Abstract
Gordonia amicalis infection has never been reported in humans. We report here the first case of G. amicalis-related cutaneous infection after a traumatic injury. The isolate was confirmed by 16S rRNA sequencing analysis, and the patient responded well to repeated debridement and antibiotic treatment.
CASE REPORT
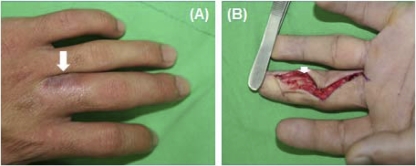
A 30-year-old man was referred to our hospital because of a chronic nonhealing wound on the middle finger of his left hand. Three months prior to this presentation, he sustained a high-pressure injection injury to the middle finger of his left hand that resulted in progressive painful swelling. He received surgical debridement at a hospital elsewhere, but the condition of the wound did not improve. On arrival at our hospital, physical examination showed a 0.5-cm by 0.5-cm ulcer on the volar side of the distal phalanx and a 1-cm by 1-cm ulcer on the dorsal side of the proximal phalanx of the middle finger of his left hand. A hard mass was also noted on the volar side of the proximal and middle phalanx (Fig. 1A). The patient did not have fever or other skin lesions. Laboratory studies revealed the following values: white blood cell count, 6.82 × 103/liter; serum urea nitrogen, 12.4 mg/dl; serum creatinine, 0.9 mg/dl; aspartate aminotransferase 16 U/liter; and sodium, 140 mmol/liter. Magnetic resonance imaging of the left hand revealed subcutaneous edema and fat stranding on the left middle finger with loculated cyst-like lesions of various sizes at the dorsoulnar aspect of the proximal portion and at the ventroulnar aspect of the distal portion of the finger. The wound on the middle finger of the left hand was debrided via a Brunner (volar zig-zag) incision. Yellowish discharge and chronic granulomatous inflammation were noted in zones I and II of the left middle finger (Fig. 1B). The debrided tissue was sent to the microbiology laboratory for bacterial, mycobacterial, and fungal cultures. There was no direct specimen Gram-stain preparation made of materials (pus or discharges).
Fig 1.
(A) A 1-cm by 1-cm ulcer with discharge on the dorsal side of the proximal phalanx of the middle finger of the left hand. (B) Yellowish discharge on the volar side of the middle phalanx beneath the neurovascular bundle.
Pathological examination of the excised tissue showed some foci of acute and chronic inflammatory cell infiltration with granulation tissue formation and fibrosis, as well as numerous empty spaces surrounded by foreign body giant cells. Several Gram-positive bacilli were visible. Antibiotics (ampicillin-sulbactam, 1,500 mg every 6 h) were administered intravenously, and the postoperative course was uneventful. The patient was discharged home 2 days later on a 7-day course of oral antibiotics (amoxicillin-clavulanate, 1 g every 12 h).
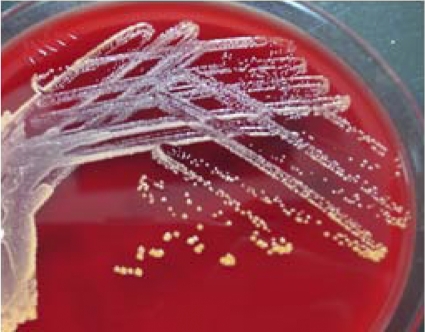
Cultures on Trypticase soy agar supplemented with 5% sheep blood (Becton Dickinson, Sparks, MD) grew small numbers of slightly orange and dry colonies and Gram-positive coryneform bacilli with rudimentary branching and weakly acid-fast bacilli after incubation for 4 days. The growth on chocolate agar (Becton Dickinson) and CDC blood agar plate (Becton Dickinson) was negative. Cultures for mycobacteria and fungi were negative. After subculture and incubation for 72 h on the blood agar plate, orange, opaque, dry, and nonhemolytic colonies without aerial hyphae were found (Fig. 2). The colonies were compatible with those for some Gordonia, Rhodococcus, and Nocardia species (10). The isolate exhibited negative biochemical reactions, including hydrolysis of casein, xanthine, hypoxanthine, and tyrosine (3). The isolate was identified as a Gordonia species by PCR-restriction fragment length polymorphism (RFLP) analysis of hsp65 (440 bp) with the presence of unique fragments of the isolates digested by HinfI (245/150 bp) (10). The isolate was further identified by partial 16S rRNA gene (980 bp) sequencing analysis as previously described (8). The accession number obtained from the GenBank database was HQ842811.1, with an identity of >99.9% as Gordonia amicalis, and the other closely related strain was Gordonia rubripertincta, with 98.9% identity. The MICs of amoxicillin-clavulanate, vancomycin, and ciprofloxacin were determined by Etest (AB Biodisk, Solna, Sweden) in accordance with the manufacturer's directions and were 1.0/0.5 μg/ml, 0.5 μg/ml, and 0.008 μg/ml, respectively. There were no MIC interpretive criteria of Gordonia isolates for defining susceptibility to antimicrobials by the Clinical and Laboratory Standards Institute.
Fig 2.
Orange, opaque, dry, and nonhemolytic colonies grew on Trypticase soy agar supplemented with 5% sheep blood after incubation for 72 h.
