Abstract
The lack of epidemiologic data on invasive Streptococcus pyogenes infections in many developing countries is concerning, as S. pyogenes infections are commonly endemic in these areas. Here we present the results of the first prospective surveillance study of invasive Streptococcus pyogenes infections in India. Fifty-four patients with invasive S. pyogenes infections were prospectively enrolled at two study sites, one in the north and one in the south of India. Sterile-site isolates were collected, and clinical information was documented using a standardized questionnaire. Available acute-phase sera were tested for their ability to inhibit superantigens produced by the patient's own isolate using a cell-based neutralizing assay. The most common clinical presentations were bacteremia without focus (30%), pneumonia (28%), and cellulitis (17%). Only two cases of streptococcal toxic shock syndrome and no cases of necrotizing fasciitis were identified. Characterization of the isolates revealed great heterogeneity, with 32 different emm subtypes and 29 different superantigen gene profiles being represented among the 49 sterile-site isolates. Analyses of acute-phase sera showed that only 20% of the cases in the north cohort had superantigen-neutralizing activity in their sera, whereas 50% of the cases from the south site had neutralizing activity. The results demonstrate that there are important differences in both clinical presentation and strain characteristics between invasive S. pyogenes infections in India and invasive S. pyogenes infections in Western countries. The findings underscore the importance of epidemiologic studies on streptococcal infections in India and have direct implications for current vaccine developments.
INTRODUCTION
Streptococcus pyogenes is a significant human pathogen capable of causing a wide spectrum of diseases ranging from uncomplicated infections of the throat and skin to severe life-threatening diseases and poststreptococcal sequelae. Streptococcal pharyngitis is one of the most common childhood diseases worldwide, accounting for several millions of cases each year. Inadequate treatment of S. pyogenes infections, predominantly throat infections, can result in the serious postinfectious sequela acute rheumatic fever, which may lead to rheumatic heart disease. The burden of poststreptococcal sequelae is great in developing countries but relatively rare in developed countries, although isolated outbreaks have been reported (5, 12). Furthermore, S. pyogenes causes invasive infections, including the two most severe invasive manifestations, streptococcal toxic shock syndrome (STSS) and necrotizing fasciitis, associated with high morbidity and mortality.
S. pyogenes invasive disease in North America and Europe has been an area of intense research since its reemergence in the late 1980s (1, 9). In contrast, these infections have not received much attention in developing countries. In an attempt to estimate the global burden of S. pyogenes infections, Carapetis et al. (5) reviewed available databases and estimated that more than 18 million people currently suffer from a serious S. pyogenes disease, with about 2 million new cases occurring each year and with an annual mortality of more than 100,000. Added to this are 111 million cases of streptococcal pyoderma and 616 million new cases of S. pyogenes pharyngitis each year. It was noteworthy that the vast majority of cases were in resource-limited countries. This report especially highlighted the fact that epidemiologic data from less developed countries are scarce, thus emphasizing the need for studies in these regions (5).
In India, the disease burden of streptococcal infections is considerable (26) and the incidence of acute rheumatic fever and rheumatic heart disease ranges from 0.3 to 5.4 per 1,000 children (24). Streptococcus pyogenes pharyngitis has a high prevalence in north India (14), whereas pyoderma is more frequent in south India (4). In light of invasive infection, this is a completely neglected field in India, and the only data available in the literature are from one retrospective study of invasive beta-hemolytic streptococcal infections (19). To advance our understanding of S. pyogenes infections in India and to obtain epidemiologic data that could direct the design of an effective vaccine, a European Commission-funded project, ASSIST, was launched in 2007. The project included surveillance of acute rheumatic fever/rheumatic heart disease as well as invasive S. pyogenes infections at study sites in north and south India. Here we present the results of the first prospective surveillance study of invasive S. pyogenes infections conducted in India, with the results showing important differences in relation to surveillance data from Western countries.
MATERIALS AND METHODS
Setting and study population.
