Abstract
Point-of-care (POC) diagnostic tests for influenza can considerably shorten the time to clinical decision making. An investigational POC test based on a multiplexed immunoassay was developed by Meso Scale Diagnostics, LLC (MSD), with the objective to make a more sensitive rapid test that can also subtype influenza A viruses (1977 H1, H3, and H5). Between February and November 2010, we conducted a prospective multicenter study at four hospitals in Vietnam and compared the performance of this test to that of the WHO/CDC real-time reverse transcriptase PCR (RT-PCR) on nasal and throat swab specimens from patients presenting with influenza-like illness. Five hundred sixty-three adults and children with a median age of 25 months were enrolled. Sensitivity and specificity of the test with combined results from nasal and throat swab samples were 74.0% (131/177) and 99.7% (351/352), respectively, compared to RT-PCR. The POC test was as sensitive for influenza virus B as for influenza virus A (74.4% [64/86] versus 73.6% [67/91]). The positivity rate was associated with lower cycle threshold values (a marker for higher viral loads), sample type (73.6% for nasal swab versus 52.4% for throat swab), and younger age. A total of 210 (18.7%) out of 1,126 MSD tests failed, and for 34 (6%) of patients, both test samples failed (these were excluded from the performance analysis). Subtyping could be assessed only for influenza virus A/H3N2, as 1977 H1N1 was not circulating at the time and no H5N1-infected patients were enrolled, and was successful only in 9/54 patients infected with H3 influenza virus who had a positive POC test result for influenza virus A. This novel POC test provided highly sensitive detection of influenza viruses A and B compared to the reported sensitivities of other rapid tests. However, 18.7% of tests failed for technical reasons and subtyping for H3 was poor. Drawbacks to the technology include the requirement for a dedicated reader instrument and the need for continual updating of subtyping antibodies within the test array.
INTRODUCTION
Rapid and reliable methods to diagnose influenza at the point of care (POC) are highly desirable for timely therapeutic and infection control measures. At present, POC testing for influenza is performed using rapid antigen tests currently available on the market. Among these are BinaxNOW! (Binax), Directigen EZ (Becton Dickinson), and QuickView (Quidel), and these have been evaluated extensively for use on both pre-2009 seasonal influenza A and B viruses (5–7, 9–11, 13–15, 18) and influenza virus A/H1N1-pdm09 (2009 H1N1) (4, 12, 17). These tests, typically lateral-flow immunoassays, are based on antigen detection in upper respiratory samples and can be used in out- and inpatient settings to diagnose influenza virus infection. The duration of these tests ranges from 10 to 30 min, and they typically target the conserved nucleoproteins of influenza virus A and B.
The current rapid tests have several limitations that have restricted their implementation at the bedside or in the microbiology laboratory: low clinical sensitivity and limited clinical specificity; subjective visual readout (in most cases) in case of unclear or vague bands, creating the potential for interobserver bias; and no ability to subtype influenza virus A.
The inability to subtype implies that none of the available tests can differentiate avian influenza virus A/H5N1 from circulating human seasonal strains. Therefore, in the event of an H5N1 pandemic (or emergence of any novel influenza virus subtype), health care professionals would need to rely on more complex and time-consuming tests such as culture or reverse transcriptase PCR (RT-PCR) to identify infected patients, leading to delays in patient treatment, infection control measures, and public health responses.
In 2006, the U.S. Centers for Disease Control and Prevention (CDC) announced an award of contracts to four companies working to develop new diagnostic tests that doctors and epidemiologists could use to quickly and accurately test patients for avian influenza virus H5N1 and other emerging influenza viruses, as well as more common seasonal influenza viruses (1). One such contract was made to Meso Scale Diagnostics, LLC (MSD; Gaithersburg, MD), for development of a multiplex in vitro immunoassay panel using monoclonal antibodies with the goal of achieving higher sensitivity than the currently available rapid tests and to subtype influenza A viruses into the prepandemic H1 (1977 H1), seasonal H3, and avian H5 subtypes.
This test was used in the United States to detect the first case of 2009 H1N1 infection to be caused by a nonsubtypeable influenza A virus, thereby alerting public health authorities and initiating further testing that eventually led to recognition of the antigenic shift and ensuing pandemic (3).