Gordonia species, previously classified as Rhodococcus species, are ubiquitous in the environment and are often found in soil and water (1, 3). Human infections caused by Gordonia species are rare. To date, at least nine Gordonia species, including G. terrae, G. bronchialis, G. polyisoprenivorans, G. rubripertincta, G. sputi, G. araii, G. effusa, G. otitidis, and G. amicalis (this report), have been reported to cause human infections (1, 2, 5, 7, 8, 10–12). Gordonia amicalis was first isolated from garden soil in Russia in 2000 and was proposed as a novel species by Kim et al. (9). In this study, the isolate was identified as G. amicalis based on the PCR-RFLP analysis of hsp65 and 16S rRNA sequencing analysis with >99% identity as defined by the Clinical and Laboratory Standards Institute (document MM18-A) (4). Further comparison of results from biochemical reactions and other sequence-based analysis might be of value for better differentiation between the two genetically close taxa G. amicalis and G. rubripertincta.
The clinical significance of G. amicalis, however, remains unknown. Herein, we demonstrated the first case of G. amicalis cutaneous infection after a traumatic injury. The clinical manifestations of human infections caused by Gordonia species include primary bacteremia, catheter-related bloodstream infection, respiratory tract infection, cutaneous infection, ocular infection, and central nervous system infection (2, 5–8, 10–15). Previously reported cases of skin and soft tissue infections (SSTI) due to Gordonia species included breast abscess and sternal wound infections (6, 10, 13–15). All of the reported cases of SSTI were caused by either G. terrae or G. bronchialis, and most of the patients required prolonged antibiotic treatment and surgical debridement (6, 10, 13–15).
The optimal antimicrobial treatment for infections due to Gordonia species remains unclear. A previous study reported that the MIC values were as low as ≤1/0.5 μg/ml for amoxicillin-clavulanic acid, ≤0.5 μg/ml for ciprofloxacin, and ≤0.5 μg/ml for vancomycin against Gordonia isolates and that the outcomes in patients treated with these three antimicrobials were favorable (10). In this study, the MIC value of G. amicalis was 1.0/0.5 μg/ml for amoxicillin-clavulanate, and our patient responded well to antibiotic therapy for 9 days and extensive debridement. More clinical isolates of Gordonia species are needed to further investigate the in vitro susceptibility and in vivo response.
To the best of our knowledge, this is the first reported case of cutaneous infection caused by G. amicalis after a traumatic injury. Combined antibiotic and surgical management were needed to treat the associated infection.
Footnotes
Published ahead of print 15 February 2012
REFERENCES
- 1. Arenskötter M, Bröker D, Steinbüchel A. 2004. Biology of the metabolically diverse genus Gordonia. Appl. Environ. Microbiol. 70:3195–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bakker XR, Spauwen PH, Volmans WM. 2004. Mycetoma of the hand caused by Gordonia terrae: a case report. J. Hand. Surg. Br. 29:188–190 [DOI] [PubMed] [Google Scholar]
- 3. Blaschke AJ, et al. 2007. Gordonia species: emerging pathogens in pediatric patients that are identified by 16S ribosomal RNA gene sequencing. Clin. Infect. Dis. 45:483–486 [DOI] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2008. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing; approved guideline. CLSI document MM18-A. Clinical and Laboratory Standards Institute, Wayne, Pa [Google Scholar]
- 5. Drancourt M, et al. 1994. Brain abscess due to Gordonia terrae in an immunocompromised child: case report and review of infections caused by G. terrae. Clin. Infect. Dis. 19:258–262 [DOI] [PubMed] [Google Scholar]
- 6. Gil-Sande E, et al. 2006. Etiological misidentification by routine biochemical tests of bacteremia caused by Gordonia terrae infection in the course of an episode of acute cholecystitis. J. Clin. Microbiol. 44:2645–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kageyama A, et al. 2006. Gordonia araii sp. nov. and Gordonia effusa sp. nov., isolated from patients in Japan. Int. J. Syst. Evol. Microbiol. 56:1817–1821 [DOI] [PubMed] [Google Scholar]
- 8. Kempf VA, et al. 2004. Gordonia polyisoprenivorans septicemia in a bone marrow transplant patient. Eur. J. Clin. Microbiol. Infect. Dis. 23:226–228 [DOI] [PubMed] [Google Scholar]
- 9. Kim SB, et al. 2000. Gordonia amicalis sp. nov., a novel dibenzothiophene-desulphurizing actinomycete. Int. Syst. Evol. Microbiol. 50:2031–2036 [DOI] [PubMed] [Google Scholar]
- 10. Lai, et al. 2010. Infections caused by Gordonia species at a medical centre in Taiwan, 1997 to 2008. Clin. Microbiol. Infect. 16:1448–1453 [DOI] [PubMed] [Google Scholar]
- 11. Renvoise A, Harle JR, Raoult D, Roux V. 2009. Gordonia sputi bacteremia. Emerg. Infect. Dis. 15:1535–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richet HM, et al. 1991. A cluster of Rhodococcus (Gordona) bronchialis sternal-wound infections after coronary artery bypass surgery. N. Engl. J. Med. 324:104–109 [DOI] [PubMed] [Google Scholar]
- 13. Sng L-H, et al. 2004. Bacteremia caused by Gordonia bronchialis in a patient with sequestrated lung. J. Clin. Microbiol. 42:2870–2871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Werno AM, Anderson TP, Chambers ST, Laird HM, Murdoch DR. 2005. Recurrent breast abscess caused by Gordonia bronchialis in an immunocompetent patient. J. Clin. Microbiol. 43:3009–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zardawi IM, Hones F, Clark DA, Holland JJ. 2004. Gordonia terrae induced suppurative granulomatous mastitis following nipple piercing. Pathology 36:275–280 [DOI] [PubMed] [Google Scholar]