Abstract
The first case of cavitary pulmonary disease caused by Purpureocillium lilacinum is described. The isolate showed atypical microscopic characteristics similar to Acremonium and Fusarium spp., which necessitated molecular identification by sequencing of multiple conserved loci. The patient responded to voriconazole, reinforcing its therapeutic efficacy for P. lilacinum infections.
CASE REPORT
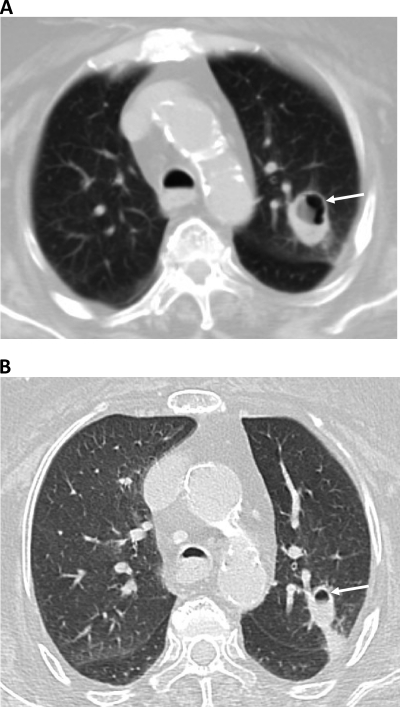
An 80-year-old asthmatic woman presented with a 3-week history of productive cough, associated with fever and pleuritic chest pain, which did not respond to a week of treatment with antibiotics and steroid therapy. She had a history of asthma, coronary artery disease, diabetes mellitus, hypertension, dyslipidemia, rheumatoid arthritis, and osteoporosis. Clinically, she presented with fever, tachypnea, tachycardia, and hypotension. Chest auscultation revealed bilateral scattered wheezes. The rest of the physical findings were unremarkable. Within 24 h of admission, her condition progressively deteriorated, requiring mechanical ventilation. As the patient was febrile, she was empirically started on ceftriaxone and clarithromycin. Her initial assessment revealed a normal white cell count, but the chest X-ray showed a consolidative lesion in the left upper lobe (LUL). The sputum culture showed heavy growth of Pseudomonas aeruginosa. Consequently, her antibiotic regimen was modified to include ciprofloxacin and meropenem for a course of 14 days, followed by a 3-week course of tazocin-ciprofloxacin. Despite an initial clinical improvement, the follow-up chest X ray showed persistence of the LUL lesion. Therefore, a computed tomography (CT) scan of the chest was performed on 7 October 2010, which revealed a cavitary lesion in the anterior segment of the LUL (Fig. 1A).
Fig 1.
(A) Initial chest computed tomography (CT) imaging showing cavitating lesion in anterior segment of lung left upper lobe (arrow). (B) Follow-up chest CT image showing regression of cavitating lesion after 1 week of voroconazole therapy.
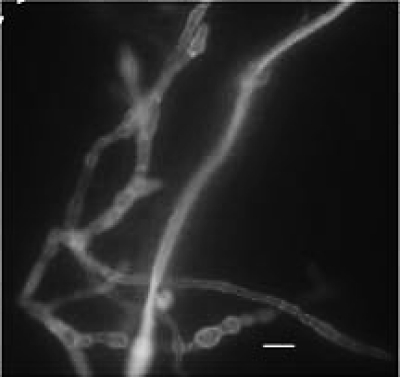
Since her condition deteriorated further, as assessed by her oxygen requirements, sputum specimens were collected on 11 and 18 October 2010 and were sent to the Mycology Reference Laboratory (MRL), Faculty of Medicine, Kuwait University. Both specimens showed septate hyphal elements when examined with calcofluor-potassium hydroxide (Fig. 2) and grew a white mold after 5 days on Sabouraud dextrose agar (Difco, Becton, Dickinson and Company, Sparks, MD). On 21 October 2010, a bronchoalveolar lavage (BAL) specimen from the LUL was obtained and sent to the MRL to establish the role of this white mold in the etiology of her cavitary lung lesion. The BAL specimen also showed septate fungal elements in the calcofluor-KOH mount (Fig. 2) and yielded a morphologically identical mold culture. The mold was provisionally identified as Acremonium or Paecilomyces. Although no defined antifungal susceptibility breakpoints exist for these organisms, the isolate demonstrated high MICs to amphotericin B (≥32 μg/ml), caspofungin (4 μg/ml), and itraconazole (≥32 μg/ml), suggesting resistance, but low MICs for posaconazole (0.5 μg/ml) and voriconazole (0.064 μg/ml), suggesting clinical efficacy. The patient was thus started on voriconazole, at 6 mg/kg of body weight at 12-h intervals for the first 24 h, followed by 4 mg/kg every 12 h. After 1 week of voriconazole therapy, the patient showed significant clinical improvement accompanied by regression in the size of the cavitary lesion (Fig. 1B). Since the patient continued to improve clinically, she was discharged on oral voriconazole (200 mg orally every 12 h). However, she died 2 weeks later apparently due to choking while she was being fed orally at home.