We conducted a prospective multicenter investigational study to evaluate the performance of the MSD influenza test (POC test) in comparison to the WHO/CDC real-time RT-PCR and virus culture in detecting and differentiating influenza virus strains, including A/H1N1 (1977), A/H3N2, A/H5N1, and B, in subjects presenting with influenza-like illness (ILI) in POC settings in Asia using nasal and throat swab specimens.
MATERIALS AND METHODS
Study sites, patient population, and sample size.
The study took place between March and November 2010 in four large referral hospitals in Vietnam (Children's Hospitals 1 and 2 [CH1 and CH2] and the Hospital for Tropical Diseases [HTD] in Ho Chi Minh City and the National Hospital for Tropical Diseases [NHTD] in Ha Noi), all part of the Southeast Asia Infectious Diseases Clinical Research Network (SEAICRN).
Any patient who presented with ILI (defined as fever [subjective or documented] and cough or sore throat), was suspected of having influenza virus infection, and had signed an informed consent form was considered eligible for enrollment. Patients who had prior nasal wash/aspirate or nasopharyngeal wash/aspirate specimens collected for routine health care purposes within the same suspected influenza virus infection episode or from whom nasal and throat swab specimens could not be collected were excluded. Enrollment was planned to continue up to a maximum of 1,500 subjects or at least until a required total number of influenza virus-positive specimens was obtained from freshly collected specimens: 30 cases of 1977 H1N1, 30 cases of H3N2, and 30 cases of influenza B. No required sample size for H5N1 was set, as this rarely causes human infections in Vietnam.
Samples.
Four specimens (two nasal swab and two throat swab specimens) were collected from each subject. One nasal swab specimen and one throat swab specimen were tested on-site with the POC test; the other swabs were placed into viral transport medium (M4 collection kit; Remel, Lenexa, KS), brought to the laboratory on-site, and aliquoted for RT-PCR and viral culture. Aliquots for viral culture were stored at −80°C and shipped every 4 months on dry ice to the virology reference lab at the Hospital for Tropical Diseases, Ho Chi Min City, Vietnam.
POC test.
The MSD influenza test (POC test) consists of single-use disposable cartridges and a small benchtop reader that processes the cartridges, eliminating possible intraobserver bias. The test was designed for nasal swab samples; however, throat swab samples were also included in the study to evaluate an alternative sample type (as H5N1 reaches higher viral loads in the pharynx than the nose). The test uses antibodies for influenza virus A and B nucleoproteins to identify virus type and antibodies for 1977 H1, H3, and H5 hemagglutinin to identify influenza virus A subtype. Each cartridge contains all reagents necessary to carry out the test panel, including controls that monitor the assay procedure and check reagent integrity. Electrochemiluminescence (ECL)-based immunoassays are performed using an integrated fluidic network. The tips of the swabs are inserted and snapped off in the cartridges, which are then inserted into the reader. Processing and analysis steps are fully automated, including extraction of the sample from the swab. The control results are automatically checked by the reader to ensure that the test result is valid. Results are reported for influenza A and influenza B viruses as negative or positive. If the influenza A virus result is positive, then subtype information is displayed; if H1, H3, and H5 antigens were not detected, the subtype result is reported not determined. Results are reported within 15 min. Tests were done within 1 h of specimen collection at room temperature or within 8 h if the specimens were stored refrigerated. As required by the study protocol, a positive control and a negative control were run each day before subject specimens were tested.
Reference testing.
