Abstract
The TaqMan real-time PCR assay was developed from the Blastomyces dermatitidis BAD1 gene promoter. The assay identified all haplotypes of B. dermatitidis and five of six positive paraffin-embedded tissues. The assay sensitivity threshold was 1 pg genomic DNA of the mold form and 2 CFU of the yeast form of B. dermatitidis. No cross-reactivity was observed against other fungal DNA. The assay allowed rapid (5-h) identification of B. dermatitidis from culture and from clinical specimens.
TEXT
Blastomyces dermatitidis is a dimorphic fungal pathogen that causes blastomycosis. The infection generally starts by inhalation of spores of the mold form of the fungus, found in the environment. Upon entry into the hosts, the spores convert to the yeast form. The infection can be self-limiting to the lungs, or it can disseminate to other body parts, mainly to bones and skin. Apart from humans, dogs are highly susceptible to B. dermatitidis infection. Blastomycosis is endemic in the midwestern and southeastern United States and around the Great Lakes (4). Interestingly, blastomycosis has also been reported in humans and dogs in New York State (6, 8), but the ecological niche of this fungus has yet to be established in this region. Similarly, blastomycosis has been reported from Colorado and Nebraska, which are all outside known zones where the infection is endemic (7, 13).
The laboratory methods most frequently used to diagnose blastomycosis include serology, direct smear, and histopathology. However, the gold standard for diagnosis remains a positive culture. Traditional confirmation of a suspect culture of B. dermatitidis by conversion to the yeast form in the laboratory can take weeks, but identification can be confirmed on the same day by Gen-Probe (Gen-Probe, Inc., San Diego, CA). The Gen-Probe test can be used only with pure cultures of B. dermatitidis (yeast or mold); hence, it has limited application. To overcome these problems, several conventional PCR assays have been developed for the identification of B. dermatitidis from clinical specimens and soil samples (2, 5). These assays used identical primer pair sequences targeting the putative promoter region of the BAD1 (earlier known as WI-1) gene, which codes for an important adhesin molecule and virulence factor (10). Recently, it has been shown that the BAD1 promoter region has a number of nucleotide polymorphisms, which resulted in the identification of four haplotypes of B. dermatitidis (11, 12). In addition to nucleotide polymorphism, a major size disparity due to two large insertions in the BAD1 promoter was also found in many B. dermatitidis strains (12). This can complicate the conventional PCR assay due to either insufficient amplification efficiency or misinterpretation as a nonspecific product.
In this study, we describe the development of a TaqMan real-time PCR assay using a specific region of the BAD1 promoter to encompass all known haplotypes of B. dermatitidis. Our results indicate that the BAD1 real-time PCR assay is highly specific and rapid, with a turnaround time of approximately 5 h for the identification of B. dermatitidis from culture and from paraffin-embedded tissues.
A total of 24 strains of B. dermatitidis, 12 formalin-fixed paraffin-embedded (FFPE) tissues, and DNA from 31 other fungal species and 5 bacterial species were part of this investigation. Blastomyces dermatitidis cultures were grown in yeast extract peptone dextrose agar (YPD) for 5 to 7 days at 30°C. Two of the B. dermatitidis strains (M799 and M808) were also converted to the yeast form on cottonseed agar at 37°C. The paraffin-embedded tissues were from different patients and were processed in the years 2002 to 2008. The tissue types included lung, lymph node, testicle, skin, skull tumor, heart, and kidney.
