trophozoites and cysts decreased in a dose-dependent way after PHMB treatments, and the results were significantly correlated (r = 0.83 and P < 0.01 for trophozoites; r = 0.60 and P < 0.01 for cysts; Spearman rank correlation test). The respiratory activities in the trophozoites and cysts treated with SCL disinfectant solutions were significantly correlated with the survival rate (r = 0.70 and P < 0.01 for trophozoites; r = 0.64 and P < 0.01 for cysts; Spearman rank correlation test). The significant correlation of the results indicated that the CTC biocidal assay can be used as an alternative method to a culture-dependent biocidal assay. The CTC biocidal assay is a rapid and simple method to test the effectiveness of disinfectant solutions against Acanthamoeba trophozoites and cysts.
INTRODUCTION
Acanthamoeba keratitis (AK) is painful and potentially blinding (22). In recent years, AK has been associated with contact lens-related corneal diseases (29). The recent increase in the incidence of AK has been attributed to several factors, including the rising number of soft contact lens (SCL) wearers and the widespread noncompliance with the cleaning and rinsing regimens for SCLs (9, 13, 17, 19). In addition, the use of SCL disinfectant solutions that are not effective is also suspected to be linked to the increase in cases of AK (6).
The situation that SCL disinfectants may not be effective against Acanthamoeba has arisen partially because there is no standardized method to evaluate the effectiveness of lens care disinfectants against Acanthamoeba. The International Organization for Standardization (ISO) has adopted the Stand Alone test (ISO 14729), a standard method for testing the disinfectant efficacy of lens care products. However, this method does not include a protocol specifically for Acanthamoeba. Thus, a standard method for testing the disinfecting efficacy of lens care products against Acanthamoeba is needed to determine the effectiveness of new disinfectant lens care products against Acanthamoeba.
Traditionally, culture-dependent methods have been used to evaluate the effectiveness of various disinfectants against Acanthamoeba (1, 2, 11, 12, 15, 20, 24). Among these, culture-dependent biocidal assays using the most-probable-number (MPN) method or the Spearman-Karber method have been considered suitable methods to quantify the number of living organisms (1, 18, 24). Although conventional culture-dependent methods have been shown to be reliable for detecting surviving organisms after exposure to disinfectants, the requirement of long-term cultivation may be limiting for the development of new disinfectants (1, 11, 18, 24). In fact, the previously reported culture-dependent biocidal assay requires 1 week for trophozoites and 3 weeks for cysts to be detected (18). Therefore, a rapid method to test the efficacy of disinfectant solutions would be useful for laboratory investigations, especially for testing the efficacy of new disinfectants.
5-Cyano-2,3-ditolyl-tetrazolium chloride (CTC) is a redox dye that is widely used to determine the respiratory activity of bacteria (27, 30). CTC is a soluble crystal that forms a nearly colorless nonfluorescent solution. In the electron transport system, tetrazolium salts function as artificial redox partners instead of the final electron acceptor, oxygen (7). Respiring bacteria placed in CTC solution will take up the CTC and reduce it to insoluble formazan (CTC formazan), which accumulates in the cells. On the other hand, dead or inactive cells show no accumulation of CTC formazan (26, 27). Because the dye competes with the terminal electron acceptor, it will eventually poison the cells once the reduction processes are completed. Therefore, CTC staining represents an index of the respiratory activity of the cell at the time of observation (14). CTC formazan emits a red fluorescence (emission peak, 630 nm) when excited by a blue light (peak, 480 nm). Thus, it is possible to distinguish fluorescence-labeled respiring active cells from inactive cells by fluorescence microscopy. It has been reported that the bacterial respiratory activity assessed by CTC staining is well correlated with bacterial viability units such as CFU (7, 25).
However, there have been few reports on the application of CTC staining for protozoans (14), and it has not been used for Acanthamoeba spp. Thus, the purpose of this study was to determine whether CTC staining can be used for rapid biocidal assay of Acanthamoeba. To accomplish this, we first investigated whether it is possible to determine the respiratory activity of Acanthamoeba by CTC staining. A biocidal assay for Acanthamoeba with CTC staining was then performed, and the respiratory activities obtained were compared to the survival rates determined by a culture method using the Spearman-Karber method.
MATERIALS AND METHODS
Acanthamoeba trophozoites and cysts.