RT-PCR for detection of influenza A and B viruses and for subtyping of influenza A viruses was done according to WHO/CDC protocols (CDC Real-Time RT-PCR Protocol for Detection and Characterization of Influenza [version 2007] and for Detection and Characterization of Swine Influenza [version 2009]) using a Superscript III one-step RT-PCR with Platinum Taq (Invitrogen, Carlsbad, CA) on a DNA Engine Peltier thermocycler platform with a Chromo4 RT-PCR detector (Bio-Rad, Hercules, CA). All laboratories participate in an external quality assurance program for influenza virus RT-PCR. Virus culture was conducted using Madin-Darby canine kidney (MDCK) cells (ATCC CCL-34) in a shell vial format with a maximum of three passage attempts per specimen, according to WHO protocols. Virus isolates were typed and subtyped using Imagen influenza A and B immunofluorescence tests (Oxoid, Cambridge, United Kingdom) according to the instructions of the manufacturer, and subtypes were confirmed by bidirectional sequencing of the viral hemagglutinin using a BigDye Terminator (version 3.1) cycle sequencing kit on an ABI 3100 genetic analyzer (Applied Biosystems, Foster City, CA) following the manufacturer's instructions using published primers for H3N2 (16) and in-house primers for 2009 H1N1 (8).
Data analysis.
The primary objective was to evaluate the performance of the POC test in comparison to virus culture and RT-PCR in detecting influenza virus A (and subtypes) and influenza virus B in nasal swabs and throat swabs. Performance was evaluated as the sensitivity and specificity of the POC test in comparison to RT-PCR and virus culture. A per patient analysis and a per sample analysis were done. In the per patient analysis, a patient is considered positive if either the nasal or the throat swab (or both) is positive and negative if both swabs are negative (or if one is negative and one has no result/failure). Patients who had failure of both swabs in the POC test were excluded, unless otherwise specified. In the per sample analysis, nasal swab and throat swab results are analyzed separately; failed POC tests were excluded from analysis (see Table 1).
Table 1.
Per patient and per sample (nasal and throat swab) performance of a point-of-care test for influenza compared to RT-PCR and viral culturea
| Analysis and test compared with POC test | n | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| Per patient analysis | |||||
| RT-PCR flu | 529 | 74.0 (131/177)b | 99.7 (351/352) | 99.2 (131/132) | 88.4 (351/397) |
| Culture flu | 163 | 76.9 (20/26) | 100 (137/137) | 100 (20/20) | 95.8 (137/143) |
| RT-PCR A | 529 | 73.6 (67/91) | 99.8 (437/438) | 98.5 (67/68) | 94.8 (437/461) |
| RT-PCR B | 529 | 74.4 (64/86) | 99.8 (442/443) | 98.5 (64/65) | 95.3 (442/464) |
| Nasal swab | |||||
| RT-PCR flu | 476 | 73.6 (117/159) | 99.7 (316/317) | 99.2 (117/118) | 88.3 (316/358) |
| Culture flu | 146 | 86.4 (19/22) | 100 (124/124) | 100 (19/19) | 97.6 (124/127) |
| RT-PCR A | 476 | 74.1 (60/81) | 99.7 (394/395) | 98.4 (60/61) | 94.9 (394/415) |
| RT-PCR B | 476 | 73.1 (57/78) | 100 (398/398) | 100 (57/57) | 95.0 (398/419) |
| Throat swab | |||||
| RT-PCR flu | 440 | 52.4 (75/143) | 99.3 (295/297) | 97.4 (75/77) | 81.3 (295/363) |
| Culture flu | 130 | 61.1 (11/18) | 100 (112/112) | 100 (11/11) | 94.1 (112/119) |
| RT-PCR A | 440 | 54.7 (41/75) | 100 (365/365) | 100 (41/41) | 91.5 (365/399) |
| RT-PCR B | 440 | 50.0 (34/68) | 99.5 (370/372) | 94.4 (34/36) | 91.6 (370/404) |
POC, point-of-care test; n, number of patients or samples; flu, influenza virus; A, influenza virus A, B, influenza virus B; NPV, negative predictive value; PPV, positive predictive value.
Data in parentheses indicate number of patients or samples positive/total number of patients or samples tested.
Tests for associations between POC test sensitivity and continuous covariates (age, day of illness, and cycle threshold [CT] value) were based on logistic regression. Data were analyzed using R (version 2.11.1; Foundation for Statistical Computing, Vienna, Austria).
Ethics.
The protocol was approved by the ethics committee (EC) of each institution, the National Institute of Allergy and Infectious Diseases (NIAID) Institutional Review Board, and the Oxford Tropical Research Ethics Committee and was conducted in accordance with good clinical practice. The investigational trial was registered at clinicaltrials.gov as NCT01089816.