Extraction of the mold form B. dermatitidis DNA was carried out in the biological safety cabinet (BSC) in the biological safety level III (BSL III) laboratory, while extraction of the yeast form B. dermatitidis DNA was carried out in the BSC in the BSL II laboratory. Mold form DNA extraction was done using the chloroform-phenol extraction method as described previously (3). Briefly, 0.5 cm mycelial mat was added to 500 μl of lysis buffer (10 mM Tris-HCl, pH 7.5, 1% SDS, 100 mM EDTA, 2% Triton X-100, and 100 mM NaCl) and 0.2 g of acid-washed glass beads (425 to 600 μm; Sigma). Samples were incubated at 70°C for 1 h and then vortexed for 30 min. Supernatants were extracted with phenol-chloroform-isoamyl alcohol (24:24:1) followed by an extraction with chloroform. The DNA from the aqueous phase was precipitated with 100% ethanol, washed with 70% ethanol, air dried, and finally resuspended in 10 mM Tris, pH 8.0. Extraction of yeast form DNA was carried out by using the MasterPure DNA/RNA extraction kit (Epicenter, Madison, WI) with a slight modification. Serially diluted yeast form cultures of B. dermatitidis were first incubated in lysis buffer containing approximately 0.2 g of glass beads for 1 h at 70°C, followed by disruption in the cell disrupter for 30 min. DNA yield and purity were determined by spectrophotometer. The yeast suspensions were also plated on YPD agar in duplicate and incubated at 30°C for 5 to 7 days to determine the CFU counts. DNA extractions from 12 paraffin-fixed tissues were carried out using both Epicenter and QIAamp DNA FFPE tissue (Qiagen, Valencia, CA) kits per the manufacturers' instructions, with minor modifications. In brief, tissues were trimmed of paraffin excess and cut into approximately 3-μm-thick sections with a sterile blade; four or five sections from each tissue were first treated with xylene; after tissue lysis, glass beads were added; and the mixture was disrupted in a cell disrupter for 30 min, followed by DNA extraction using reagents provided in the kit.
DNA from fungal species was extracted using the method described above for the mold form B. dermatitidis. The DNA from fungal species included Coccidioides immitis, C. posadasii, Chrysosporium spp., Histoplasma capsulatum, a Malbranchea sp., Paracoccidioides brasiliensis, Sporothrix schenckii, Alternaria alternata, Aspergillus flavus, A. fumigatus, A. versicolor, Epidermophyton floccosum, Fusarium oxysporum, Geomyces pannorum, Microsporum gypseum, Penicillium verrucosum, Trichophyton rubrum, T. mentagrophytes, Candida albicans, C. glabrata, C. inconspicua, C. krusei, C. lusitaniae, C. parapsilosis, C. tropicalis, Cryptococcus albidus, C. laurentii, C. neoformans var. grubii, C. neoformans var. neoformans, C. gattii, and Saccharomyces cerevisiae. DNA from bacterial species included Bacillus megaterium, Escherichia coli, Nocardia farcinica, Pseudomonas aeruginosa, and Streptococcus pneumoniae.
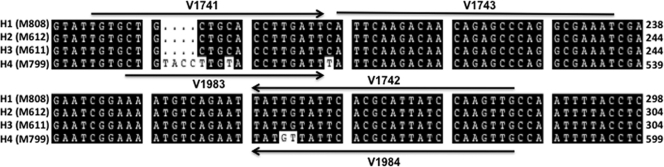
Primers for conventional PCR V1642 (forward) 5′-AAGTGGCTGGGTAGTTATACGCTAC-3′ and V1643 (reverse) 5′-TAGGTTGCTGATTCCATAAGTCAGG-3′ were designed from the BAD1 gene promoter as described previously (5). Primers and probe for the real-time PCR assay were designed from the promoter region of the BAD1 gene using Primer Express software, version 3.0 (Applied Biosystems, Foster City, CA). The choice of BAD1 promoter was based upon an earlier finding that this region is unique to B. dermatitidis by conventional PCR (5). In order to encompass all of the known haplotypes of B. dermatitidis (9), two forward and two reverse primers were designed (see Fig. 2). Oligonucleotide sequences of the primers and probe are as follows: V1741 (forward), 5′-TGTGCTGCTGCACCTTGATT-3′; V1983 (forward) 5′-CTGTACCTTGTACCTTGATT-3′; V1742 (reverse), 5′-CAACTTGGATAATGCGTGAATACAATA-3′; V1984 (reverse), 5′CAACTTGGATAATGCGTGAATAACATA-3′; and probe V1743, 5′-/56JOEN/ATTCAAGACAACAGAGCCCAGGCG AAAT-black hole quencher 1-3′.