We used Acanthamoeba castellanii (ATCC 50370) for this study. Trophozoites were cultured in peptone-yeast extract-glucose (PYG) medium (ATCC medium 712) in tissue culture flasks (Becton Dickinson, Tokyo, Japan) at 25°C. Encystment was induced by transferring the trophozoites from PYG medium to Neff's constant-pH encystment medium (23) and incubating the trophozoites for at least 2 weeks at 25°C. All procedures involving the organisms were carried out in biosafety level 2 laboratories.
CTC staining.
Acanthamoeba trophozoites were collected from the solutions in the flasks by centrifugation. Centrifugation was carried out for 10 min at 150 × g throughout the experiments for both trophozoites and cysts. The trophozoites were counted with a hemocytometer under a phase-contrast microscope, and they were suspended in phosphate-buffered saline (PBS) at 2 × 106 trophozoites in 1.8 ml of PBS. The amoeba suspension was divided into two portions (900 μl each), and 100 μl of H2O was added to one portion and 100 μl of sodium azide solution (20 mg/ml) was added to the other portion. The organisms that had their respiration inhibited by sodium azide were used as negative controls (14). CTC staining was performed on each portion by use of a Bacstain-CTC rapid staining kit (Dojindo Laboratories, Kumamoto, Japan) for 30 min at 25°C according to the manufacturer's instructions. After staining, the samples were fixed by adding 1 ml of paraformaldehyde (4% in PBS) for 30 min at 4°C.
Acanthamoeba cysts were collected from the solutions in the flasks by centrifugation and then counted with a hemocytometer under a phase-contrast microscope. They were then suspended in 10 ml of PYG medium (2 × 106 cysts in 10 ml of PYG medium) and preincubated for 16 h at 25°C to restore the respiratory activity of the organisms. After preincubation, the cysts were collected by centrifugation, stained by CTC with or without sodium azide, and fixed as described above.
The fixed trophozoites and cysts were collected by centrifugation and suspended in 1 ml of PBS. Two hundred microliters of the amoeba suspension was transferred to each well of 96-well glass-bottom plates (Asahi Techno Glass, Chiba, Japan) and examined by fluorescence microscopy (excitation wavelength, 480 nm; emission wavelength, 630 nm).
Disinfectant treatments for Acanthamoeba.
Polyhexamethylene biguanide (PHMB) was diluted with one-quarter-strength (1/4) Ringer's solution (Nihon Pharmaceutical Co., Ltd., Tokyo, Japan) to final concentrations of 0.1, 0.2, 0.5, 1, 2, 5, and 10 ppm for trophozoites and 1, 10, 100, and 300 ppm for cysts. Commercial SCL disinfectant solutions used were Complete Double Moist (AMO, Inc.) (MPS1), Optifree Plus (Alcon Japan, Ltd.) (MPS2), Renu Fresh (Bausch and Lomb Japan Company, Ltd.) (MPS3), Bioclen First Care, and CT (Ophtecs Corp.) (povidone iodine solution). MPS1 contained 1.0 ppm PHMB as the disinfectant. MPS3 contained 1.1 ppm PHMB, and MPS2 contained 11 ppm polydronium chloride (Polyquad). The povidone iodine solution was made by adding the attached disinfecting neutralizing tablet to the solution at the onset of disinfection according to the manufacturer's instructions.
The trophozoites or cysts were collected from the solutions in the flasks, and after centrifugation, the organisms were suspended in 1/4 Ringer's solution at a concentration of 5 × 106 organisms/ml. A 400-μl sample of the amoeba suspension was added to 40 ml of each disinfectant solution, to a final concentration of 5 × 104 organisms/ml. Control samples were also prepared in 1/4 Ringer's solution. Subsequently, each sample was incubated at 25°C for 4 h in a 50-ml conical tube (Becton Dickinson). After exposure to the disinfectant, the amoebal respiratory activity was determined by the CTC biocidal assay and the survival rate was determined by the culture-dependent biocidal assay using the Spearman-Karber method.
CTC biocidal assay.