RESULTS
Between February and November 2010, a total of 569 patients were enrolled into the study (NHTD, 142; HTD, 78; CH1, 167; CH2, 182). The study was stopped in November 2010 before the target number of enrolled or positive patients was fully achieved because influenza virus 1977 H1N1 was no longer circulating in Vietnam and the POC test was not designed to subtype the novel 2009 H1N1 which had become dominant within the study population, whereas the target number of positive cases of influenza virus A/H3 and B had been met. At this time, virus culture attempts were also stopped; samples from a total of 173 patients had been cultured. Three patients withdrew from the study, and for 3 patients, RT-PCR was not performed. Among the remaining 563 patients, 184 were positive for influenza viruses by RT-PCR (94 for influenza virus A [18 for 2009 H1N1, 76 for H3N2] and 90 for influenza virus B).
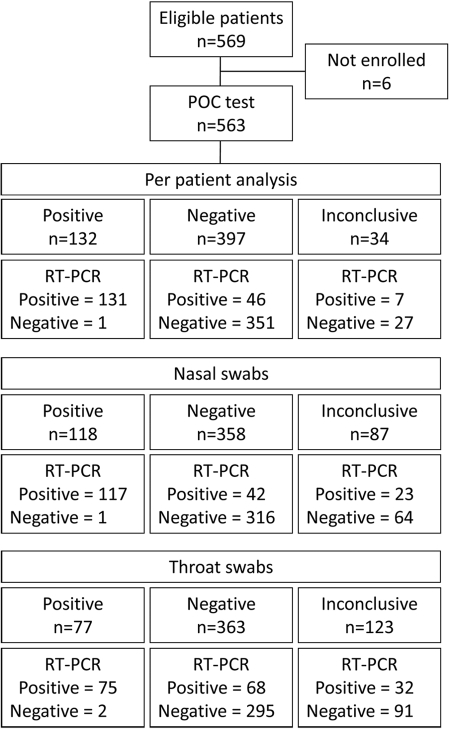
For 34 patients (6%), the POC test failed to yield a conclusive result for either influenza A or B virus (failure of both nasal and throat swabs); there were 87 (15%) failures for the nasal swab and 123 (22%) for the throat swab. Failures were due to instrument failure (n = 4), internal control failure (n = 17), test failure (n = 162), or other failures (n = 27). Failures were excluded from the analysis of the performance of the POC test compared to RT-PCR or viral culture, unless otherwise specified. A flow diagram of the study is presented in Fig. 1.
Fig 1.
Flow diagram of the clinical validation of a point-of-care test compared to RT-PCR for influenza virus. Each patient had a nasal swab and a throat swab specimen taken. The diagram shows results when analyzed per patient and for nasal and throat swabs separately. In the per patient analysis, a patient was considered positive if the nasal swab or the throat swab (or both) was positive for influenza virus (A or B). Patients were treated as negative if both the nasal and throat swabs were negative for influenza virus (A and B) or—for the POC test—if one swab specimen was negative and the other was inconclusive.
Viral culture was conducted for 173 of enrolled patients. When analyzed per patient, agreement between RT-PCR and viral culture was high (κ = 0.937): 30/173 (9 for influenza virus A and 21 for influenza virus B) patients had at least one positive sample by RT-PCR, and 27 (8 for influenza virus A and 19 for influenza virus B) of these patients were also positive by culture. When analyzed per sample, among 30 nasal swab specimens and 30 throat swab specimens positive by RT-PCR, 24 (7 for influenza virus A and 17 for influenza virus B) and 24 (8 for influenza virus A and 16 for influenza virus B) were also positive by culture, respectively (κ = 0.869 and 0.869). No RT-PCR-negative samples were positive by culture.
Patient data.
The median age of the patients was 25 months (range, 1 month to 70 years; interquartile ratio [IQR], 12 months to 22 years), with a female/male ratio of 0.48 (n = 268):0.52 (n = 295). The median day of illness at collection of samples was 3 (IQR, 2 to 5).
Per patient analysis.