Fig 2.
Primer and probe design from the BAD1 promoter region. Multiple alignments of BAD1 promoter sequences of B. dermatitidis strains comprising four haplotypes revealed a number of nucleotide deletions and mutations. A representative sequence is shown for each of the four B. dermatitidis haplotypes (H1 to H4). This information was used for the design of two forward (V1741 and V1983) and two reverse (V1742 and V1984) primers and a probe (V1743).
The conventional PCR was set up using conditions previously described (5). For real-time PCR, each reaction mixture contained 1× LightCycler Fast Start DNA master hybridization probe mix (Roche Applied Science, Indianapolis, IN), 4 mM MgCl2, a 1 μM concentration of each primer, a 0.25 μM concentration of probe, and 2 μl of genomic DNA from the mold or the yeast form or 5 μl of tissue DNA in a final volume of 20 μl. Parallel to each PCR assay, inhibitory PCR was also performed by incorporating 1 ng of B. dermatitidis DNA into each tissue DNA sample. The PCR cycling conditions were 10 min at 95°C followed by 15 s at 95°C and 60 s at 60°C for 40 cycles. Each sample was tested in duplicate, and the results were averaged to obtain the cycle threshold (Ct)—i.e., the point at which sample fluorescence rises above the background level. A Ct value of >40 was considered negative, while a Ct value of <40 was considered positive. Each sample run also utilized a non-DNA template control. The PCR and data analyses were performed on an IQ5 real-time PCR detection system (Bio-Rad, Hercules, CA).
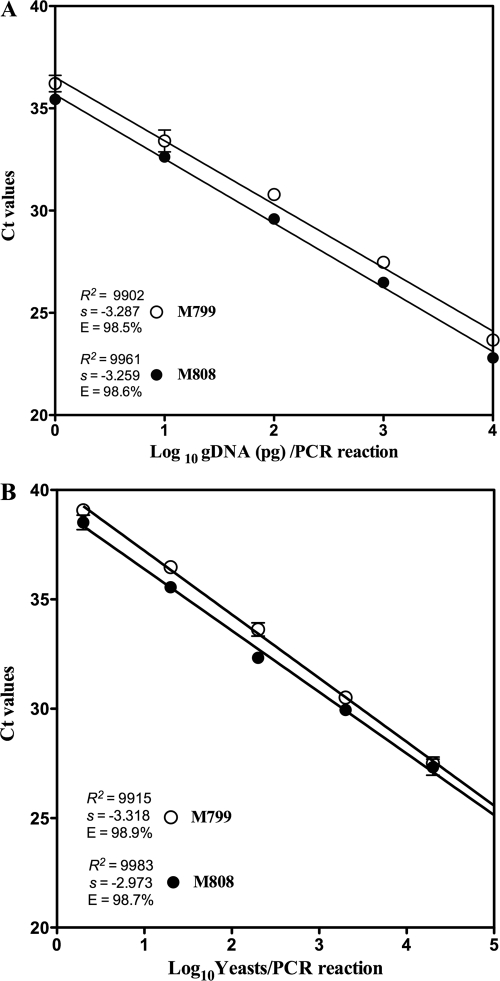
The conventional PCR produced markedly different BAD1 amplicon sizes of 363 and 663 bp when genomic DNA (gDNA) of different B. dermatitidis strains was used (Fig. 1). These results were not totally unexpected, as it has been reported previously that there is a significant size and sequence variation in the BAD1 gene amplicon. The differences are the basis of four haplotypes of B. dermatitidis (12). The sequence analysis of the BAD1 gene through a BLAST search revealed that the 24 strains of B. dermatitidis used in the present study belonged to all known H1 to H4 haplotypes (Table 1). Multiple alignments of the BAD1 gene revealed a region with the least nucleotide variations, which were used for the design of primers and probe in the present study (Fig. 2). Initial standardizations were done with strain M808, which represented the H1 to H3 haplotypes and strain M799, which represented the H4 haplotype. The results from 10-fold serial dilution of gDNA of the mold form of M808 and M799 revealed linearity over four logs, with a correlation coefficient of 0.99 and an amplification efficiency of >98%. The limit of detection (LOD) was 1 pg gDNA per PCR, which was within the linear range of the standard curve for both strains of B. dermatitidis (Fig. 3A). Similarly, the assay was linear over several logs when the yeast form of the culture was used. The assay could detect the DNA equivalent of 2 yeast cells per PCR for both