Surveillance of invasive S. pyogenes infections was established at the two ASSIST study sites, the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, in the north of India, and the Christian Medical College (CMC), Vellore, in the south of India. All consenting patients with invasive S. pyogenes infection (n = 56) identified at the two sites during the study period (January 2007 to February 2010) were enrolled in the study. Invasive cases were defined as those with isolation of S. pyogenes from normally sterile sites, and cases of STSS were defined according to published definition criteria (30). Identification of cases was achieved by daily contact with the microbiology laboratory serving the hospital. Isolates were collected (n = 49), and clinical information obtained from the patients' medical records was documented in a standardized questionnaire designed to harmonize with data collected in the recently published StrepEuro study of invasive S. pyogenes infections in Europe (15). Acute-phase sera were obtained from 10 and 22 invasive cases from the north and south sites, respectively.
The ethics of the study were approved by the human research ethics committees at the respective hospitals in India, as well as in Stockholm, Sweden. Written informed consent was obtained from all enrolled patients.
Strain characterization: emm sequencing and superantigen gene profile.
Clinical isolates were confirmed to be S. pyogenes by use of conventional bacteriologic techniques. Genomic DNA was isolated from strains grown overnight in Todd-Hewitt medium plus yeast extract (THY) using zirconia beads in combination with a DNeasy kit (Qiagen, Hilden, Germany). Direct sequencing of the 5′ end of the emm gene was done as detailed at http://www.cdc.gov/ncidod/biotech/strep/M-ProteinGene_typing.htm, using the oligonucleotides emm_fwd (5′-TATTCGCTTAGAAAATTAA-3′) and emm_rev (5′-GCAAGTTCTTCAGCTTGTTT-3′). The emm type was obtained as described at http://www.cdc.gov/ncidod/biotech/strep/strepblast.htm by BLAST comparison to emm sequences in the database.
Superantigen genes were detected by PCR using the primers listed in Table S1 in the supplemental material. As positive controls for the respective genes, the sequenced strains MGAS315, MGAS6708, and MGAS8232 were used.
Proliferation and neutralization assays.
Supernatants were prepared from overnight bacterial cultures in Todd-Hewitt broth supplemented with yeast extract (THY) and tested for mitogenic activity in proliferation assays using peripheral blood mononuclear cells (PBMCs) from healthy donors as detailed elsewhere (22).
Patient sera were tested for their ability to inhibit superantigens produced by the patients' own isolates by use of a cell-based neutralizing assay previously described in detail (10, 22, 29). In brief, PBMCs from healthy donors were stimulated with superantigen-containing bacterial culture supernatants (SUPs; 1:50 dilution) in the presence of 2.5% heat-inactivated patient serum (PS) supplemented with 2.5% fetal calf serum (FCS) or 5% FCS. After 72 h, [3H]thymidine uptake was determined. Phytohemagglutinin-L (1 μg/ml; Sigma) was included as a control for nonspecific toxicity and PBMC quality. All samples were assayed in triplicate, and the data are presented as mean counts per minute of [3H]thymidine uptake ± standard deviation (SD). Serum neutralizing activity was calculated by the equation [1 − (cpmPS + SUP − cpmPS + medium)/(cpmFCS + SUP − cpmFCS + medium)] × 100. Significant neutralizing activity was defined as 50% inhibition of proliferative responses, as previously established (22). The analyses also included sera from 58 healthy controls (median age, 18 years; provided by PGIMER), which were tested for neutralizing activity against SUPs from two selected isolates (isolates 01-1400-01 and 01-1400-08), both from the north India study site, as that was the region of the controls. Intravenous polyspecific immunoglobulin (IVIG; 2.5 mg/ml; Baxter) was tested for neutralizing activity against bacterial culture supernatants using medium supplemented with 5% FCS.
Statistical analyses.
Data were analyzed using Prism software (version 5.01; Graphpad). The D'Agostino and Pearson omnibus normality test was used to assess the Gaussian distribution. Student's t test or the Mann-Whitney U test was used to compare the two patient cohorts, and the χ2 test or Fisher's exact test was used to compare the distribution of categorical data.