In the per patient analysis, a patient was considered positive if the nasal or the throat (or both) swab was positive for influenza virus A or B. Patients were treated as negative if both the nasal and throat swabs were negative for influenza virus A and B or—for the POC test—if one swab was negative and the other failed.
Overall sensitivity for combined detection of influenza viruses A and B compared to RT-PCR was 74.0% (131/177; 95% confidence interval [CI], 67.1 to 79.9%), with a specificity of 99.7% and corresponding positive predictive value (PPV) and negative predictive value (NPV) of 99.2 and 88.4%, respectively. If failures were considered negative, sensitivity dropped slightly to 71.2% (95% CI, 64.3 to 77.3%). Overall sensitivity compared to culture in patients where both test results were available was 76.9% (20/26; 95% CI, 57.9 to 89.0%). Values were similar for detection of influenza viruses A and B (73.6% [67/91] versus 74.4% [64/86]). Results are displayed in Table 1.
Among 73 patients positive for H3 by RT-PCR (and for whom a POC test result was available), 54 were also positive with the POC test for influenza virus A, but only 9 were correctly subtyped as H3 (less than 20% of positive patients) and 1 of these was subtyped as both H3 and H1. The performance for the other subtypes could not be determined. Among 18 patients positive for 2009 H1N1 by RT-PCR, 13 were also positive with the POC test for influenza virus A; the POC test did not have the ability to subtype 2009 H1N1 viruses, but isolates from 2 of those patients were incorrectly subtyped as H3. An additional RT-PCR/culture-negative patient was positive by the POC test for influenza virus A, which subtyped as H1 and H3.
There was no association between day of illness at sample collection and positivity rate of the POC test among RT-PCR-positive samples (P = 0.93; data not shown); the positivity rate for the POC test was higher among children under age 15 years with a positive RT-PCR result (P = 0.001).
Per sample analysis.
In the per sample analysis, the overall sensitivity of the POC test for combined detection of influenza viruses A and B compared to RT-PCR was 73.6% (117/159) in nasal swabs and 52.4% (75/143) in throat swabs, and compared to culture, these values were 86.4% (19/22) and 61.1% (11/18), respectively, in samples where both culture and POC results were available (Table 1). Results for influenza viruses A and B were very similar. CT values of the RT-PCR (which are inversely correlated to log viral load) were higher in throat swabs than in nasal swabs, which may provide an explanation for the large difference in their sensitivity. Sensitivity was strongly associated with CT values for both nasal and throat swabs and for both influenza virus A and influenza virus B (Table 2). Values for specificity, PPV, and NPV were similar to those for the per patient analysis. If failures were taken into account, sensitivities dropped to 64.3% and 42.9% for nasal and throat swabs, respectively.
Table 2.
Performance of a point-of-care test for influenza in nasal and throat swabs compared to RT-PCR stratified for CT value of PCR resulta
| Virus and POC test result | No. of samples with the following CT value: |
P | |||
|---|---|---|---|---|---|
| 0–25 | 25–30 | 30–35 | 35 or higher | ||
| Influenza A virus | |||||
| Nasal pos | 32 | 18 | 6 | 4 | |
| Nasal neg | 0 | 1 | 11 | 9 | <0.0001 |
| Throat pos | 12 | 21 | 7 | 1 | |
| Throat neg | 0 | 8 | 10 | 16 | <0.0001 |
| Influenza B virus | |||||
| Nasal pos | 38 | 16 | 3 | 0 | |
| Nasal neg | 4 | 10 | 6 | 1 | 0.0004 |
| Throat pos | 20 | 8 | 5 | 0 | |
| Throat neg | 11 | 14 | 8 | 1 | 0.03 |
Numbers of samples with a positive RT-PCR result are displayed per range of CT values with the corresponding numbers of positive and negative POC test results and the P value of that association. Nasal, nasal swab specimen; throat, throat swab specimen; pos, positive; neg, negative.
DISCUSSION
Point-of-care tests with a time to result of about 15 min have been available for diagnosis of influenza for many years. Clinical and laboratory evaluation of these tests on other sample sets have shown limited sensitivities of between 50 and 80% and generally good specificity compared to virus culture or RT-PCR as the “gold standard” (4–7, 9–15, 17, 18). For clinical practice, the CDC recommends that POC tests be relied on only to rule influenza in and not to rule it out and only in case of outbreaks or during influenza season (2).