strains M808 and M799 (Fig. 3B). These results indicated that the real-time PCR assay targeting the BAD1 gene is highly sensitive for the identification of both the mold and the yeast forms of B. dermatitidis. To further establish the reproducibility of the assay, approximately 1 to 10 ng of gDNA extracted from 22 additional strains encompassing all four haplotypes of B. dermatitidis was tested in a real-time PCR assay (Table 1). The results produced cycle threshold (Ct) values of <30, thereby confirming that the real-time PCR assay can identify all the B. dermatitidis strains irrespective of their haplotypes. Previous DNA-based identification studies suggested some evidence of genetic diversity among B. dermatitidis strains (11, 14). Recently, PCR and sequence analyses of the putative promoter region of the BAD1 gene, a surface adhesion and essential virulence factor of B. dermatitidis, revealed polymorphisms, which led to the identification of four haplotypes of this fungal pathogen (12). Thus, it is possible that genetic diversity in B. dermatitidis could influence disease presentation and progression, response to antifungal therapy, and efficacy of laboratory diagnostic tests. In this context, the present investigation, where real-time PCR assay is able to identify all known haplotypes of B. dermatitidis, is crucial.
Fig 1.
Conventional PCR of the BAD1 promoter region from B. dermatitidis strains. The conventional primer pair within the BAD1 (WI-1) putative promoter region (5) yielded two size products of either 363 or 663 bp from different B. dermatitidis strains. First and last lanes, 100-bp marker; NTC, non-DNA template control; numbers preceded by the letter M are various B. dermatitidis strains.
Table 1.
Blastomyces dermatitidis strains positive by real-time PCR
| Collection strain no.c | Origin | BAD1 size (bp)a | Haplotypeb |
|---|---|---|---|
| M611 | Human, New York | 363 | H3 |
| M612 | Human, New York | 363 | H2 |
| M613 | Human, New York | 363 | H2 |
| M799 (ATCC MYA-2585) | Dog, Wisconsin | 663 | H4 |
| M800 (ATCC 56920) | Bat, India | 363 | H2 |
| M801 (ATCC 48938) | Bat, India | 663 | H4 |
| M802 (ATCC 26198) | Soil, Kentucky | 363 | H3 |
| M803 | Soil, Wisconsin | 663 | H4 |
| M804 | Sea lion, Illinois | 363 | H3 |
| M805 | Polar bear, Tennessee | 363 | H3 |
| M806 | Dog, Tennessee | 363 | H3 |
| M807 | Dog, Tennessee | 363 | H1 |
| M808 | Dog, Tennessee | 363 | H1 |
| M809 | Dog, Tennessee | 363 | H1 |
| M810 | Dog, Tennessee | 363 | H3 |
| M811 | Dog, Tennessee | 363 | H1 |
| M812 | Cat, Tennessee | 363 | H1 |
| M813 | Sea lion, Tennessee | 363 | H1 |
| M814 | Dog, Tennessee | 363 | H3 |
| M815 | Dog, Tennessee | 363 | H3 |
| M816 | Dog, Tennessee | 363 | H2 |
| M1060 (ATCC MYA-2586) | Soil, Wisconsin | 363 | H3 |
| M1062 | Human, Wisconsin | 363 | H3 |
| M1063 | Human, Wisconsin | 363 | H3 |
Amplicon size in base pairs, based on conventional PCR.
Haplotype determination based on BLAST search of BAD1 sequences.
ATCC, American Type Culture Collection.
Fig 3.