RESULTS
A total of 56 invasive cases were enrolled at the two Indian study sites, including 26 and 30 patients from the north and south sites, respectively. Sterile-site isolates were obtained from blood (n = 41, 73%), pleural fluid (n = 4), synovial fluid (n = 3), tissue aspirates (n = 3), peritoneal fluid (n = 2), bile (n = 1), and vitreous fluid (n = 2). Upon further analyses, two isolates were reclassified as group G streptococcus, and consequently, the two cases were excluded from the study. The median age of all invasive cases was 30 years (range, 1 day to 80 years), with 24% being ≤4 years old (Table 1 and Fig. 1). The two cohorts differed significantly with respect to age, as children and adolescents were more common among patients enrolled at the north site than the south site (Table 1 and Fig. 1).
Table 1.
Demographics and characteristics of invasive cases at the two Indian study sites
| Characteristic | Result by invasive case source |
P valuea | |
|---|---|---|---|
| North India (n = 24) | South India (n = 30) | ||
| Median (range) age | 8.5 (4 days–60 yr) | 51 (1 day–80 yr) | 0.0001 |
| % male sex | 54.0 | 66.7 | NS |
| No. (%) of cases with a fatal outcome | 4 (16.7) | 2 (6.7) | NS |
| % with an underlying condition | 37.5 | 53.3 | NS |
| No. (%) of cases with the following clinical diagnosis: | |||
| Bacteremia, no focal infection | 9 (37.5) | 7 (23.3) | NS |
| Pneumonia | 8 (33.3) | 7 (23.3) | NS |
| Cellulitis | 3 (12.5) | 6 (20) | NS |
| Septic arthritis | 0 | 4 (13.3) | NS |
| Meningitis | 3 (12.5) | 0 | NS |
| STSS | 2 (8.3) | 0 | NS |
| Necrotizing fasciitis | 0 | 0 | NS |
| Otherb | 3 (12.5) | 3 (10) | NS |
NS, not significant; significant, P < 0.05 using the Mann-Whitney U test or Fisher's exact test.
Including empyema, endophthalmitis, puerperal sepsis, nephrotic syndrome, peritonitis, and follicular cholecystitis.
Fig 1.
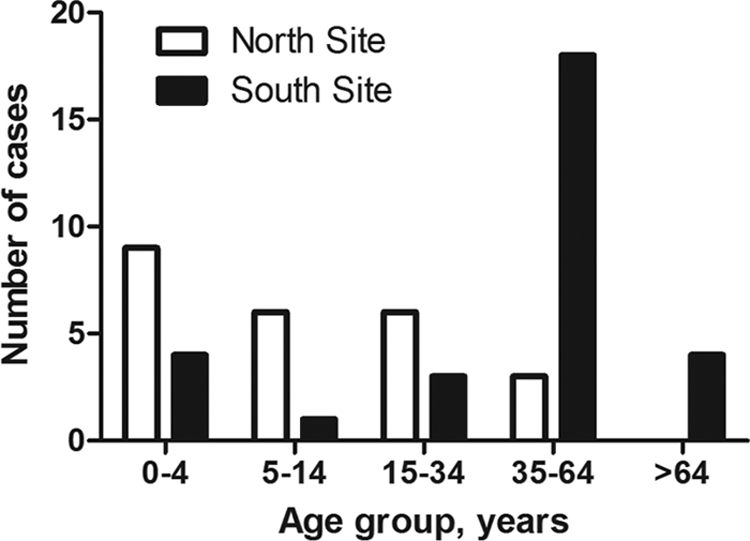
Age distribution among invasive S. pyogenes cases enrolled at the north and south study sites in India.
Clinical characteristics of invasive cases.
Forty-six percent of the invasive cases reported underlying conditions, including cancer (13%), chronic liver disease (7.4%), malnourishment (7.4%), surgery (5.6%), and burns (5.6%). The most common clinical diagnoses were bacteremia with no identified focus (30%) and pneumonia (28%), followed by cellulitis (17%). Septic arthritis cases were found only in the south site patient cohort, whereas meningitis cases were exclusively represented in the north cohort (Table 1). No necrotizing fasciitis cases and only two cases of STSS were identified. The two STSS cases were both children who presented with cellulitis and with meningitis and pneumonia. Nine patients were treated at the intensive care unit (16.7%). A fatal outcome was recorded in 6 of the 54 invasive cases, including 1 of the STSS cases, for a fatality rate of 11.1%. However, it should be noted that the study did not include a day 28 follow-up, and many patients declined hospitalization due to the associated cost; hence, the recorded fatalities are likely underestimated.