The MSD influenza test was developed to be more sensitive than currently available POC tests for detection of influenza viruses A and B and to be able to subtype influenza virus A, especially avian H5N1 viruses. The test takes approximately 15 min to complete and requires a reader to assess the results. We evaluated the performance of this test on 563 patients (children and adults) from whom nasal and throat swab specimens were taken and who presented with ILI to four hospitals in Vietnam. Combining the results of the two swabs resulted in a sensitivity of 74.0% (131/177) compared to RT-PCR and a sensitivity of 76.9% (20/26) compared to culture. These numbers are in the high range of reported sensitivities (50 to 80%) for POC tests that were evaluated in other studies. Specificity was at least 99% in all analyses.
There was a significant correlation between CT values of the RT-PCR and positivity rate of the POC test. Furthermore, positivity rates were higher in children than in adults, as has been reported before (9, 13, 15, 17). The test was designed for use on nasal swabs, and, indeed, nasal swabs had a much higher diagnostic yield than throat swabs (73.6% versus 52.4%); this has also been reported for other POC tests (14), and, furthermore, although positivity rates for RT-PCR in nasal and throat swabs were similar, median CT values in positive throat swabs were higher than those in positive nasal swabs. Combining the results of nasal and throat swabs did not change the sensitivity (74.0% versus 73.6%), but a larger number of patients were positive compared to the number when nasal swabs alone were used (177 versus 159). Sensitivities for influenza viruses A and B were similar, whereas previous POC test evaluations have reported lower sensitivities for influenza virus B (30 to 50%) than influenza virus A (6, 7, 9, 14).
The MSD influenza test was designed to be able to subtype influenza virus A into 1977 (prepandemic) H1, H3, and (avian) H5. Among the enrolled patients were no patients with 1977 H1N1 or avian H5N1, and only subtyping of H3N2 could be evaluated. Subtyping was poor, as virus from only 9 out of 54 patients positive for H3 by RT-PCR and positive for influenza virus A by the POC test was correctly subtyped as H3. In addition, virus in two 2009 H1N1-infected patients was incorrectly subtyped as H3.
The limitations of this study were that no patients with 1977 H1N1 or with avian H5N1 were enrolled and instead patients infected with a novel subtype of influenza virus A (2009 H1N1, detected as influenza virus A but not subtyped by the test) for which the test was not validated were enrolled and that—due to time and funding constraints—only a subset of samples was cultured.
In summary, the sensitivity of the MSD influenza test to detect influenza viruses was high and is in the upper range of what has previously been reported for other POC tests. The test was as sensitive for influenza virus B as for influenza virus A, whereas previous reports on other POC tests consistently showed lower sensitivity for influenza virus B. The POC test is easy to use and, unlike lateral-flow tests, does not require manual sample extraction. Drawbacks are that the test cannot be used at bedside, as it requires a reader, that there was a high number of test failures, and that subtyping—although determined only for H3—was poor and requires improvement.
ACKNOWLEDGMENTS
The study doctors, nurses, lab technicians, and administrators at the four enrolling hospitals and staff at the Clinical Trials Unit and the virology reference lab at the Hospital for Tropical Diseases, at the Network Coordination Centre in Jakarta, Indonesia, and at the National Institute of Allergy and Infectious Diseases who participated in this study are gratefully acknowledged for their contributions. Meso Scale Diagnostics is acknowledged for participating in study design and reviewing of the manuscript.
