Abstract
The epidemiology of new acquisition of antibiotic-resistant organisms (AROs) in community-based skilled nursing facilities (SNFs) is not well studied. To define the incidence, persistence of, and time to new colonization with methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and ceftazidime-resistant (CAZr) and ciprofloxacin-resistant (CIPr) Gram-negative bacteria (GNB) in SNFs, SNF residents were enrolled and specimens from the nares, oropharynx, groin, perianal area, and wounds were prospectively cultured monthly. Standard microbiological tests were used to identify MRSA, VRE, and CAZr and CIPr GNB. Residents with at least 3 months of follow-up were included in the analysis. Colonized residents were categorized as having either preexisting or new acquisition. The time to colonization for new acquisition of AROs was calculated. Eighty-two residents met the eligibility criteria. New acquisition of AROs was common. For example, of the 59 residents colonized with CIPr GNB, 28 (47%) were colonized with CIPr GNB at the start of the study (96% persistent and 4% intermittent), and 31 (53%) acquired CIPr GNB at the facility (61% persistent). The time to new acquisition was shortest for CIPr GNB, at a mean of 75.5 days; the time to new acquisition for MRSA was 126.6 days (P = 0.007 versus CIPr GNB), that for CAZr was 176.0 days (P = 0.0001 versus CIPr GNB), and that for VRE was 186.0 days (P = 0.0004 versus CIPr GNB). Functional status was significantly associated with new acquisition of AROs (odds ratio [OR], 1.24; P = 0.01). New acquisition of AROs, in particular CIPr GNB and MRSA, is common in SNFs. CIPr GNB are acquired rapidly. Additional longitudinal studies to investigate risk factors for ARO acquisition are required.
INTRODUCTION
The prevalence of antibiotic-resistant organisms (AROs), such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus spp. (VRE), and resistant Gram-negative bacteria (GNB) has been well studied (4, 11, 18, 28, 30). It is estimated that one-third of the 1.6 million skilled nursing facility (SNF) residents in the United States are colonized with at least one ARO. MRSA is the most common ARO studied, and cross-sectional point prevalence studies in SNFs show a wide range of colonization rates, with 10 to 50% of residents being colonized with MRSA (20, 22, 26, 31, 33). Multidrug-resistant (MDR) Gram-negative bacteria have been found to colonize over 20% of residents of long-term care facilities (23), while prevalence rates for VRE are found to be lower at 4 to 9.6% (2, 3, 26). The presence of indwelling devices, functional impairment, prior hospitalization, and antimicrobial usage are all considered to increase the risk of multiple ARO colonization (7, 12, 20, 32, 35, 36).
In contrast to the large quantity of cross-sectional data available, there are limited prospective studies that document new acquisition rates in SNFs. In one prospective study by Bradley et al. (5), specimens from multiple body sites were cultured monthly for a year, and it was found that 25% of SNF veterans were colonized with MRSA upon initial culturing. Over the course of the year of study, 10% of admitted residents newly acquired MRSA at the facility. Another study by Stone et al. (29) found a 48% prevalence of MRSA on weekly cultures obtained over an 8-week period in a long-term care facility. Only 29% of newly admitted residents were colonized on their initial culture, indicating a relatively large number of new acquisitions at the facility. A recent prospective study documented that 39% of long-term care residents acquired at least 1 MDR Gram-negative organism during a 1-year sampling period (24). Other short-term prospective studies documented new acquisition of ceftazidime-resistant (CAZr) GNB in 22 of 86 (25.6%) colonized surgical intensive care unit patients during their stay over the 5-month study period (10) and new acquisition of VRE in 6 of 32 (19%) chronic hemodialysis patients for whom cultures were obtained every 5 days until discharge (9). A more recent prospective study with hemodialysis patients found that 22 (40%) of the 55 who had follow-up cultures acquired a new ARO, with totals of 20, 15, and 13% of patients acquiring MDR GNB, VRE, and MRSA, respectively (27).
The main objective of this prospective study was to further characterize new acquisition of multiple AROs in community-based SNFs, with a focus on defining the time to acquisition and persistence of colonization. To define the dynamics of new ARO acquisition in residents of SNFs more precisely, residents were followed for a period of at least 90 days and up to 1 year.
MATERIALS AND METHODS
Study design and population.