Fig 2.
Potassium hydroxide (10%)-calcofluor (0.1%) mount of bronchoalveolar lavage showing septate hyphal elements. Bar, 5 μm.
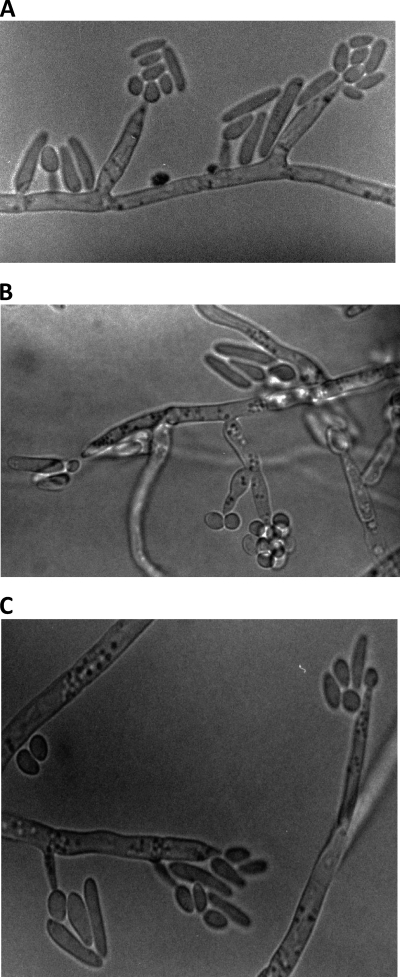
Colonies of our isolate (MF3411/10) on Sabouraud dextrose agar attained a diameter of 42 mm after 7 days of incubation at 30°C. The isolate showed restricted growth at 37°C, and it failed to grow at 40°C. Colonies consisted of a basal felt and sectors of floccose white aerial mycelium, which remained white after extended incubation. The colony reverse was colorless. Microscopic characteristics of the isolate are depicted in Fig. 3A to C. In slide culture at 30°C, straight or simply branched conidiophores of various lengths (6.2 to 52.0 by 1.8 to 2.6 μm) arising from the hyphae were observed. No verticillate branches, as seen in typical cultures of Purpureocillium lilacinum, were observed. Cylindrical, mostly Acremonium-like phialides, tapering toward the apex (3.2 to 22.4 by 1.5 to 3.2 μm) were observed (Fig. 3C). Hyaline conidia, mostly fusiform and ellipsoidal to subglobose and rarely globose (1.6 to 12.2 by 2.0 by 3.8 μm), were produced either singly or in clusters (Fig. 3B). Fusiform-shaped conidia were mostly formed on Acremonium-like conidiophores in a “slimy head” (Fig. 3A and C). The isolate has been deposited in the CBS-KNAW Biodiversity Center as CBS129077.
Fig 3.
(A to C) One-week-old slide culture of P. lilacinum (MF3411/10) grown on Sabouraud dextrose agar at 30°C showing Acremonium-like phialides and fusiform conidia.