Microbiologic findings.
From the 54 invasive S. pyogenes cases, 49 isolates were retrieved and further characterized with respect to emm type and toxin gene profiling (Table 2). The emm sequencing revealed a great heterogeneity among the isolates, with 32 different emm types being represented in the cohort. The most common subtypes were emm49.0 (n = 4), emm74.0 (n = 4), emm80.0 (n = 4), emm12.0 (n = 3), and emm28.5 (n = 3). The fatal cases were caused by various subtypes, including the two emm1-2.2 isolates and one isolate each of emm28.5, emm74.0, and emm80.0. The type distribution differed between isolates from the north and south sites, with all isolates of the emm12.0 (n = 3), emm28.5 (n = 3), and emm49.0 (n = 4) subtypes being identified in the south cohort, whereas emm74.0 (n = 3), emm80.0 (n = 3), and emm1-2.2 (n = 2) were predominantly in the north isolate cohort.
Table 2.
Superantigen gene profiles related to emm subtypes of invasive S. pyogenes isolates
| Profile no. | emm type (no. of isolates) | No. (%) of isolates | Presence of superantigen |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| speA | speC | speG | speH | speI | speJ | speK | speL | speM | ssa | smeZ | |||
| 1 | 76.0, 80.0 (3), 105.0, 112.2 | 6 (12) | − | − | + | − | − | + | − | − | − | − | + |
| 2 | 63.0, 78.3, 113.0 (2), st6735.0 | 5 (10) | − | − | + | − | − | − | − | − | − | − | + |
| 3 | 42.0 (2), 58.8, stNS554.0 | 4 (8) | − | + | + | − | − | − | − | − | − | − | + |
| 4 | 12.0 (2), 118.0 | 3 (6) | − | + | + | − | − | − | − | − | − | + | + |
| 5 | 28.5 (3) | 3 (6) | − | − | − | − | − | − | − | − | − | − | + |
| 6 | 6.35, 74.0 (2) | 3 (6) | + | + | + | − | − | − | − | − | − | − | + |
| 7 | 11.0, 43.3 | 2 (4) | − | + | + | − | − | − | − | + | − | − | + |
| 8 | 49.0 (2) | 2 (4) | + | − | + | + | + | − | − | − | − | − | − |
| 9 | 1–2.2 | 1 (2) | + | − | + | + | + | + | − | + | + | + | + |
| 10 | 1–2.2 | 1 (2) | + | − | + | − | − | + | − | − | − | − | + |
| 11 | 4.5 | 1 (2) | − | − | − | − | − | − | + | − | − | + | + |
| 12 | 4.5 | 1 (2) | − | − | − | − | − | − | − | + | + | + | + |
| 13 | 9.0 | 1 (2) | − | − | + | − | − | − | + | − | − | − | + |
| 14 | 9.0 | 1 (2) | − | + | + | − | − | − | + | − | − | − | + |
| 15 | 12.0 | 1 (2) | − | + | + | + | + | − | − | − | − | + | + |
| 16 | 18.8 | 1 (2) | + | − | + | − | − | − | − | − | − | − | + |
| 17 | 44.0 | 1 (2) | − | + | + | + | − | + | − | − | − | − | − |
| 18 | 49.0 | 1 (2) | − | − | + | − | − | − | − | + | − | − | − |
| 19 | 49.0 | 1 (2) | − | − | + | + | + | − | − | + | + | − | − |
| 20 | 71.1 | 1 (2) | + | − | + | − | − | + | − | − | + | − | + |
| 21 | 74.0 | 1 (2) | + | + | + | + | + | − | − | + | + | + | + |
| 22 | 74.0 | 1 (2) | + | + | + | + | + | − | − | + | − | − | + |
| 23 | 79.1 | 1 (2) | − | + | + | − | − | − | − | − | − | − | − |
| 24 | 80.0 | 1 (2) | − | − | + | − | − | + | + | − | − | − | + |
| 25 | 85.0 | 1 (2) | − | − | + | + | + | − | − | − | − | − | + |
| 26 | 91.0 | 1 (2) | − | + | + | + | + | + | − | − | − | − | + |
| 27 | 103.0 | 1 (2) | − | − | + | + | − | + | + | − | − | + | + |
| 28 | 109.1 | 1 (2) | + | − | − | − | − | − | − | − | − | − | + |
| 29 | st2147.0 | 1 (2) | + | − | + | − | − | − | + | − | − | + | + |
| Gene frequency (%a) | 49 | 34 | 38 | 86 | 34 | 28 | 28 | 21 | 24 | 17 | 28 | 83 | |
Percentage related to number of profiles.