We conducted a large prospective microbial study involving 15 SNFs located in the southeast region of Michigan. The goal of this parent study was to define the attributable fraction of device-associated infections and ARO colonization. The project was approved by the University of Michigan and Veterans Affairs Ann Arbor Healthcare System Institutional Review Boards. Any resident with an indwelling device and a randomly selected control were considered to be eligible for this study. Of 483 eligible residents, 178 (37%) were enrolled in the parent study. The main reasons for nonenrollment were refusal to give informed consent by the residents (23%) or their family or legal guardian (32%), inability to contact family or legal guardian (21%), and discharge from the facility or device discontinuation (23%). We obtained written informed consent from all enrolled residents or from the individual holding a durable power of attorney. Each resident was enrolled for up to 1 year, and monthly cultures were obtained. Clinical and demographic data were obtained by chart review.
Of the 178 patients enrolled in the parent study, 90 residents had an indwelling device (a urinary catheter, feeding tube, or both) and 88 residents did not. In order to achieve our stated objective in this substudy of defining new acquisition in residents who stay in a facility long-term, we included only residents who had a minimum of 90 days of follow-up. Eighty two residents (46%) from 12 SNFs qualified for this analysis, including 21 residents (25.6%) with an indwelling device.
Baseline demographic and clinical data.
Baseline information was obtained from the resident's chart, such as age, weight, gender, presence of an indwelling device, Charlson's comorbidity score, functional status, hospitalization, antibiotic usage, and duration of residence at the facility. At each subsequent visit, information on hospitalization and antibiotic usage was gathered. The Lawton and Brody Physical Self Maintenance Scale (PSMS) was used to assess functional status (15), and mean values for Charlson's comorbidity score were calculated to assess comorbidity (6). Residents were considered hospitalized if they stayed at the hospital for at least one night. The use of any antibiotic was included in quantifying antibiotic usage.
Microbiological methods.
Cultures were obtained from the nares, oropharynx, groin, and perianal area during each monthly visit. If present, specimens from wounds and skin around feeding tube or suprapubic catheter sites were also cultured using Culturette swabs (Becton Dickinson Inc., Sparks, MD). Standard microbiological methods were used to identify positive colonization with Staphylococcus aureus, vancomycin-resistant Enterococcus spp. (VRE), and GNB. S. aureus isolates were further tested for methicillin resistance using oxacillin screening plates, and GNB isolates were tested for both ceftazidime and ciprofloxacin resistance by disc diffusion (Sensi-Disc; BD BBL, Sparks, MD). Strains that showed intermediate sensitivity to CIP or CAZ were considered to be resistant.
Definitions of preexisting colonization, new acquisition, intermittent colonization, and persistent colonization.
Colonized residents were divided into two categories: those with a resistant organism present at the start of the study, or having “preexisting colonization,” and those with a resistant organism acquired at the facility during the time of the study, or having “new acquisition.” Adhering to the Society for Healthcare Epidemiology of America recommendation for measuring AROs (8), residents with new ARO acquisitions were those with no positive culture for that organism recovered on the first visit (day 0) followed by a positive culture on any subsequent visit. Each type of ARO was evaluated independently for new acquisition. For example, a resident with preexisting MRSA could have a new acquisition of ciprofloxacin resistance (CIPr) during the study period.
For each category, the status of the colonization was defined as either persistent or intermittent carriage. Definitions for persistent and intermittent carriage vary widely in the literature (14, 16, 21, 23, 25, 34). We used the definitions established by Muder et al. (21), as these were the most appropriate for prospective studies in the SNF setting. Under this definition, an intermittent carriage is defined as two or more negative cultures after a single positive culture for any of the organisms, whereas persistence is defined as two or more positive cultures separated by fewer than two negative cultures. If there was no culture taken after a single positive culture, it was conservatively classified as an intermittent colonization.
The time to acquisition was calculated for each new acquisition, measured by the visit day that the organism was first identified in culturing samples.
Statistical analysis.
Colonization rates were calculated by estimating the percentage of residents who were colonized with the organism at any anatomic site at any time in the study. Student t tests were performed to analyze differences in mean functional status and Charlson's comorbidity score. Residents who newly acquired an organism were considered to have prior hospitalization or antibiotic usage if these events occurred at any time before the first identification of colonization. To be conservative, noncolonized residents were considered to have prior hospitalization or to have taken antibiotics if these events occurred at any point during the study. Residents with a preexisting colonization were considered to have prior hospitalization or antibiotic usage if these events occurred within 30 days prior to the start of the study. For hospitalization and antibiotic usage, chi-square tests were performed to compare the probabilities between colonized and noncolonized residents. Statistical analysis was performed using STATA 10, and P values of ≤0.05 were considered significant. We also compared residents who newly acquired any ARO with residents not colonized with any ARO at any point, using a multivariate logistic regression model to assess the risk factors leading to new acquisition. Risk factors included in the analysis were functional status, Charlson's comorbidity score, prior hospitalization, and antibiotic usage. Residents with a preexisting colonization who did not acquire any other new ARO during the study period were excluded from the multivariate analysis.