The Etest (bioMérieux, Marcy l'Etoile, France) was employed to determine MICs of five antifungal agents as described previously (17). Briefly, the test was performed on RPMI 1640 medium supplemented with 2% glucose, and the pH was adjusted to 7.0 with 0.165 M morpholinepropanesulfonic acid buffer. The isolate was cultivated on a potato dextrose agar slant (Difco, Becton, Dickinson and Company, Sparks, MD) for 7 days at 30°C. Conidia were harvested in 2 ml sterile normal saline, and clumps were allowed to settle. The plates were inoculated by dipping a sterile swab into the conidial suspension and streaking it uniformly over the agar surface. Plates were allowed to dry at room temperature for 15 min before Etest strips were applied. MICs were read after 48 h of incubation at 35°C where the border of the inhibition ellipse intersected the scale on the antifungal strip. Microcolonies within the inhibition zone for caspofungin were ignored.
Genomic DNA from the patient's isolate (MF3411/10) was prepared as described previously and used as the template for PCR amplification (2). The divergent domains (D1/D2) of the 28S rRNA gene were amplified with the NL-1 and NL-4 primers, the internal transcribed spacer (ITS) region (ITS1, 5.8S rRNA, and ITS2) of ribosomal DNA (rDNA) was amplified with the ITS1 and ITS4 primers (3), and the variable region of the β-tubulin (benA) gene was amplified by using the BTUBF (5′-TGGTAACCAAATCGGTGCTGCTT-3′) and BTUBR (5′-GCACCCTCAGTGTAGTGACCCT-3′) primers, while the variable region of the calmodulin gene was amplified by using the Cmd5 (5′-GTCTCCGAGTACAAGGAGGC-3′) and Cmd6 (5′-TCGCCGATRGAGGTCATRACGTG-3′) primers, and the amplicons were sequenced as described previously (3, 16, 17). The sequencing primers included BTUFS1 (5′-TAACCAAATCGGTGCTGCTTTCTG-3′) and BTURS (5′-CCTCAGTGTAGTGACCCTTGGC-3′) for the β-tubulin amplicon and CMDFS (5′-TCCGAGTACAAGGAGGCCTTC-3′) and CMDRS (5′-GATAGAGGTCATRACGTGRCGCA-3′) for the calmodulin gene fragment. GenBank basic local alignment search tool (BLAST) searches (http://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastHome) were performed for species identification.
The ITS sequence of our isolate exhibited 100% identity with the corresponding sequences from the type strain (CBS 284.36) and several other strains (ATCC 10114, CBS 431.87, CBS 432.87, CBS 226.73B, and CBS 100379) of P. lilacinum. The partial 28S rRNA gene sequence was also 100% identical to that of P. lilacinum CBS 284.36 and ATCC 10114 and differed at one nucleotide position from the sequences of CBS 431.87 and CBS 101068. The partial β-tubulin gene sequence also showed only 1 nucleotide difference from the sequence of P. lilacinum strains CBS 284.36 and CBS 432.87. The calmodulin gene sequence of our isolate also exhibited 100% identity to the sequence of the type strain of P. lilacinum (CBS 284.36).
The case described here is unique in three respects. First, it describes P. lilacinum as a cause of cavitary pulmonary disease, a clinical presentation, which to our knowledge, has not been described previously. Second, the isolate presented atypical morphological features, which were more akin to Acremonium spp. than to P. lilacinum, thus requiring molecular identification. Third, it documents the clinical efficacy of voriconazole in the treatment of a cavitary Purpureocillium infection.
Purpureocillium lilacinum (formerly Paecilomyces lilacinus) is a hyaline hyphomycete with a ubiquitous distribution (19). It occurs mainly in soil and decaying vegetable matter as a saprobe (7). P. lilacinum is an increasingly recognized agent of hyalohyphomycosis, capable of causing a wide spectrum of clinical manifestations in immunocompromised and immunocompetent individuals (4, 29, 34, 36, 39). Although ocular and cutaneous/subcutaneous infections are the most familiar clinical presentations, it is also encountered in cases of fungemia and deep-seated/systemic infections (28, 29). Infections with P. lilacinum present diagnostic and therapeutic challenges since its morphology in tissue is indistinguishable from those of Aspergillus and other agents of hyalohyphomycosis (36) and because it exhibits reduced susceptibility to amphotericin B (29).