Exotoxin gene profiling of the 49 invasive isolates identified 29 distinct profiles with unique combinations of superantigen genes evenly distributed among the 32 different emm subtypes (Table 2). The 29 profiles contained between 1 and 9 superantigen genes per profile, with the greatest variation occurring in genes encoding prophage. There were five profiles that harbored only one or two exotoxin genes. Profile 5 harbored only smeZ and was exclusively represented by emm28.0 strains. speG and smeZ were the most common superantigen genes, being present in 86% and 83% of the profiles, respectively.
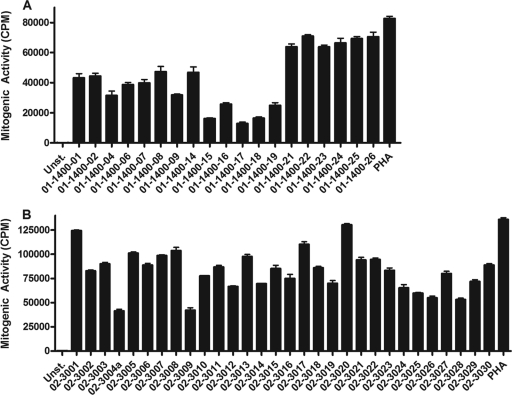
Superantigen-containing culture supernatants from all isolates were potent triggers of proliferative responses, and there was no statistical difference between the cohorts (Fig. 2). There was no relation between degree of proliferative response and the emm subtype, number of superantigen genes harbored, or superantigen profile.
Fig 2.
Mitogenic activity induced by overnight bacterial culture supernatants. Peripheral blood mononuclear cells from healthy donors were stimulated with bacterial supernatants prepared from invasive isolates collected at the north site (A) and south site (B). Proliferative responses were determined after 72 h of culture by measurement of [3H]thymidine uptake. The data are shown as mean counts per minute ± SD of triplicate experiments for unstimulated cells (unst.) and cells stimulated with supernatants of all invasive isolates (indicated by patient identifier) or phytohemagglutinin (PHA) as a positive control.
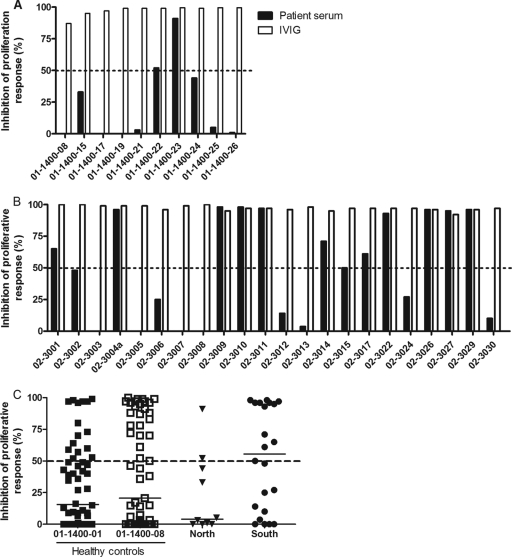
Superantigen-neutralizing activity in acute-phase sera.