RESULTS
Demographics.
The demographics of the subpopulation used for analysis are shown in Table 1, by groups with and without indwelling devices. Indwelling devices were present in 26% of the population with at least 90 days of follow-up (total population, n = 82). Residents with indwelling devices were younger, had more comorbidities, were more functionally dependent, and had a shorter follow-up period than residents without them.
Table 1.
Baseline data for the 82 SNF residents enrolled in the study
| Characteristic | Value for SNF residents: |
P value | |
|---|---|---|---|
| Without indwelling device (n = 61) | With indwelling device (n = 21) | ||
| Age (yr, mean ± SD) | 83.49 ± 9.85 | 77.52 ± 13.88 | 0.03 |
| Wt (lb, mean ± SD) | 157.46 ± 43.41 | 160.60 ± 46.12 | 0.78 |
| No. (%) male | 12 (20) | 10 (48) | |
| No. (%) white | 59 (97) | 16 (76) | |
| Comorbidity score (mean ± SD) | 2.25 ± 1.59 | 3.00 ± 1.52 | 0.06 |
| PSMS (mean ± SD) | 19.31 ± 5.19 | 24.00 ± 4.95 | <0.01 |
| Follow-up days in the study (mean ± SD) | 278.36 ± 81.82 | 228.57 ± 95.57 | 0.02 |
| Admission time in facility (mo, mean ± SD) | 39.10 ± 44.54 | 45.98 ± 101.23 | 0.67 |
| No. (%) with: | |||
| Prior hospitalization | 7 (11) | 12 (57) | <0.001 |
| Antibiotic usage | 43 (70) | 17 (81) | 0.35 |
| Ciprofloxacin usage | 12 (20) | 4 (19) | 0.95 |
Colonization with antibiotic-resistant organisms.
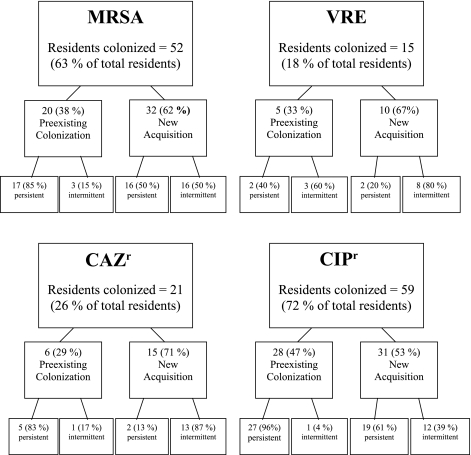
Overall, CIPr GNB were the most prevalent ARO, colonizing 59 of 82 residents in this study (72%) (Fig. 1). A significant proportion, 31 of 59 (53%) residents colonized, newly acquired the organism at the facility. Of these new acquisitions, 19 (61%) remained persistently colonized on subsequent cultures. Of the 28 (47%) residents with preexisting colonization, 27 (96%) remained colonized at subsequent visits. Escherichia coli and Proteus mirabilis were the two most common CIPr GNB persistently colonizing residents, often cocolonizing the same resident (Table 2).
Fig 1.
Flow chart displaying the total number of residents colonized and the percentage of colonization for each organism. Unless otherwise stated, the percentages in parentheses represent the percentage of residents colonized out of the number of residents colonized in that group with that organism (e.g., 17 [85%] of 20 preexisting colonizations were intermittent). MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci; CAZr, ceftazidime-resistant Gram-negative bacillus; CIPr, ciprofloxacin-resistant Gram-negative bacillus.
Table 2.
Numbers of SNF residents with persistent CIPr GNB colonization, by species
| No. and name(s) of species present | No. of residents with: |
|
|---|---|---|
| Preexisting colonization (n = 27) | New acquisition (n = 19) | |
| 1 | ||
| Escherichia coli | 10 | 3 |
| Proteus mirabilis | 2 | 4 |
| Pseudomonas aeruginosa | 1 | 0 |
| Providencia stuartii | 2 | 0 |
| Morganella morganii | 1 | 4 |
| 2 | ||
| E. coli, P. mirabilis | 7 | 4 |
| E. coli, P. stuartii | 0 | 1 |
| P. mirabilis, P. stuartii | 0 | 1 |
| P. mirabilis, Pseudomonas fluorescens | 1 | 0 |
| 3 | ||
| E. coli, P. mirabilis, M. morganii | 0 | 1 |
| E. coli, P. mirabilis, K. pneumoniae | 1 | 0 |
| ≥4 | 1 | 0 |
| No single persistent species | 1 | 1 |
MRSA colonized 52 residents (63%), 32 of whom (62%) newly acquired the organism at the facility, with equal numbers of residents being colonized persistently or intermittently. Of those with preexisting colonization, most (17 [85%]) remained colonized at subsequent visits.