Among upper respiratory tract infections, P. lilacinum has been implicated in the etiology of invasive rhinitis (6) and sinusitis (12, 23, 31, 32, 35, 36). Pulmonary infections due to P. lilacinum are rare. In this context, the present report is noteworthy as it describes the first case of cavitary pulmonary disease, thus extending the spectrum of clinical presentations known to be associated with P. lilacinum. So far, pulmonary infections due to P. lilacinum have been reported in four cases (Table 1) (9, 19, 22, 26). The first report of chest involvement was a case of empyema reported in 1972 in a 20-year-old male from Malta with no known predisposing condition (9). The second case was reported in a 58-year-old female with history of collagen lung disease who was receiving corticosteroid therapy (22). The fungus was isolated from pleural drainage. The third case involved a patient with acute lymphoblastic leukemia, where infection from the lung apparently disseminated to other organs and the fungus was isolated from blood (19). The fourth case was reported in a 57-year-old healthy man, who developed a coin lesion in the right hilum. A culture of pus obtained from the abscess following a right middle lobe lobectomy yielded P. lilacinum. The patient recovered without antifungal therapy (26). Two additional cases of pulmonary involvement, where the isolates were identified only to the genus level, have also been described (13, 37). One of these involved a 12-year-old Pakistani boy with chronic granulomatous disease in which the isolate was recovered from a lung biopsy specimen (37). The patient was treated with amphotericin B, followed by itraconazole and the withdrawal of prednisolone. The second case involved a 41-year-old female with a history of cough and hemoptysis (13). The isolate was cultured from a needle aspirate obtained from a pulmonary lesion with a radiologic diagnosis of mycetoma. Based on the DNA sequence of the ITS region, the isolate could only be identified up to the genus level due to poor sequence identity with available sequences of pathogenic Paecilomyces species, including P. lilacinum (13). The patient received voriconazole for 6 months, followed by resection of the fungal ball. The latter two reports underscore the difficulties in identifying Paecilomyces spp. Pulmonary infections due to P. lilacinum have also been reported in animals (30).
Table 1.
Summary of salient findings in cases of pulmonary P. lilacinum infection
| Case no. | Reference | Country | Age (yr)/sexa | Clinical presentation | Predisposing factor(s) | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Fenech and Mallia (9) | Malta | 20/M | Pleural effusion, no evidence of dissemination | Not known | Amphotericin B | Recovered |
| 2 | Mormede et al. (22) | France | 58/F | Pleural effusion, diffuse reticonodular lesion | Interstitial lung disease, corticosteroids, recurrent pulmonary infections | Antibiotics, anti-inflammatory, no antifungal given | Died of hepatic complications |
| 3 | Liu et al. (19) | United States | Not available | Lung disseminated | Acute lymphoblastic leukemia | Not available | Recovered |
| 4 | Ono et al. (26) | Japan | 57/M | Lung abscess | Not available | Lobectomy | Recovered |
| 5 | Present case | Kuwait | 80/F | Fever, pleuritic chest pain | Asthma, diabetes, rheumatoid arthritis | Voriconazole | Improved but then died due to other causes |
M, male; F, female.
The etiologic significance of P. lilacinum in our patient is apparent from both the fact that the same fungus was isolated from two sputum specimens and a BAL sample, all collected within a 10-day period, and the fact that the patient responded to voriconazole therapy. A noteworthy feature of our isolate is its atypical microscopic morphology characterized by the formation of Acremonium-like conidia in the absence of verticillate branches with whorls of phialides that characterize typical strains. This made a definitive morphological identification impossible. Unequivocal identification was established by DNA sequencing of four highly conserved genes. Consistent with our isolate, Okada et al. (25) demonstrated that P. lilacinum was able to form Acremonium-like conidiophores in submerged cultures, as well as on the agar surface, with dimorphic characteristics. Acremonium-like conidiophores, phialides, and conidia (formed in “slimy heads”) were described by Luangsa-Ard et al. (20) when placing Paecilomyces lilacinus in the new genus Purpureocillium. This Acremonium state resembles members of the Fusarium solani species complex (FSSC), which are major agents of fungal keratitis. Interestingly, the most frequent manifestation of P. lilacinum is also keratitis (29), suggesting that these fungi may share similar pathogenesis or pathogenic mechanisms. Additionally, members of the FSSC and P. lilacinum may be misidentified in histopathological sections due to their similar morphological appearances (19). It is possible that like Fusarium spp., P. lilacinum may also form intravascular budding structures or phialoconidia through adventitious sporulation and thus facilitate its hematogenous dissemination to deeper tissues (19, 24). Thus, cutaneous lesions due to P. lilacinum may be caused not only by direct inoculation but may also result from hematogenous or lymphatic spread (15). It is worth noting that creams or lotions contaminated with P. lilacinum have resulted in outbreaks of cutaneous and disseminated disease (15, 27).