This project has been funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, MD, under contract number HHSN272-2009-00001l. Other funding came from the Biomedical Advanced Research and Development Authority, Washington, DC (HHS200-2007-19346), and the Wellcome Trust of Great Britain (grant 077078/Z/05/Z). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 22 February 2012
REFERENCES
- 1. Anonymous 2006, posting date CDC awards $11.4 million to develop new rapid diagnostic tests for avian influenza. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/media/pressrel/r061204.htm [Google Scholar]
- 2. Anonymous 2011, posting date Guidance for clinicians on the use of rapid influenza diagnostic tests. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm [Google Scholar]
- 3. Anonymous 2009. Swine influenza A (H1N1) infection in two children—Southern California, March-April 2009. MMWR Morb. Mortal. Wkly. Rep. 58:400–402 [PubMed] [Google Scholar]
- 4. Babin SM, Hsieh YH, Rothman RE, Gaydos CA. 2011. A meta-analysis of point-of-care laboratory tests in the diagnosis of novel 2009 swine-lineage pandemic influenza A (H1N1). Diagn. Microbiol. Infect. Dis. 69:410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biggs C, et al. 2010. Performance of influenza rapid antigen testing in influenza in emergency department patients. Emerg. Med. J. 27:5–7 [DOI] [PubMed] [Google Scholar]
- 6. Booth S, Baleriola C, Rawlinson WD. 2006. Comparison of two rapid influenza A/B test kits with reference methods showing high specificity and sensitivity for influenza A infection. J. Med. Virol. 78:619–622 [DOI] [PubMed] [Google Scholar]
- 7. Cruz AT, Cazacu AC, Greer JM, Demmler GJ. 2008. Rapid assays for the diagnosis of influenza A and B viruses in patients evaluated at a large tertiary care children's hospital during two consecutive winter seasons. J. Clin. Virol. 41:143–147 [DOI] [PubMed] [Google Scholar]
- 8. Hien TT, et al. 2010. Early pandemic influenza (2009 H1N1) in Ho Chi Minh City, Vietnam: a clinical virological and epidemiological analysis. PLoS Med. 7:e1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hurt AC, Alexander R, Hibbert J, Deed N, Barr IG. 2007. Performance of six influenza rapid tests in detecting human influenza in clinical specimens. J. Clin. Virol. 39:132–135 [DOI] [PubMed] [Google Scholar]
- 10. Landry ML, Cohen S, Ferguson D. 2008. Real-time PCR compared to Binax NOW and cytospin-immunofluorescence for detection of influenza in hospitalized patients. J. Clin. Virol. 43:148–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liao RS, Tomalty LL, Majury A, Zoutman DE. 2009. Comparison of viral isolation and multiplex real-time reverse transcription-PCR for confirmation of respiratory syncytial virus and influenza virus detection by antigen immunoassays. J. Clin. Microbiol. 47:527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lucas PM, et al. 2011. Diagnosis of 2009 pandemic influenza A (pH1N1) and seasonal influenza using rapid influenza antigen tests, San Antonio, Texas, April-June 2009. Clin. Infect. Dis. 52(Suppl. 1):S116–S122 [DOI] [PubMed] [Google Scholar]
- 13. Rouleau I, Charest H, Douville-Fradet M, Skowronski DM, De Serres G. 2009. Field performance of a rapid diagnostic test for influenza in an ambulatory setting. J. Clin. Microbiol. 47:2699–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smit M, Beynon KA, Murdoch DR, Jennings LC. 2007. Comparison of the NOW Influenza A & B, NOW Flu A, NOW Flu B, and Directigen Flu A+B assays, and immunofluorescence with viral culture for the detection of influenza A and B viruses. Diagn. Microbiol. Infect. Dis. 57:67–70 [DOI] [PubMed] [Google Scholar]
- 15. Steininger C, Redlberger M, Graninger W, Kundi M, Popow-Kraupp T. 2009. Near-patient assays for diagnosis of influenza virus infection in adult patients. Clin. Microbiol. Infect. 15:267–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tran TH, et al. 2004. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 350:1179–1188 [DOI] [PubMed] [Google Scholar]
- 17. Uyeki TM, et al. 2009. Low sensitivity of rapid diagnostic test for influenza. Clin. Infect. Dis. 48:e89–e92 [DOI] [PubMed] [Google Scholar]
- 18. Weitzel T, Schnabel E, Dieckmann S, Borner U, Schweiger B. 2007. Evaluation of a new point-of-care test for influenza A and B virus in travellers with influenza-like symptoms. Clin. Microbiol. Infect. 13:665–669 [DOI] [PubMed] [Google Scholar]