CAZr GNB had a lower prevalence, colonizing 21 residents (26%). A majority of colonization with CAZr GNB (15 [71%]) was newly acquired at the facility, with 2 (13%) residents being persistently colonized. VRE were the least prevalent of all the AROs, colonizing only 15 residents (18%). Ten residents newly acquired VRE (67%), 2 of whom (20%) were colonized persistently.
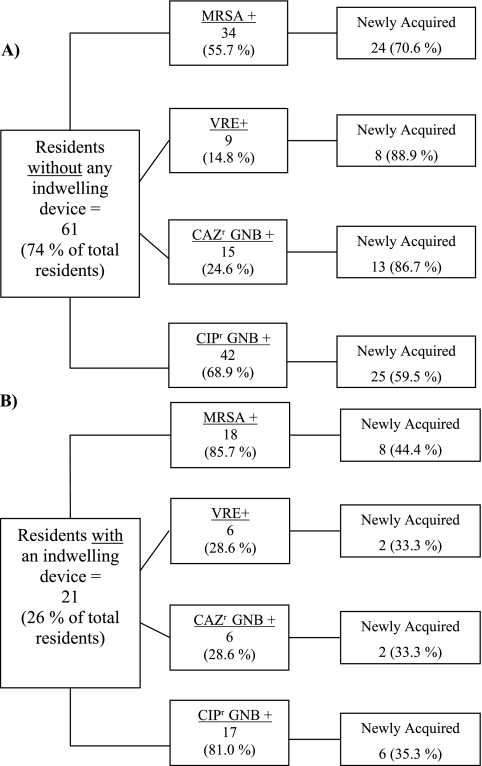
In the group with devices, MRSA was the most prevalent ARO, colonizing 18 (85.7%) residents with devices at any time during the study (Fig. 2B). CIPr GNB were also prevalent, colonizing 17 (81.0%) residents with devices, while VRE and CAZr GNB each colonized 6 (28.6%) residents with devices. In comparison, in residents without an indwelling device, CIPr GNB were the most prevalent ARO (42 [68.9%]), followed by MRSA (34 [55.7%]), CAZr GNB (15 [24.6%]), and VRE (9 [14.8%]). Due to higher rates of preexisting colonization, residents with indwelling devices had lower rates of new ARO acquisition. In contrast, the majority of colonized residents without devices newly acquired the ARO during the study period (Fig. 2A). As expected, groin and perirectal areas gave the highest yield to identify new colonization with both CIPr and CAZr GNB (Table 3).
Fig 2.
The total numbers of residents in the groups without (A) and with (B) indwelling devices are displayed in the largest, leftmost boxes, followed by the numbers and percentages of those residents colonized with the antibiotic-resistant organism. To the right of these boxes, the numbers of the colonizing organisms that were newly acquired are indicated, along with the corresponding percentage. MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci; CAZr GNB, ceftazidime-resistant Gram-negative bacillus; CIPr, ciprofloxacin-resistant Gram-negative bacillus.
Table 3.