Purpureocillium lilacinum and P. variotii are the two clinically most important members of the genus (4, 14, 15, 29). Because of morphological similarities, their accurate identification is crucial as they exhibit different susceptibilities to antifungal agents (1). Recently, Castelli et al. (5) reported antifungal susceptibility profiles for P. lilacinum (n = 27) and P. variotii (n = 31) in which 20 of the isolates were identified by molecular methods. The results of this study indicated that amphotericin B, itraconazole, and echinocandins exhibit reduced susceptibility, whereas voriconazole and posaconazole show good activity against P. lilacinum. This finding is also consistent with the susceptibility profile of our isolate. In contrast, P. variotii isolates were susceptible to amphotericin B, itraconazole, and echinocandins, but showed reduced susceptibility to voriconazole (5). Similar susceptibility results were reported by Gonzalez et al. (11), where voriconazole, posaconazole, and ravuconazole demonstrated good in vitro activity against P. lilacinum. Pastor and Guarro (29) summarized available in vitro susceptibility data on P. lilacinum. Voriconazole and posaconazole were found to possess maximum activity, whereas amphotericin B was least active. Echinocandins have shown variable in vitro activity against P. lilacinum (5, 8, 29, 38). Voriconazole, consistent with its in vitro activity against P. lilacinum, has been used successfully for treatment of several cases with different clinical manifestations, including the present case (6, 10, 18, 21). The efficacy of voriconazole has also been demonstrated in an experimental murine model of P. lilacinum infection in comparison to amphotericin B (33). However, it is important to recognize that strains of P. lilacinum showing in vitro resistance to voriconazole have also been reported (5, 29). Clinical experience with posaconazole or other newer triazoles does not exist.
In conclusion, the first case of cavitary pulmonary disease caused by P. lilacinum is described. Since the isolate presented atypical, Acremonium-like morphological characteristics, a definitive identification was obtained by sequencing multiple loci. The patient was successfully treated with voriconazole.
Nucleotide sequence accession numbers.
The DNA sequence data of our isolate (MF3411/10, CBS 129077, or UTHSC 11-93) have been deposited in the EMBL data bank under accession no. FR822391, FR822392, HE648327, and HE648328.