The Acanthamoeba trophozoites or cysts that were treated with the disinfectant solutions or 1/4 Ringer's solution (control) were collected by centrifugation and stained and fixed as described above. Organisms that were stained with CTC following fixation were collected by centrifugation and suspended in 1 ml of PBS. A 200-μl sample of the amoeba suspension was transferred to each well of a 96-well plate (Corning International Inc., Tokyo, Japan), and the fluorescence intensity was measured with a fluorescence microplate reader (FlexStation 3; Molecular Devices, Sunnyvale, CA) (excitation wavelength, 480 nm; emission wavelength, 630 nm). The samples that had sodium azide added to inhibit respiration were used as negative controls. To normalize the fluorescence intensity, the fluorescence intensity of the negative control was subtracted from the value of the test sample (14). Respiratory activity is presented as a percentage of the 1/4 Ringer's solution control level.
Culture-dependent biocidal assay using the Spearman-Karber method.
The culture-dependent biocidal assay using the Spearman-Karber method was performed as described in detail previously (18). Briefly, after exposure to disinfectant or 1/4 Ringer's solution, 100 μl of the test solution was mixed with 900 μl of Dey-Engley neutralizing broth (Sigma, St. Louis, MO) and with 10-fold serial dilutions of each test solution in PYG medium. This resulted in four dilutions, with theoretical maximum final concentrations of 5 × 103, 5 × 102, 5 × 101, and 5 × 100 amoeba/ml. Four 200-μl samples of each dilution were transferred to separate wells in a 96-well plate (Corning International Inc., Tokyo, Japan) and incubated at 25°C. Samples containing trophozoites were incubated for 1 week, while those containing cysts were incubated for 3 weeks. At the end of the incubation period, amoebal growth in the wells was confirmed using a phase-contrast microscope. The number of surviving organisms was counted for each test solution by using the Spearman-Karber equation as described previously (10). The survival rate is presented as a percentage of that in the 1/4 Ringer's solution control.
Statistical analysis.
The Spearman rank correlation coefficient was used to determine the relationship between respiratory activity (CTC biocidal assay) and survival rate (culture-dependent biocidal assay).
RESULTS
CTC staining.
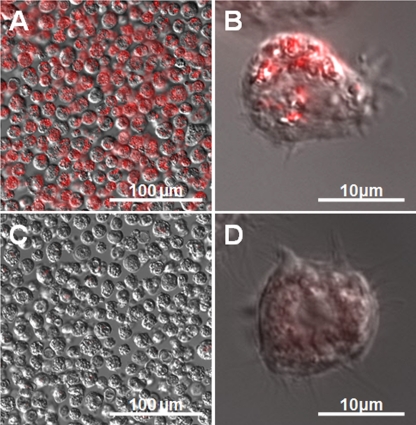
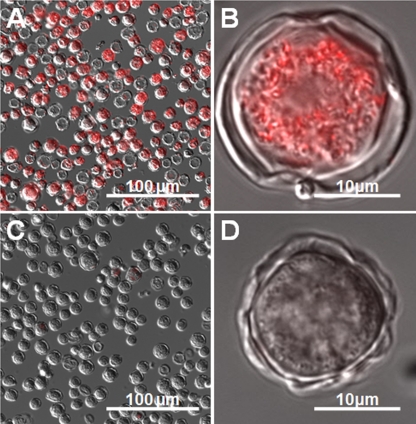
Our results showed that CTC formazan, which has a red fluorescence when excited by blue light, accumulated inside Acanthamoeba castellanii trophozoites (Fig. 1A and B) but not in trophozoites exposed to sodium azide (Fig. 1C and D). The accumulation of CTC formazan was also observed in Acanthamoeba cysts after 16 h of preincubation in PYG medium (Fig. 2A and B) but not in cysts exposed to sodium azide (Fig. 2C and D). On the other hand, no CTC formazan accumulation was observed in Acanthamoeba cysts without preincubation in PYG medium (data not shown), indicating that the respiratory activity of the dormant cysts was restored by 16 h of preincubation in PYG medium.
Fig 1.
Acanthamoeba castellanii trophozoites stained with CTC. Merged fluorescence images of red-fluorescing CTC formazan and differential interference contrast images of trophozoites stained with CTC are shown. (A) CTC formazan accumulates within trophozoites, as shown by red fluorescence. (B) Trophozoite from panel A at higher magnification. (C) Inhibition of respiration of trophozoites by addition of sodium azide. (D) Respiration-inhibited trophozoite from panel C at higher magnification.