Anatomic sites colonized with resistant GNB, by device status
| Anatomic site | % of residentsa: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| With indwelling device (n = 52 anatomic sites in 17 residents) |
Without indwelling device (n = 85 anatomic sites in 44 residents) |
|||||||||
| CAZr GNB only | CIPr GNB only | CIPr and CAZr GNB | Cocolonized | Total | CAZr GNB only | CIPr GNB only | CIPr and CAZr GNB | Cocolonized | Total | |
| Nares | 0 | 3.8 | 1.9 | 0 | 5.8 | 0 | 3.5 | 0 | 0 | 3.5 |
| Oropharynx | 0 | 11.5 | 1.9 | 1.9 | 15.4 | 0 | 2.4 | 1.2 | 0 | 3.5 |
| Groin | 1.9 | 19.2 | 1.9 | 5.8 | 28.8 | 1.2 | 29.4 | 0 | 12.9 | 43.5 |
| Rectum | 1.9 | 21.2 | 0 | 5.8 | 28.8 | 2.4 | 40.0 | 0 | 4.7 | 47.1 |
| Wound | 1.9 | 1.9 | 3.8 | 0 | 7.7 | 1.2 | 0 | 0 | 1.2 | 2.4 |
| Device site | 0 | 11.5 | 0 | 1.9 | 13.5 | 0 | 0 | 0 | 0 | 0 |
| Total | 5.8 | 69.2 | 9.6 | 15.4 | 100 | 4.7 | 75.3 | 1.2 | 18.8 | 100 |
For each group, n is the total number of anatomic sites colonized in that group. Data represent the percentage of colonization at that anatomic site out of the total number of anatomic sites colonized for that group. For the group with a device, there were 52 sites colonized in 17 residents (out of 21; 4 were not colonized) with a total of 134 follow-up months. For the group without a device, there were 85 sites colonized in 44 residents (out of 61; 17 not colonized) with a total of 396 follow-up months. CIPr and CAZr GNB, GNB resistant to both classes of antibiotics. Cocolonized, two or more classes of resistant GNB identified at the same site (i.e., CAZr GNB and CIPr GNB).
Time to new acquisition of ARO.
Average time to new acquisition in all residents was the shortest for CIPr GNB, at 75.5 days (standard deviation [SD], ±65.7) (Table 4). The time to new acquisition for MRSA was 126.6 days (±79.1), which was significantly longer than that for CIPr GNB (P = 0.007). CAZr GNB and VRE also had significantly longer average times to new acquisition, at 176.0 days (± 94.1; P = 0.0004 versus CIPr GNB) and 186.0 days (±108.4; P = 0.0001 versus CIPr GNB).
Table 4.
Average time to new acquisition of AROs
| Organism(s) | Days to acquisition (avg ± SD) for residents: |
P value (without device vs with device) | ||
|---|---|---|---|---|
| All (n = 82) | Without indwelling device (n = 61) | With indwelling device (n = 21) | ||
| MRSA | 126.6 ± 79.1 | 143.8 ± 78.1 | 75.0 ± 60.0 | 0.03 |
| VRE | 186.0 ± 108.4 | 176.3 ± 113.9 | 225.0 ± 106.1 | 0.60 |
| CAZr GNB | 176.0 ± 94.1 | 182.3 ± 90.4 | 135.0 ± 148.5 | 0.53 |
| CIPr GNB | 75.5 ± 65.7 | 74.4 ± 66.0 | 80.0 ± 70.1 | 0.85 |
In residents with devices, the times to new acquisition for both MRSA and CIPr GNB (75.0 and 80.0 days, respectively) were shorter than those for CAZr GNB or VRE (135.0 and 225.0 days, respectively). Residents with devices acquired MRSA more rapidly than those without devices (Table 4) (P = 0.03). In residents without devices, CIPr GNB had the shortest mean time to new acquisition, at 74.4 days. The times to new acquisition in residents without devices for MRSA, VRE, and CAZr GNB were considerably longer, at 143.8, 176.3, and 182.3 days, respectively.
Risk factors for new acquisition of ARO.
We compared residents who newly acquired any ARO (n = 57 [70.0%]) with residents who were not colonized with any ARO at any point (n = 11 [13.4%]). Residents with a preexisting colonization who did not acquire any other new AROs were excluded (Table 5). Compared with noncolonized residents, colonized residents with a new acquisition were more functionally dependent (mean PSMS = 20.9 versus 15.9). This was the only significant risk factor identified using a multivariate logistic regression model (odds ratio [OR], 1.24; 95% confidence interval [CI], 1.05 to 1.46; P = 0.01). Prior hospitalization, antibiotic usage, Charlson's comorbidity score, and device use were not statistically significant.
Table 5.
Risk factors for not being colonized versus having new acquisition of AROs
| Risk factor | Value for residents: |
|
|---|---|---|
| Not colonized with any ARO (n = 11) | Having new acquisition of any ARO (n = 57) | |
| PSMS, mean ± SD | 15.9 ± 5.61 | 20.9 ± 5.35a |
| Charlson's comorbidity score, mean ± SD | 2.36 ± 2.34 | 2.51 ± 1.51 |
| Any hospital visit, no./total (%) | 1/11 (9) | 16/57 (28) |
| Any antibiotic use, no./total (%) | 6/11 (55) | 42/57 (74) |
| Device use, no./total (%) | 1/11 (9) | 14/57 (25) |
P ≤ 0.05.