As a lack of protective humoral immunity against superantigens has been shown to be a risk factor for invasive S. pyogenes infections in Europe and in Canada (2, 18, 22), it was of interest to assess protective antibody levels among Indian patients. Available acute-phase sera were tested for their ability to inhibit superantigens produced by the patient's own isolate using a cell-based neutralizing assay previously employed in epidemiologic studies (2, 18, 22, 29). Only 20% of the cases in the north cohort had superantigen-neutralizing activity in their sera (Fig. 3A), whereas 50% of the cases from the south cohort showed neutralizing activity (Fig. 3B). Equally low neutralizing activities were seen in the healthy controls, with 26% and 40% showing neutralizing activity against isolates 01-1400-01 and 01-1400-08, respectively (Fig. 3C). IVIG was included as a source of neutralizing superantigen antibodies that completely inhibits the superantigenic activity of invasive S. pyogenes isolates in Europe and in Canada (7, 13, 21). Similarly, superantigens produced by Indian invasive isolates were completely neutralized by IVIG (Fig. 3).
Fig 3.
Superantigen-neutralizing activity in acute-phase patient sera and in healthy controls. Peripheral blood mononuclear cells from healthy donors were stimulated with bacterial culture supernatants in the presence or absence of patient sera, and proliferative responses were assessed. The data are presented as percent inhibition of bacterial supernatant in the presence of patient serum (2.5%) or IVIG (2.5 mg/ml) in relation to the response obtained in the presence of fetal calf serum. Serum from each patient was tested for neutralizing activity against the patient's own isolate, whereas healthy control sera were analyzed using two selected isolates, as indicated. The dashed lines indicate 50% inhibition of mitogenic activity, which is considered significant neutralizing activity. Results for samples from the north site (A) and from the south site (B) are shown. (C) Compiled data for controls and patients. The horizontal lines denote median values in each group. Phytohemagglutinin was included as a control for nonspecific toxicity and in the presence of patient sera induced responses as high as those obtained with FCS.
DISCUSSION
Here we present the results of the first prospective study of invasive S. pyogenes infections in India, including infections at two study sites, one in the north and one in the south. At both sites, the most common clinical presentations were bacteremia without focus, pneumonia, and cellulitis. Three meningitis cases, all from the north site, were reported, consistent with children being more prevalent in this patient cohort than that at the south site. In contrast, four septic arthritis cases were found in the south patient cohort, whereas none was found in the north. It was noteworthy that only two STSS cases and no cases of necrotizing fasciitis were identified, numbers which are considerably less than those that have been reported from Western countries (8, 15). Equally low numbers were reported in a retrospective study of beta-hemolytic streptococcal infections in India, in which 3 cases of STSS were found among 225 invasive cases and there was no mention of necrotizing fasciitis cases (19). In contrast, results from the 2003-2004 European surveillance covering 11 countries reported 13% and 8% of cases with STSS and necrotizing fasciitis, respectively (15), and similar figures have been observed in Canada (8, 11) and the United States (23). Two recent prospective studies from developing countries, including one from New Caledonia, which reported 43% of cases with necrotizing fasciitis but only low frequencies of STSS (3%) (16), and one from Fiji, where 5% and 7% of cases with STSS and necrotizing fasciitis, respectively, were noted (27).
The lack of necrotizing fasciitis cases was particularly unexpected, considering that the south of India, like many other tropical settings, is known to have a high prevalence of streptococcal skin infections. It is recognized that different S. pyogenes emm types are associated with specific tissue tropism (3) and particular disease manifestations, such as the association between emm1 and emm3 with STSS and necrotizing fasciitis cases (reviewed in reference 9). emm subtyping of the Indian invasive isolates revealed great heterogeneity, with a total of 32 different subtypes being represented among the 49 isolates. Importantly, there were no emm1 or emm3 types among the invasive isolates, nor were there any emm15 types that were predominant in the study from New Caledonia and responsible for 19% of the necrotizing fasciitis cases (16). Thus, the emm distribution indicates a lack of types previously found to be associated with STSS and necrotizing fasciitis, which might explain the low number of cases with these severe manifestations in this material. However, the small size of the patient material does not allow robust conclusions, and future studies are warranted to confirm these data.