Fig 2.
Acanthamoeba castellanii cysts stained with CTC. Merged fluorescence images of red-fluorescing CTC formazan and differential interference contrast images of cysts stained with CTC are shown. (A) CTC formazan accumulates within cysts, as shown by red fluorescence. (B) Cyst from panel A at higher magnification. (C) Inhibition of respiration of cysts by addition of sodium azide. (D) Respiration-inhibited cyst from panel C at higher magnification.
CTC biocidal assay.
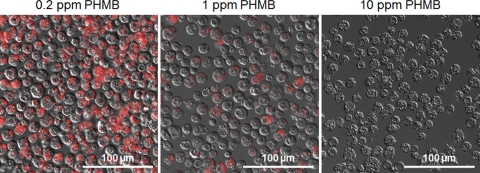
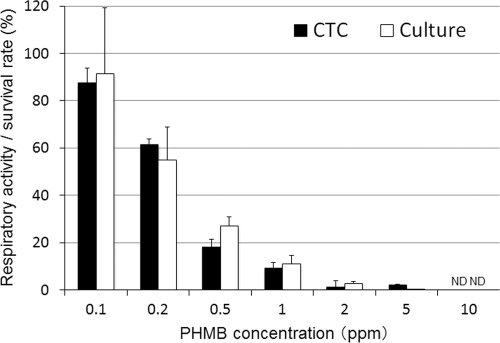
The trophozoites exposed to 0.2 ppm of PHMB appeared red by fluorescence, indicating that most of the trophozoites were respiring (Fig. 3). On the other hand, fluorescence was not observed in the trophozoites exposed to 10 ppm of PHMB, and only a weak fluorescence was detected in the trophozoites exposed to 1 ppm of PHMB (Fig. 3). The respiratory activity determined by the CTC biocidal assay and the survival rate determined by the culture-dependent biocidal assay for trophozoites treated with PHMB are shown in Fig. 4. The respiratory activities were 87.5%, 61.3%, 18.0%, 9.1%, 1.2%, 2.0%, and 0% of the control level and the survival rates were 91.4%, 54.9%, 27.0%, 11.1%, 2.7%, 0.02%, and 0% of the control level for samples treated with 0.1, 0.2, 0.5, 1, 2, 5, and 10 ppm PHMB, respectively. Thus, the respiratory activity (CTC biocidal assay) and the survival rate (culture-dependent biocidal assay) were reduced after PHMB treatment, in a dose-dependent manner, and a significant positive correlation between the respiratory activity and the survival rate was found for trophozoites treated with PHMB (r = 0.83 and P < 0.01; Spearman rank correlation test).
Fig 3.
CTC staining of PHMB-treated Acanthamoeba trophozoites. Merged fluorescence images of red-fluorescing CTC formazan and differential interference contrast images of trophozoites stained with CTC after PHMB treatments are shown. CTC staining was performed on trophozoites treated with PHMB (0.2, 1, or 10 ppm) for 4 h at 25°C.
Fig 4.
Respiratory activity and survival rate of Acanthamoeba trophozoites after PHMB treatment. The respiratory activity (CTC biocidal assay) was compared with the survival rate (culture-dependent biocidal assay) for trophozoites after treatment with PHMB (25°C, 4 h). Error bars represent the standard errors of the means for four experiments. ND, not detected (<1%).
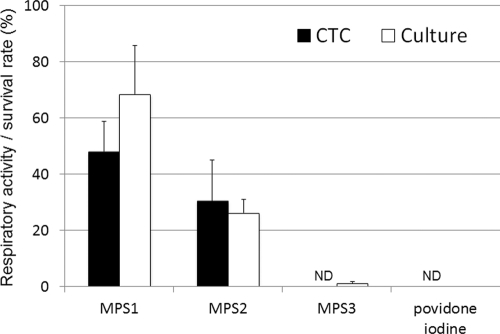
The amoebal respiratory activities and survival rates after a 4-h exposure to SCL disinfectant solutions are shown in Fig. 5. The respiratory activities of the trophozoites were 48.0%, 30.4%, 0%, and 0% of the control level and the survival rates were 68.5%, 26.2%, 1.1%, and 0.04% of the control level for the samples treated with MPS1, MPS2, MPS3, and povidone iodine solution, respectively. There was a significant positive correlation between the respiratory activity (CTC biocidal assay) and the survival rate (culture-dependent biocidal assay) for the trophozoites treated with SCL disinfectant solutions (r = 0.70 and P < 0.01; Spearman rank correlation test).