DISCUSSION
In this prospective study, we showed that ARO colonization rates have increased considerably in recent years and that new acquisition of AROs is common. The proportion of residents with a new acquisition exceeded the proportion of residents who were colonized at the start of the study, for all AROs. Residents with an indwelling device had higher rates of preexisting colonization. In contrast, residents without devices had a higher percentage of new acquisition than those without devices.
CIPr GNB were the most prevalent ARO, colonizing 72% of residents during the study period. An earlier study, published in 1998, on CIPr in an SNF found low colonization rates, with a mean prevalence of 2.6% (17). Since then, there appears to have been a rapid rise in colonization with CIPr GNB. The time to new acquisition for all residents was shortest for CIPr GNB, at 75.5 days, suggesting a relatively rapid acquisition of CIPr. CIPr E. coli and P. mirabilis seem to be of particular concern, as they were found to colonize residents persistently over long periods of time. We chose to test all GNB separately for ciprofloxacin and ceftazidime resistance in order to understand the magnitude of resistant GNB. These antimicrobials were chosen to reflect prescribing practices in the SNF setting, in which fluoroquinolone and cephalosporin use is common (3). Our results suggest that resistant GNB are a major concern in SNFs and should prompt reevaluation of empirical treatment for common infections.
We also evaluated the roles of various risk factors in the acquisition of ARO colonization. As expected, residents with indwelling devices had greater ARO colonization rates. They also had a shorter time to new acquisition of MRSA, which to our knowledge has not been previously reported. Impaired functional status is also a risk factor for MRSA colonization and infections (1, 5, 7). Our findings confirm this, demonstrating that residents who newly acquired an ARO were more functionally impaired than those residents who were not colonized. It can be hypothesized that residents with impaired functional status require more assistance in activities of daily living, therefore increasing the likelihood of transmission. Hospitalization is also correlated to an increased risk of colonization and infection with AROs in SNFs (2, 13, 35, 36), although this was not significant, possibly due to our sample size. Antibiotic usage was frequent and was not correlated to colonization or new acquisition of AROs. Further efforts should be made to investigate the link between antibiotic usage and acquisition of AROs.
A major strength of our study is that we prospectively cultured specimens from multiple anatomic sites of residents for extended periods of time. As a result, we have been able to perform a more comprehensive assessment of new ARO acquisition and thereby ARO transmission in SNF residents. Rates of prevalence of ARO colonization have been extensively described, but the studies have often been performed at a single SNF, used clinical cultures, or described colonization at a single anatomic site (2, 16, 23, 24). It is increasingly recognized that colonization at multiple anatomic sites is common (19), and therefore the multisite sampling may account for the higher rates of colonization.
A limitation of our study is that new acquisition of AROs in SNFs may have been underestimated due to the fact that we did not enroll solely new admissions to the facility. Therefore, baseline colonization could represent undetected acquisition at the facility before enrollment for some residents. New acquisition may also have been overestimated, as it is possible that residents had a preexisting colonization that was not found on the initial culture. However, with a mean time to acquisition of between 70 and 150 days, multiple repeated negative cultures were obtained; therefore, it is likely that true new ARO acquisition was indeed frequent. New acquisition found after a hospitalization during the follow-up period could reflect ARO acquisition from the hospital or the SNF. Molecular typing of ARO strains was not performed. It is thus impossible to identify whether the newly acquired organisms were acquired due to horizontal transmission or to a change in the resident's endogenous flora as a result of antibiotic pressure. It is also not possible to determine whether persistent carriage of a certain ARO was the result of chronic carriage of the same strain or represented intermittent carriage of different strains.
Limitations notwithstanding, the high prevalence of new acquisition of AROs in SNFs in our study suggests a growing need to develop more effective and targeted infection prevention protocols. Infection prevention strategies should also account for multisite colonization, both within the SNF and upon admission to acute care facilities. This study furthers our knowledge that acquisition of AROs in SNFs is common, particularly acquisition of CIPr GNB and MRSA. CIPr GNB are acquired rapidly and are more likely to persist after initial colonization. This adds to the growing concern that resistant GNB are a greater threat than MRSA and VRE. Further research into the epidemiology of specific resistant GNB, such as P. mirabilis and E. coli, is needed to investigate the factors that lead to persistent colonization. Infection prevention strategies in SNFs need to be adapted to prevent the spread and new acquisition of all AROs, particularly resistant GNB.
ACKNOWLEDGMENTS
We thank the staff and residents of all participating nursing homes.