Standard curve of the B. dermatitidis real-time PCR assay. Linear regression analysis was carried out for the BAD1 target in a B. dermatitidis real-time PCR assay using GraphPad Prism software (La Jolla, CA). Ct values were calculated from duplicate wells and were plotted against serial 10-fold dilutions of gDNA (1 to 10,000 pg) from the mold form of the culture (A), and 10-fold serial dilutions of gDNA corresponding to 2 to 20,000 CFU from the yeast form of the culture (B). The slope (s), correlation coefficient (R2), and amplification efficiency (E) are shown. The choice of B. dermatitidis strains M808 and M799 is based upon their significant difference in BAD1 size and nucleotide composition.
B. dermatitidis real-time PCR assay specificity was analyzed with various fungal and bacterial DNAs. The absence of any signal indicated that this assay was 100% specific for the detection of B. dermatitidis from other cultured fungal or bacterial isolates.
To demonstrate the further utility of the B. dermatitidis real-time PCR assay, we used 12 paraffin-embedded tissue samples, including 6 positive and 6 negative for B. dermatitidis or any other fungal organisms by histopathology. Only three of the six positive tissue samples (50%) tested positive for the BAD1 target when DNA was extracted using the QIAamp DNA FFPE kit, whereas five of the six positive tissue samples (83%) tested positive when DNA was extracted using the MasterPure (Epicenter, Madison, WI) DNA extraction kit (Table 2). The DNA extracted with QIAamp revealed the presence of inhibitors, which inhibited PCR from 15 to 100%, while no inhibitors were seen in DNA extracted with the MasterPure kit. We speculate either that the column provided in the QIAamp kit is inefficient in removal of inhibitors present in the tissues or that some of the reagents used during column washing steps are trapped in the column and possibly eluted with DNA. Overall, our results indicated that the MasterPure kit is superior to the QIAamp kit for detection of the BAD1 target by real-time PCR assay. Paraffin-embedded tissue has historically been viewed as an insensitive source for PCR assay (9). This was not the case in the present study, as the real-time PCR assay could detect the BAD1 target from five of the six tissues. These results are noteworthy considering that only a few yeast cells are seen in all but one tissue sample by histopathology (details not shown). Also, tissue age did not seem to be a limiting factor, as even a tissue sample as old as 8 years was positive for B. dermatitidis by real-time PCR assay. Interestingly, one histopathology-positive tissue sample tested negative by real-time PCR. This sample came from skull tumor tissue, which is not unusual, as B. dermatitidis is known to disseminate to skin and bones. Perhaps the bone sample was not adequately extracted for DNA to be positive by real-time PCR assay. Overall, our results clearly indicated that the real-time PCR assay is highly sensitive for the identification of B. dermatitidis from paraffin-embedded tissue samples. Furthermore, the assay appears to be highly specific, as none of the negative tissue samples yielded positive results.
Table 2.
Detection of B. dermatitidis from paraffin-embedded tissues by histopathology and real-time PCR
| Histopathology result (n = 12) | Real-time PCR result (n = 12) |
||
|---|---|---|---|
| Interpretation | No. with interpretation by kit from: |
||
| Qiagen | Epicenter | ||
| Positive (n = 6) | Positive | 3 | 5 |
| Negative | 3 | 1 | |
| With inhibitors | 3 | 0 | |
| Negative (n = 6) | Positive | 0 | 0 |
| Negative | 6 | 6 | |
| With inhibitors | 1 | 0 | |
In summary, the TaqMan real-time PCR assay targeting the BAD1 gene is highly sensitive and specific for the identification of B. dermatitidis in culture and in clinical specimens. As considerable genetic diversity is observed among B. dermatitidis strains, the real-time PCR assay is a valuable diagnostic tool for correct identification of all known haplotypes of B. dermatitidis. Moreover, DNA extraction to final analysis can be completed in 5 h, which makes this a rapid assay, with a turnaround time of less than 1 day. Recently, Babady et al. (1) described a real-time PCR assay utilizing a hybridization probe targeting the histidine kinase gene of B. dermatitidis. The distinguishing features of our assay include the use of a hydrolysis probe, higher sensitivity, with a LOD of two yeast cells, inclusion of all known haplotypes of B. dermatitidis, and a high degree of success with formalin-fixed tissue samples. In summary, the availability of these two real-time PCR assays is likely to advance rapid identification of B. dermatitidis in clinical laboratories.