Fig 5.
Respiratory activity and survival rate of Acanthamoeba trophozoites after treatment with SCL disinfectant solutions. The respiratory activity (CTC biocidal assay) was compared with the survival rate (culture-dependent biocidal assay) for trophozoites after treatment with SCL disinfectant solutions (25°C, 4 h). Error bars represent the standard errors of the means for four experiments. ND, not detected (<1%).
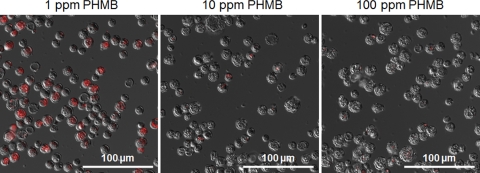
The fluorescence signals from cysts that were stained with CTC after the PHMB treatments were decreased in proportion to the PHMB concentration (Fig. 6). The cysts exposed to 1 ppm of PHMB appeared red by fluorescence, indicating that most of the cysts maintained their respiratory activity. A weak fluorescence was observed after exposure of cysts to 10 ppm or 100 ppm PHMB, although this was partially due to autofluorescence of the cysts.
Fig 6.
CTC staining of PHMB-treated Acanthamoeba cysts. Merged fluorescence images of red-fluorescing CTC formazan and differential interference contrast images of cysts stained with CTC after PHMB treatments are shown. CTC staining was performed on cysts treated with PHMB (1, 10, or 100 ppm) for 4 h at 25°C.
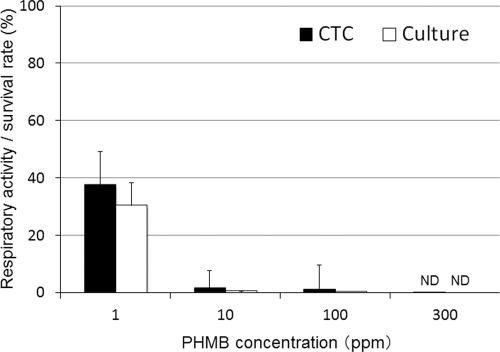
The respiratory activities and survival rates of the cysts after the PHMB treatments (4 h) are shown in Fig. 7. The respiratory activities were 37.6%, 1.5%, 1%, and 0% of the control level and the survival rates were 30.3%, 0.4%, 0.01%, and 0% of the control level for cysts treated with 1, 10, 100, and 300 ppm of PHMB, respectively. Thus, the respiratory activity (CTC biocidal assay) and survival rate (culture-dependent biocidal assay) were reduced after PHMB treatment, in a dose-dependent manner. A significant positive correlation between the respiratory activity and the survival rate was present for the cysts treated with PHMB (r = 0.60 and P < 0.01; Spearman rank correlation test).
Fig 7.
Respiratory activity and survival rate of Acanthamoeba cysts after PHMB treatment. The respiratory activity (CTC biocidal assay) was compared with the survival rate (culture-dependent biocidal assay) for cysts after treatment with PHMB (25°C, 4 h). Error bars represent the standard errors of the means for four experiments. ND, not detected (<1%).
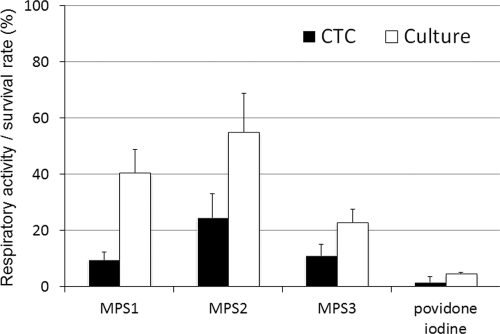
The respiratory activities and survival rates of the cysts after a 4-h exposure to SCL disinfectant solutions are shown in Fig. 8. The respiratory activities were 9.8%, 24.4%, 11.0%, and 1.5% of the control level and the survival rates were 40.5%, 54.9%, 22.8%, and 4.4% of the control level for the cysts treated with MPS1, MPS2, MPS3, and povidone iodine solution, respectively. There was a significant positive correlation between the respiratory activity (CTC biocidal assay) and the survival rate (culture-dependent biocidal assay) for cysts treated with SCL disinfectant solutions (r = 0.64 and P < 0.01; Spearman rank correlation test).