L. Mody and this work were supported by National Institute of Aging grant K23 AG028943, an ASP/AGS T. Franklin Williams Research Scholarship, National Institute of Aging grant R01AG032298, and The Claude D. Pepper Older Americans Independence Center and Michigan Institute for Clinical and Health Research, University of Michigan Pilot Grant Program.
Footnotes
Published ahead of print 29 February 2012
REFERENCES
- 1. Anderson DJ, et al. 2008. Poor functional status as a risk factor for surgical site infection due to methicillin-resistant Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 29:832–839 [DOI] [PubMed] [Google Scholar]
- 2. Benenson S, et al. 2009. Vancomycin-resistant enterococci in long-term care facilities. Infect. Control Hosp. Epidemiol. 30:786–789 [DOI] [PubMed] [Google Scholar]
- 3. Benoit SR, et al. 2008. Factors associated with antimicrobial use in nursing homes: a multilevel model. J. Am. Geriatr. Soc. 56:2039–2044 [DOI] [PubMed] [Google Scholar]
- 4. Bonomo RA. 2000. Multiple antibiotic-resistant bacteria in long-term-care facilities: an emerging problem in the practice of infectious diseases. Clin. Infect. Dis. 31:1414–1422 [DOI] [PubMed] [Google Scholar]
- 5. Bradley SF, et al. 1991. Methicillin-resistant Staphylococcus aureus: colonization and infection in a long-term care facility. Ann. Intern. Med. 115:417–422 [DOI] [PubMed] [Google Scholar]
- 6. Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40:373–383 [DOI] [PubMed] [Google Scholar]
- 7. Chen T, et al. 2010. Poor functional status is an independent predictor of surgical site infections due to methicillin-resistant Staphylococcus aureus in older adults. J. Am. Geriatr. Soc. 58:527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen AL, et al. 2008. Recommendations for metrics for multidrug-resistant organisms in healthcare settings: SHEA/HICPAC position paper. Infect. Control Hosp. Epidemiol. 29:901–913 [DOI] [PubMed] [Google Scholar]
- 9. D'Agata E, et al. 2001. Vancomycin-resistant enterococci among chronic hemodialysis patients: a prospective study of acquisition. Clin. Infect. Dis. 32:23–29 [DOI] [PubMed] [Google Scholar]
- 10. D'Agata E, Venkataraman L, DeGirolami P, Samore M. 1997. Molecular epidemiology of acquisition of ceftazidime-resistant gram-negative bacilli in a nonoutbreak setting. J. Clin. Microbiol. 35:2602–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Esposito S, Leone S, Noviello S, Ianniello F, Fiore M. 2007. Antibiotic resistance in long-term care facilities. New Microbiol. 30:326–331 [PubMed] [Google Scholar]
- 12. Eun SH, et al. 2006. The point prevalence and associated risk factors of nasal mehicillin-resistant Staphylococcus aureus colonisation in eight geriatric hospitals in Korea. Clin. Microbiol. Infect. 12:81–83 [DOI] [PubMed] [Google Scholar]
- 13. Ho PL. 2003. Carriage of methicillin-resistant Staphylococcus aureus, ceftazidime-resistant gram-negative bacilli and vancomycin-resistant enterococci before and after intensive care unit admission. Crit. Care Med. 31:1175–1182 [DOI] [PubMed] [Google Scholar]
- 14. Kluytmans J, Belkum A, Verbrugh H. 1997. Nasal carraige of Staphylococcus aureus: epidemiology, underlying mechanisms and associated risks. Clin. Microbiol. Rev. 10:505–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lawton MP, Brody EM. 1969. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontology 9:179–186 [PubMed] [Google Scholar]
- 16. Lederer SR, Riedelsdorf G, Schiffl H. 2007. Nasal carriage of methicillin-resistant Staphylococcus aureus: the prevalence, patients at risk and the effect of elimination on outcomes among outclinic haemodialysis patients. Eur. J. Med. Res. 12:284–288 [PubMed] [Google Scholar]
- 17. Lee YL, et al. 1998. Low-level colonization and infection with ciprofloxacin-resistant gram-negative bacilli in a skilled nursing facility. Am. J. Infect. Control. 26:552–557 [DOI] [PubMed] [Google Scholar]
- 18. Mody L, Bradley SF, Strausbaugh LJ, Muder RR. 2001. Prevalence of ceftriaxone- and ceftazidime-resistant gram-negative bacteria in long-term-care facilities. Infect. Control Hosp. Epidemiol. 22:193–194 [DOI] [PubMed] [Google Scholar]