The heterogeneity in S. pyogenes emm types is in agreement with other studies from developing countries and indigenous populations, all of which report highly diverse emm types among throat, skin, and invasive isolates (16, 25, 27). Not only are the isolates more heterogenic, but also the emm types are strikingly different from those in the Western population (17, 28). This has implications for the coverage of the 26-valent vaccine that has completed clinical phase I and II trials (20), inasmuch as only 22% of the Indian isolates in this study are accounted for in the vaccine, compared to 69% and 76% for isolates in Europe and the United States, respectively (17, 23). However, it should be noted that a recent report of a 30-valent M-protein-based vaccine showed that vaccine-elicited bactericidal antibodies cross-reacted to some extent with nonvaccine serotypes, indicating that the efficacy may extend beyond the included serotypes (6).
Similar heterogeneity in the superantigen gene profile was seen, with 29 distinct profiles identified. Despite different profiles, all isolates were prominent triggers of T cell activation, and the degree of response was not associated with either the number of superantigen genes, the presence of smeZ, or particular profiles or emm types. Lack of superantigen-neutralizing antibodies has been identified to be a significant risk factor for invasive S. pyogenes infections in Western countries (2, 18, 22, 29). We hypothesized that the antibody levels should be higher in India, considering the endemic nature and thus likely greater exposure to streptococcal infections in this area. However, analyses of acute-phase sera and sera from healthy controls revealed surprisingly low levels of superantigen-neutralizing activity in both patients and controls. Thus, despite an expected high exposure to streptococcal infections, the Indian cases were as susceptible to streptococcal superantigens as the Western population. Here we assessed neutralizing activity only in a functional cell-based assay, but in future studies it will be of interest to investigate enzyme-linked immunosorbent assay (ELISA) titers of antibodies against superantigens as well as other streptococcal factors to address how neutralization relates to prior exposure. It has previously been shown that ELISA titers of antibodies to streptococcal superantigens frequently do not correlate with neutralizing activity (22), but the underlying rationale for this as of yet remains unknown.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the European Union's 6th Framework Program (grant number 032390), Torsten and Ragnar Söderberg's Foundation, the Swedish Research Council (grant number 12610), and Karolinska University Hospital.
We thank Jonathan Sampath Franklyne for important contributions to this work.
Footnotes
Published ahead of print 22 February 2012
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1. Aziz RK, Kotb M. 2008. Rise and persistence of global M1T1 clone of Streptococcus pyogenes. Emerg. Infect. Dis. 14:1511–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basma H, et al. 1999. Risk factors in the pathogenesis of invasive group A streptococcal infections: role of protective humoral immunity. Infect. Immun. 67:1871–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bessen DE, Sotir CM, Readdy TL, Hollingshead SK. 1996. Genetic correlates of throat and skin isolates of group A streptococci. J. Infect. Dis. 173:896–900 [DOI] [PubMed] [Google Scholar]
- 4. Brahmadathan KN, Koshi G. 1988. Epidemiology of streptococcal pyoderma in an orphanage community of a tropical country. J. Trop. Med. Hyg. 91:306–314 [PubMed] [Google Scholar]
- 5. Carapetis J, Steer AC, Mulholland EK, Weber M. 2005. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5:685–694 [DOI] [PubMed] [Google Scholar]
- 6. Dale JB, Penfound TA, Chiang EY, Walton WJ. 2011. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine 29:8175–8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Darenberg J, et al. 2003. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome—an European randomized double-blind placebo-controlled trial. Clin. Infect. Dis. 37:333–340 [DOI] [PubMed] [Google Scholar]
- 8. Davies DH, et al. 1996. Invasive group A streptococcal infections in Ontario, Canada. N. Engl. J. Med. 135:547–554 [DOI] [PubMed] [Google Scholar]
- 9. Efstratiou A. 2000. Group A streptococci in the 1990s. J. Antimicrob. Chemother. 45:3–12 [DOI] [PubMed] [Google Scholar]