Fig 8.
Respiratory activity and survival rate of Acanthamoeba cysts after treatment with SCL disinfectant solutions. The respiratory activity (CTC biocidal assay) was compared with the survival rate (culture-dependent biocidal assay) for cysts after treatment with SCL disinfectant solutions (25°C, 4 h). Error bars represent the standard errors of the means for four experiments.
DISCUSSION
Our CTC staining results showed that the red fluorescent compound formazan, which represents the respiratory activity of Acanthamoeba organisms, accumulated in trophozoites and cysts. The CTC biocidal assay demonstrated clearly that the respiratory activity of Acanthamoeba trophozoites and cysts was decreased in a dose-dependent way after exposure to PHMB.
Conventional culture-dependent methods have been used to evaluate the effectiveness of various disinfectants against Acanthamoeba (1, 2, 11, 12, 15, 20, 24). Among these, methods using the MPN method or the Spearman-Karber method have been considered suitable methods to quantify the number of living organisms (1, 18, 24) because they are simple, reliable, and reproducible for standardized efficacy tests (1, 18).
Our results showed that the respiratory activity determined by the CTC biocidal assay was significantly correlated with the survival rate determined by culture-dependent biocidal assay for both trophozoites and cysts treated with PHMB and SCL disinfectants. These results indicate that the respiratory activities determined by the CTC biocidal assay are strongly correlated with the number of living Acanthamoeba organisms. It has been well documented that CTC is a good estimator of bacterial viability, and thus the CTC biocidal assay can be used as an alternative method to conventional culture-dependent methods to assay the number of living Acanthamoeba organisms.
Although the culture-dependent biocidal assay has been accepted as an efficient assay to test the disinfecting properties of anti-Acanthamoeba solutions, detection of surviving Acanthamoeba organisms requires 1 to 3 weeks of cultivation for trophozoites and cysts, respectively. On the other hand, the CTC biocidal assay requires only about 2 h for Acanthamoeba trophozoites and 18 h for cysts. The CTC biocidal assay requires 30 min for staining and 30 min for fixation following the disinfectant treatment, and it also requires 16 h of preincubation before CTC staining for cysts. In addition, by using a fluorescence microplate reader to measure the fluorescence intensity of CTC-stained samples, we were able to analyze multiple samples in a very short time. Although a good correlation was found between the two assay methods within the range of about 2 log units (>1%), the range of sensitivity of the CTC biocidal assay is about 2 log units, while the range of sensitivity of the culture-dependent method is 3 log units or more. Therefore, the CTC biocidal assay can be used for rapid testing and screening of new disinfectants, and the culture method might be necessary to confirm the final results.
However, unstained Acanthamoeba organisms also have weak autofluorescence, and the autofluorescence levels varied after either trophozoites or cysts were treated with different disinfectants (data not shown). To overcome this problem, the samples that had sodium azide added to inhibit respiration were used as negative controls. Sodium azide is known to inhibit the respiratory activity of bacteria (28), and it has been reported that exposure to sodium azide (2 mg/ml) inhibits CTC reduction by protozoa, while sodium azide does not affect autofluorescence (14). Thus, to normalize the fluorescence intensity, the fluorescence intensity of the negative control was subtracted from the value of the test sample.
The efficacies of other rapid staining methods for detecting living or dead Acanthamoeba organisms have been examined (3, 16). Propidium iodide (PI) penetrates cells with damaged membranes and binds to DNA, and it also stains dead cells (21). Although its effectiveness has been correlated with that of methylene blue staining (3), a significant correlation has never been reported because of the difficulty in estimating the number of living cells by staining dead cells. Fluorescein diacetate (FDA), on the other hand, is hydrolyzed by intracellular esterases and stains live cells (8). However, it also stains dead cells because of the presence of residual esterase activity (4, 5). Thus, this method overestimates the number of living amoebae (16). These shortcomings are overcome by the CTC biocidal assay.