- 19. Mody L, Kauffman CA, Donabedian S, Zervos M, Bradley SF. 2008. Epidemiology of Staphylococcus aureus colonization in nursing home residents. Clin. Infect. Dis. 46:1368–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mody L, Maheshwari S, Galecki A, Kauffman CA, Bradley SF. 2007. Indwelling device use and antibiotic resistance in nursing homes: identifying a high-risk group. J. Am. Geriatr. Soc. 55:1921–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muder RR, Brennen C, Drenning SD, Stout JE, Wagener MM. 1991. Methicillin resistant staphylococcal colonization and infection in a long-term care facility. Ann. Intern. Med. 114:107–112 [DOI] [PubMed] [Google Scholar]
- 22. Murphy S, et al. 1992. Methicillin-resitant Staphylococcus aureus colonization in a long-term-care facility. J. Am. Geriatr. Soc. 40:213–217 [DOI] [PubMed] [Google Scholar]
- 23. O'Fallon E, Gautam S, D'Agata EMC. 2009. Colonization with multidrug-resistant gram-negative bacteria: prolonged duration and frequent cocolonization. Clin. Infect. Dis. 48:1375–1381 [DOI] [PubMed] [Google Scholar]
- 24. O'Fallon E, Kandall R, Schreiber R, D'Agata EMC. 2010. Acquisition of multidrug-resistant gram-negative bacteria: incidence and risk factors within a long-term care population. Infect. Control Hosp. Epidemiol. 31:1148–1153 [DOI] [PubMed] [Google Scholar]
- 25. Peacock SJ, de Silva I, Lowy FD. 2001. What determines nasal carraige of Staphylococcus aureus? Trends Microbiol. 9:605–610 [DOI] [PubMed] [Google Scholar]
- 26. Pop-Vicas A, Mitchell SL, Kandel R, Schreiber R, D'Agata EM. 2008. Multidrug-resistant gram-negative bacteria in a long-term care facility: prevalence and risk factors. J. Am. Geriatr. Soc. 56:1276–1280 [DOI] [PubMed] [Google Scholar]
- 27. Pop-Vicas A, Strom J, Stanley K, D'Agata EMC. 2008. Multidrug-resistant gram-negative bacteria among patients who require chronic hemoodialysis. Clin. J. Am. Soc. Nephrol. 3:752–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2006. Healthcare Infection Control Practice Advisory Committee. Management of multidrug-resistant organisms in healthcare settings. http://www.cdc.gov/ncidod/dhqp/pdf/ar/MDROGuideline2006.pdf Accessed 3 May 2010 [DOI] [PubMed]
- 29. Stone ND, et al. 2008, Importance of bacterial burden among methicillin-resistant Staphylococcus aureus carriers in a long-term care facility. Infect. Control Hosp. Epidemiol. 29:143–148 [DOI] [PubMed] [Google Scholar]
- 30. Strausbaugh LJ, Crossley KB, Nurse BA, Thrupp LD. 1996. Antimicrobial resistance in long-term-care facilities. Infect. Control Hosp. Epidemiol. 17:129–140 [DOI] [PubMed] [Google Scholar]
- 31. Strausbaugh LJ, Jacobson C, Sewell DL, Potter S, Ward TT. 1991. Methicillin-resistant Staphylococcus aureus in extended-care facilities: experience in a Veterans' Affairs nursing home and a review of the literature. Infect. Control Hosp. Epidemiol. 12:36–45 [DOI] [PubMed] [Google Scholar]
- 32. Tacconelli E, et al. 2009. Antibiotic usage and risk of colonization and infection with antibiotic-resistant bacteria: a hospital population-based study. Antimicrob. Agents Chemother. 53:4264–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trick WE, et al. 2001. Colonization of skilled-care facility residents with antimicrobial-resistant pathogens. J. Am. Geriatr. Soc. 49:270–276 [DOI] [PubMed] [Google Scholar]
- 34. VandenBergh MFQ, et al. 1999. Follow-up of Staphylococcus aureus nasal carraige after 8 years: redefining the persistent carrier state. J. Clin. Microbiol. 37:3133–3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. von Baum H, Schmidt C, Svoboda D, Bock-Hensley O, Wendt C. 2002. Risk factors for methicillin-resistant Staphylococcus aureus carriage in residents of german nursing homes. Infect. Control Hosp. Epidemiol. 23:511–515 [DOI] [PubMed] [Google Scholar]
- 36. Vovko P, et al. 2005. Risk factors for colonization with methicillin-resistant Staphylococcus aureus in a long-term-care facility in slovenia. Infect. Control Hosp. Epidemiol. 26:191–195 [DOI] [PubMed] [Google Scholar]