Footnotes
Published ahead of print 8 February 2012
REFERENCES
- 1. Aguilar C, Pujol I, Sala J, Guarro J. 1998. Antifungal susceptibilities of Paecilomyces species. Antimicrob. Agents Chemother. 42:1601–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmad S, Khan ZU, Theyyathel AM. 2007. Diagnostic value of DNA, (1–3)-beta-D-glucan, and galactomannan detection in serum and bronchoalveolar lavage of mice experimentally infected with Aspergillus terreus. Diagn. Microbiol. Infect. Dis. 59:165–171 [DOI] [PubMed] [Google Scholar]
- 3. Al-Sweih N, Ahmad S, Khan ZU, Khan S, Chandy R. 2005. Prevalence of Candida dubliniensis among germ tube-positive Candida isolates in a maternity hospital in Kuwait. Mycoses 48:347–351 [DOI] [PubMed] [Google Scholar]
- 4. Antas PR, Brito MM, Peixoto E, Ponte CG, Borba CM. 2012. Neglected and emerging fungal infections: review of hyalohyphomycosis by Paecilomyces lilacinus focusing in disease burden, in vitro antifungal susceptibility and management. Microbes Infect. 14:1–8 [DOI] [PubMed] [Google Scholar]
- 5. Castelli MV, et al. 2008. Susceptibility testing and molecular classification of Paecilomyces spp. Antimicrob. Agents Chemother. 52:2926–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ciecko SC, Scher R. 2010. Invasive fungal rhinitis caused by Paecilomyces lilacinus infection: report of a case and a novel treatment. Ear Nose Throat J. 89:594–595 [PubMed] [Google Scholar]
- 7. de Hoog G, Guarro J, Gené J, Figueras MJ. 2000. Atlas of clinical fungi, 2nd ed, p 794–809 Reus, Utrecht, The Netherlands [Google Scholar]
- 8. Del Poeta M, Schell WA, Perfect JR. 1997. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob. Agents Chemother. 41:1835–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fenech FF, Mallia CP. 1972. Pleural effusion caused by Penicillium lilacinum. Br. J. Dis. Chest 66:284–290 [DOI] [PubMed] [Google Scholar]
- 10. Garzoni C, Garbino J. 2008. New azoles as first line therapy for Paecilomyces lilacinus in transplant patients. Transpl. Infect. Dis. 10:149–150 [DOI] [PubMed] [Google Scholar]
- 11. González GM, Fothergill AW, Sutton DA, Rinaldi MG, Loebenberg D. 2005. In vitro activities of new and established triazoles against opportunistic filamentous and dimorphic fungi. Med. Mycol. 43:281–284 [DOI] [PubMed] [Google Scholar]
- 12. Gucalp R, et al. 1996. Paecilomyces sinusitis in an immunocompromised adult patient: case report and review. Clin. Infect. Dis. 23:391–393 [DOI] [PubMed] [Google Scholar]
- 13. Gutiérrez F, et al. 2005. Pulmonary mycetoma caused by an atypical isolate of Paecilomyces species in an immunocompetent individual: case report and literature review of Paecilomyces lung infections. Eur. J. Clin. Microbiol. Infect. Dis. 24:607–611 [DOI] [PubMed] [Google Scholar]
- 14. Houbraken J, Verweij PE, Rijs AJ, Borman AM, Samson RA. 2010. Identification of Paecilomyces variotii in clinical samples and settings. J. Clin. Microbiol. 48:2754–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Itin PH, et al. 1998. Cutaneous manifestations of Paecilomyces lilacinus infection induced by a contaminated skin lotion in patients who are severely immunosuppressed. J. Am. Acad. Dermatol. 39:401–409 [DOI] [PubMed] [Google Scholar]
- 16. Khan Z, et al. 2010. Cryptococcus randhawai sp. nov., a novel anamorphic basidiomycetous yeast isolated from tree trunk hollow of Ficus religiosa (peepal tree) from New Delhi, India. Antonie Van Leeuwenhoek 97:253–259 [DOI] [PubMed] [Google Scholar]
- 17. Khan Z, et al. 2011. Acremonium kiliense: reappraisal of its clinical significance. J. Clin. Microbiol. 49:2342–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Labriola L, Ercam VB, Swinne D, Jadoul M. 2009. Successful treatment with voriconazole of prolonged Paecilomyces lilacinus fungemia in a chronic hemodialyzed patient. Clin. Nephrol. 71:355–358 [DOI] [PubMed] [Google Scholar]