ACKNOWLEDGMENTS
We thank Gene M. Scalarone (Idaho State University, Pocatello, ID) for kindly providing several strains of B. dermatitidis. We also thank Media & Molecular Genetic Cores and the Bacteriology Laboratory of the Wadsworth Center for providing culture media, DNA sequencing, and bacterial DNA stocks, respectively.
This study was supported in part by the Clinical Laboratory Reference System (CLRS) and the Global Infectious Diseases Training and Research Grant (15-0292-01) to Dale Morse of Wadsworth Center.
Footnotes
Published ahead of print 7 March 2012
REFERENCES
- 1. Babady NE, et al. 2011. Detection of Blastomyces dermatitidis and Histoplasma capsulatum from culture isolates and clinical specimens by use of real-time PCR. J. Clin. Microbiol. 49:3204–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bialek R, et al. 2003. Nested PCR assays for detection of Blastomyces dermatitidis DNA in paraffin-embedded canine tissue. J. Clin. Microbiol. 41:205–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bolano A, et al. 2001. Rapid methods to extract DNA and RNA from Cryptococcus neoformans. FEMS Yeast Res. 1:221–224 [DOI] [PubMed] [Google Scholar]
- 4. Bradsher RW, Chapman SW, Pappas PG. 2003. Blastomycosis. Infect. Dis. Clin. North Am. 17:21–40, vii [DOI] [PubMed] [Google Scholar]
- 5. Burgess JW, Schwan WR, Volk TJ. 2006. PCR-based detection of DNA from the human pathogen Blastomyces dermatitidis from natural soil samples. Med. Mycol. 44:741–748 [DOI] [PubMed] [Google Scholar]
- 6. Cote E, Barr SC, Allen C, Eaglefeather E. 1997. Blastomycosis in six dogs in New York State. J. Am. Vet. Med. Assoc. 210:502–504 [PubMed] [Google Scholar]
- 7. De Groote MA, Bjerke R, Smith H, Rhodes LV., III 2000. Expanding epidemiology of blastomycosis: clinical features and investigation of 2 cases in Colorado. Clin. Infect. Dis. 30:582–584 [DOI] [PubMed] [Google Scholar]
- 8. Gaspar I, Fenstermacher WA, Lingeman LR. 1932. Systemic blastomycosis, with report of a fatal case. Radiology 18:305–315 [Google Scholar]
- 9. Gilbert MTP, et al. 2007. The isolation of nucleic acids from fixed, paraffin-embedded tissues—which methods are useful when? PLoS One 2:e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hogan LH, Josvai S, Klein BS. 1995. Genomic cloning, characterization, and functional analysis of the major surface adhesin WI-1 on Blastomyces dermatitidis yeasts. J. Biol. Chem. 270:30725–30732 [DOI] [PubMed] [Google Scholar]
- 11. McCullough MJ, DiSalvo AF, Clemons KV, Park P, Stevens DA. 2000. Molecular epidemiology of Blastomyces dermatitidis. Clin. Infect. Dis. 30:328–335 [DOI] [PubMed] [Google Scholar]
- 12. Meece JK, et al. 2010. Genetic diversity in Blastomyces dermatitidis: implications for PCR detection in clinical and environmental samples. Med. Mycol. 48:285–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Veligandla SR, et al. 2002. Delayed diagnosis of osseous blastomycosis in two patients following environmental exposure in nonendemic areas. Am. J. Clin. Pathol. 118:536–541 [DOI] [PubMed] [Google Scholar]
- 14. Yates-Siilata KE, Sander DM, Keath EJ. 1995. Genetic diversity in clinical isolates of the dimorphic fungus Blastomyces dermatitidis detected by a PCR-based random amplified polymorphic DNA assay. J. Clin. Microbiol. 33:2171–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]