There is a concern about the preincubation step for Acanthamoeba cysts to restore respiratory activity. In our preliminary experiments, no fluorescence signal of CTC formazan was detected in the cysts after CTC staining because the cysts were dormant and may have had little or no respiratory activity. CTC formazan accumulation was observed in most of the cysts after 16 h of preincubation, indicating that 16 h of preincubation was necessary to restore the respiratory activity of Acanthamoeba cysts. In addition, the 16-h preincubation period did not lead to any proliferation of Acanthamoeba. Thus, 16 h of preincubation is an appropriate duration for Acanthamoeba cysts in the CTC biocidal assay. The strong correlation between the results of the CTC biocidal assay and those of the culture-dependent biocidal assay suggests that the results determined by CTC biocidal assay most likely represent the number of living Acanthamoeba cysts, although the results may not reflect the exact respiratory activity of Acanthamoeba cysts.
The respiratory activities of cysts treated with SCL disinfectant solutions tended to be lower than the survival rates. This suggests that the recovery of respiratory activity is delayed in living cysts. A difference in encystment rates between different SCL disinfectant solutions has been described because of the different ingredients in SCL disinfectant solutions (20). Thus, our results suggest that the ingredients in SCL disinfectant solutions may affect the recovery of respiratory activity in cysts, and the preincubation time may be different for each SCL disinfectant solution. A further optimization of the preincubation time might be necessary for each SCL disinfectant solution for CTC biocidal assay for cysts.
In conclusion, CTC staining can be used to detect the respiratory activity of Acanthamoeba trophozoites and cysts, and the CTC biocidal assay can be a rapid and simple method to assay the effectiveness of a disinfectant agent against Acanthamoeba.
ACKNOWLEDGMENTS
This study was supported by a grant from the Ministry of Health, Labor and Welfare, Japan.
Footnotes
Published ahead of print 15 February 2012
REFERENCES
- 1. Beattie TK, Seal DV, Tomlinson A, McFadyen AK, Grimason AM. 2003. Determination of amoebicidal activities of multipurpose contact lens solutions by using a most probable number enumeration technique. J. Clin. Microbiol. 41:2992–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borazjani RN, Kilvington S. 2005. Efficacy of multipurpose solutions against Acanthamoeba species. Cont. Lens Anterior Eye 28:169–175 [DOI] [PubMed] [Google Scholar]
- 3. Borazjani RN, May LL, Noble JA, Avery SV, Ahearn DG. 2000. Flow cytometry for determination of the efficacy of contact lens disinfecting solutions against Acanthamoeba spp. Appl. Environ. Microbiol. 66:1057–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Breeuwer P, Drocourt JL, Rombouts FM, Abee T. 1994. Energy-dependent, carrier-mediated extrusion of carboxyfluorescein from Saccharomyces cerevisiae allows rapid assessment of cell viability by flow cytometry. Appl. Environ. Microbiol. 60:1467–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Catala P, et al. 1999. Effectiveness of CSE to counterstain particles and dead bacterial cells with permeabilised membranes: application to viability assessment in waters. FEMS Microbiol. Lett. 178:219–226 [DOI] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention 2007. Acanthamoeba keratitis multiple states, 2005–2007. MMWR Morb. Mortal. Wkly. Rep. 56:532–534 [PubMed] [Google Scholar]
- 7. Creach V, Baudoux AC, Bertru G, Rouzic BL. 2003. Direct estimate of active bacteria: CTC use and limitations. J. Microbiol. Methods 52:19–28 [DOI] [PubMed] [Google Scholar]
- 8. Diaper JP, Edwards C. 1994. Survival of Staphylococcus aureus in lakewater monitored by flow cytometry. Microbiology 140:35–42 [DOI] [PubMed] [Google Scholar]
- 9. Gray TB, Cursons RT, Sherwan JF, Rose PR. 1995. Acanthamoeba, bacterial, and fungal contamination of contact lens storage cases. Br. J. Ophthalmol. 79:601–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamilton M, Russo R, Thurston R. 1977. Trimmed Speaman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 11:714–719 [Google Scholar]