- 19. Liu K, Howell DN, Perfect JR, Schell WA. 1998. Morphologic criteria for the preliminary identification of Fusarium, Paecilomyces, and Acremonium species by histopathology. Am. J. Clin. Pathol. 109:45–54 [DOI] [PubMed] [Google Scholar]
- 20. Luangsa-Ard J, et al. 2011. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol. Lett. 321:141–149 [DOI] [PubMed] [Google Scholar]
- 21. Martin CA, Roberts S, Greenberg RN. 2002. Voriconazole treatment of disseminated Paecilomyces infection in a patient with acquired immunodeficiency syndrome. Clin. Infect. Dis. 35:e78–e81 [DOI] [PubMed] [Google Scholar]
- 22. Mormede M, et al. 1984. Isolement d'un Paecilomyces (P. lilacinus) à partir d'un épanchement pleural. Méd. Malad. Infect. 14:76–78 [Google Scholar]
- 23. Nayak DR, Balakrishnan R, Nainani S, Siddique S. 2000. Paecilomyces fungus infection of the paranasal sinuses. Int. J. Pediatr. Otorhinolaryngol. 52:183–187 [DOI] [PubMed] [Google Scholar]
- 24. Nucci M, Anaissie E. 2007. Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 20:695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Okada G, Sakai N, Yamagishi M. 1995. Acremonium-like submerged conidiation in Paecilomyces nostocoides and P. lilacinus. Mycoscience 36:345–351 [Google Scholar]
- 26. Ono N, Sato K, Yokomise H, Tamura K. 1999. Lung abscess caused by Paecilomyces lilacinus. Respiration 66:85–87 [DOI] [PubMed] [Google Scholar]
- 27. Orth B, et al. 1996. Outbreak of invasive mycoses caused by Paecilomyces lilacinus from a contaminated skin lotion. Ann. Intern. Med. 125:799–806 [DOI] [PubMed] [Google Scholar]
- 28. Ounissi M, et al. 2009. Hyalohyphomycosis caused by Paecilomyces lilacinus after kidney transplantation. Transplant. Proc. 41:2917–2919 [DOI] [PubMed] [Google Scholar]
- 29. Pastor FJ, Guarro J. 2006. Clinical manifestations, treatment and outcome of Paecilomyces lilacinus infections. Clin. Microbiol. Infect. 12:948–960 [DOI] [PubMed] [Google Scholar]
- 30. Pawloski DR, Brunker JD, Singh K, Sutton DA. 2010. Pulmonary Paecilomyces lilacinus infection in a cat. J. Am. Anim. Hosp. Assoc. 46:197–202 [DOI] [PubMed] [Google Scholar]
- 31. Permi HS, et al. 2011. A rare case of fungal maxillary sinusitis due to Paecilomyces lilacinus in an immunocompetent host presenting as a subcutaneous swelling. J. Lab. Physicians 3:46–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rockhill RC, Klein MD. 1980. Paecilomyces lilacinus as the cause of chronic maxillary sinusitis. J. Clin. Microbiol. 11:737–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodríguez MM, Pastor FJ, Serena C, Guarro J. 2010. Efficacy of voriconazole in a murine model of invasive paecilomycosis. Int. J. Antimicrob. Agents 35:362–365 [DOI] [PubMed] [Google Scholar]
- 34. Rosmaninho A, et al. 2010. Paecilomyces lilacinus in transplant patients: an emerging infection. Eur. J. Dermatol. 20:643–644 [DOI] [PubMed] [Google Scholar]
- 35. Rowley SD, Strom CG. 1982. Paecilomyces fungus infection of the maxillary sinus. Laryngoscope 92:332–334 [DOI] [PubMed] [Google Scholar]
- 36. Saberhagen C, Klotz SA, Bartholomew W, Drews D, Dixon A. 1997. Infection due to Paecilomyces lilacinus: a challenging clinical identification. Clin. Infect. Dis. 25:1411–1413 [DOI] [PubMed] [Google Scholar]
- 37. Sillevis Smitt JH, Leusen JH, Stas HG, Teeuw AH, Weening RS. 1997. Chronic bullous disease of childhood and a Paecilomyces lung infection in chronic granulomatous disease. Arch. Dis. Child. 77:150–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uchida K, Nishiyama Y, Yokota N, Yamaguchi H. 2000. In vitro antifungal activity of a novel lipopeptide antifungal agent, FK463, against various fungal pathogens. J. Antibiot. (Tokyo) 53:1175–1181 [DOI] [PubMed] [Google Scholar]
- 39. Van Schooneveld T, et al. 2008. Paecilomyces lilacinus infection in a liver transplant patient: case report and review of the literature. Transpl. Infect. Dis. 10:117–122 [DOI] [PubMed] [Google Scholar]