- 10. Ekelund K, Skinhoj P, Madsen J, Konradsen HB. 2005. Invasive group A, B, C and G streptococcal infections in Denmark 1999-2002: epidemiological and clinical aspects. Clin. Microbiol. Infect. 11:569–576 [DOI] [PubMed] [Google Scholar]
- 11. Hollm-Delgado M-G, Allard R, Pilon PA. 2005. Invasive group A streptococcal infections, clinical manifestations and their predictors, Montreal 1995-2001. Emerg. Infect. Dis. 11:77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackson SJ, Steer AC, Campbell H. 2011. Systematic review: estimation of global burden of non-suppurative sequelae of upper respiratory tract infection: rheumatic fever and post-streptococcal glomerulonephritis. Trop. Med. Int. Health 16:2–11 [DOI] [PubMed] [Google Scholar]
- 13. Kaul R, et al. 1999. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome—a comparative observational study. Clin. Infect. Dis. 28:800–807 [DOI] [PubMed] [Google Scholar]
- 14. Kumar R, et al. 2009. Epidemiology of group A streptococcal pharyngitis & impetigo: a cross-sectional & follow up study in a rural community of northern India. Indian J. Med. Res. 130:765–771 [PubMed] [Google Scholar]
- 15. Lamagni TL, et al. 2008. Epidemiology of severe Streptococcus pyogenes disease in Europe. J. Clin. Microbiol. 46:2359–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le Hello S, et al. 2010. Clinical and microbial characteristics of invasive Streptococcus pyogenes disease in New Caledonia, a region in Oceania with a high incidence of acute rheumatic fever. J. Clin. Microbiol. 48:526–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luca-Harari B, et al. 2009. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J. Clin. Microbiol. 47:1155–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mascini EM, et al. 2000. Invasive group A streptococcal disease in the Netherlands: evidence for a protective role of anti-exotoxin A antibodies. J. Infect. Dis. 181:631–638 [DOI] [PubMed] [Google Scholar]
- 19. Mathur P, et al. 2002. Invasive beta-haemolytic streptococcal infections in a tertiary care hospital in northern India. J. Med. Microbiol. 51:791–792 [DOI] [PubMed] [Google Scholar]
- 20. McNeil SA, et al. 2005. Safety and immunogenicity of 26-valent group A streptococcus vaccine in healthy adult volunteers. Clin. Infect. Dis. 41:1114–1122 [DOI] [PubMed] [Google Scholar]
- 21. Norrby-Teglund A, et al. 1998. Varying titres of neutralizing antibodies to streptococcal superantigens in different preparations of normal polyspecific immunoglobulin G (IVIG): implications for therapeutic efficacy. Clin. Infect. Dis. 26:631–638 [DOI] [PubMed] [Google Scholar]
- 22. Norrby-Teglund A, Pauksens K, Holm SE, Norgren M. 1994. Relation between low capacity of human sera to inhibit streptococcal mitogens and serious manifestation of disease. J. Infect. Dis. 170:585–591 [DOI] [PubMed] [Google Scholar]
- 23. O'Loughlin RE, et al. 2007. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin. Infect. Dis. 45:853–862 [DOI] [PubMed] [Google Scholar]
- 24. Padmavati S. 2001. Rheumatic fever and rheumatic heart disease in India at the turn of the century. Indian Heart J. 53:35–37 [PubMed] [Google Scholar]
- 25. Sagar V, Kumar R, Ganguly NK, Chakraborti A. 2008. Comparative analysis of emm type pattern of group A Streptococcus throat and skin isolates from India and their association with closely related SIC, a streptococcal virulence factor. BMC Microbiol. 8:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shet A, Kaplan E. 2004. Addressing the burden of group A streptococcal disease in India. Indian J. Pediatr. 71:41–48 [DOI] [PubMed] [Google Scholar]
- 27. Steer AC, et al. 2009. Prospective surveillance of invasive group A streptococcal disease, Fiji, 2005-2007. Emerg. Infect. Dis. 15:216–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steer AC, et al. 2009. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect. Dis. 9:611–616 [DOI] [PubMed] [Google Scholar]
- 29. Vikerfors A, et al. 2009. Severe group A streptococcal infections in Uppsala County, Sweden: clinical and molecular characterization of a case cluster from 2006-2007. Scand. J. Infect. Dis. 41:822–830 [DOI] [PubMed] [Google Scholar]
- 30. Working Group on Severe Streptococcal Infections 1993. Defining the group A streptococcal toxic shock syndrome. Rationale and consensus definition. JAMA 269:390–391 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.