- 11. Hiti K, Walochnik J, Haller-Schober EM, Faschinger C, Aspock H. 2002. Viability of Acanthamoeba after exposure to a multipurpose disinfecting contact lens solution and two hydrogen peroxide systems. Br. J. Ophthalmol. 86:144–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hugo ER, McLaughlin WR, OH KH, Tuovinen OH. 1991. Quantitative enumeration of Acanthamoeba for evaluation of cyst inactivation in contact lens care solutions. Invest. Ophthalmol. Vis. Sci. 32:655–657 [PubMed] [Google Scholar]
- 13. Illingworth CD, Cook SD. 1998. Acanthamoeba keratitis. Surv. Ophthalmol. 42:493–508 [DOI] [PubMed] [Google Scholar]
- 14. Iturriaga R, Zhang S, Sonek GJ, Stibbs H. 2001. Detection of respiratory enzyme activity in Giardia cysts and Cryptosporidium oocysts using redox dyes and immunofluorescence techniques. J. Microbiol. Methods 46:19–28 [DOI] [PubMed] [Google Scholar]
- 15. Johnston SP, et al. 2009. Resistance of Acanthamoeba cysts to disinfection in multiple contact lens solutions. J. Clin. Microbiol. 47:2040–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khunkitti W, Avery SV, Lloyd D, Furr JR, Russell AD. 1997. Effects of biocides on Acanthamoeba castellanii as measured by flow cytometry and plaque assay. J. Antimicrob. Chemother. 40:227–233 [DOI] [PubMed] [Google Scholar]
- 17. Kilvington S, et al. 2004. Acanthamoeba keratitis: the role of domestic tap water contamination in the United Kingdom. Invest. Ophthalmol. Vis. Sci. 45:165–169 [DOI] [PubMed] [Google Scholar]
- 18. Kobayashi T, et al. 2011. Efficacy of commercial soft contact lens disinfectant solutions against Acanthamoeba. Jpn. J. Ophthalmol. 55:547–557 [DOI] [PubMed] [Google Scholar]
- 19. Larkin DF, Kilvington S, Easty DL. 1990. Contamination of contact lens storage cases by Acanthamoeba and bacteria. Br. J. Ophthalmol. 74:133–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lonnen J, Heaselgrave W, Nomachi M, Mori O, Santodomingo-Rubido J. 2010. Disinfection efficacy and encystment rate of soft contact lens multipurpose solutions against Acanthamoeba. Eye Contact Lens 36:26–32 [DOI] [PubMed] [Google Scholar]
- 21. Lopez-Amoros R, Comas J, Vives-Rego J. 1995. Flow cytometric assessment of Escherichia coli and Salmonella typhimurium starvation-survival in seawater using rhodamine 123, propidium iodide, and oxonol. Appl. Environ. Microbiol. 61:2521–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marciano-Cabral F, Cabral G. 2003. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16:273–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Neff R, Ray S, Benton W, Wilborn M. 1964. Induction of synchronous encystment (differentiation) in Acanthamoeba sp. Methods Cell Physiol. 1:55–83 [Google Scholar]
- 24. Perrine D, et al. 1995. Amoebicidal efficiencies of various diamidines against two strains of Acanthamoeba polyphaga. Antimicrob. Agents Chemother. 39:339–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pyle BH, Broadaway SC, McFeters GA. 1995. Factors affecting the determination of respiratory activity on the basis of cyanoditolyl tetrazolium chloride reduction with membrane filtration. Appl. Environ. Microbiol. 61:4304–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodriguez GG, Phipps D, Ishiguro K, Ridgway HF. 1992. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl. Environ. Microbiol. 58:1801–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith JJ, Howington JP, McFeters GA. 1994. Survival, physiological response and recovery of enteric bacteria exposed to a polar marine environment. Appl. Environ. Microbiol. 60:2977–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith JJ, McFeters GA. 1997. Mechanisms of INT (2-(4-iodo-phenyl)-3-(4-nitrophenyl)-5-phenyl tetrazolium chloride), and CTC (5-cyano-2,3-ditolyl-tetrazolium chloride) reduction in Escherichia coli K-12. J. Microbiol. Methods 29:161–175 [DOI] [PubMed] [Google Scholar]
- 29. Thebpatiphat N, et al. 2007. Acanthamoeba keratitis: a parasite on the rise. Cornea 26:701–706 [DOI] [PubMed] [Google Scholar]
- 30. Winding A, Binnerup SJ, Sorensen J. 1994. Viability of indigenous soil bacteria assayed by respiratory activity and growth. Appl. Environ. Microbiol. 60:2869–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]