Abstract
In a low-incidence setting, health care workers (HCW) are at a higher risk of tuberculosis than the general population. The suboptimal sensitivity of the QuantiFERON-TB Gold In-Tube (QFT) test remains a critical issue when identifying occupational latent tuberculosis infection (LTBI) in HCW. The aim of this study was to identify additional biomarkers in order to overcome the limits of gamma interferon (IFN-γ) release assays (IGRAs) and improve the performance of LTBI diagnosis within this population. Seventy Bacille Calmette-Guérin-vaccinated HCW regularly exposed to Mycobacterium tuberculosis were grouped according to QFT results into an LTBI-positive group (positive QFT, n = 8), an LTBI-negative group (normal QFT and negative tuberculin skin test [TST], n = 21), and an undetermined group (subpositive QFT and/or positive TST, n = 41). The secretion of 22 cytokines in response to QFT-specific stimulation was quantified using a multiparameter-based immunoassay. As a result, thresholds discriminating LTBI-positive from LTBI-negative HCW were established by comparing areas under the receiver operating characteristic curves for interleukin-2 (IL-2), IL-15, IFN-γ-induced protein 10 (IP-10), and the monokine induced by IFN-γ (MIG), which are biomarkers differentially secreted by the two groups. The combination of IL-15 and MIG provided a sensitivity of 100% and a specificity of 94.1% in distinguishing LTBI-positive from LTBI-negative HCW. When using IL-15 and MIG among the undetermined group, 6/45 HCW could be classified in the LTBI-positive group. The use of additional biomarkers after IGRA screening could improve the diagnosis of LTBI. The performance of these biomarkers and their use in combination with TST and/or QFT, as well as the cost-effectiveness of such a diagnostic strategy, should be evaluated in further larger clinical trials.
INTRODUCTION
In countries with a low incidence of tuberculosis (TB), health care workers (HCW) are at a higher risk of contracting TB than the general population is (11, 15, 28). In this context, TB remains a significant occupational health problem. HCW working in a high-risk TB environment have long been screened periodically for latent TB infection (LTBI) using chest X-rays and the tuberculin skin test (TST). The TST has an estimated sensitivity of 78% (17), but its specificity is poor, mainly due to the cross-reactive immune responses to Bacille Calmette-Guérin (BCG) in a vaccinated population such as HCW. Recently, gamma interferon (IFN-γ) release assays (IGRAs), which are based on T-cell measurements of antimycobacterial immunity, have emerged as an alternative to the TST for diagnosing LTBI. IGRAs are not confounded by cross-reactivity because peptides used for T-cell stimulation are expressed by Mycobacterium tuberculosis and not by the BCG strains or by most of the nontuberculous mycobacteria (3). Furthermore, unlike the TST, IGRAs can be repeated without a sensitization risk or a boosting effect, which makes them attractive tests for TB control programs, particularly in an occupational setting. The use of IGRAs for HCW screening instead of the TST is recommended in the United States (14). In France, the current TB infection control policy indicates that IGRAs can be used for the LTBI pre-employment screening of HCW as a replacement for the TST (4).
The QuantiFERON test, manufactured by Cellestis, is one of these IGRAs quantifying the IFN-γ secreted in response to M. tuberculosis-specific RD1 antigens derived from the 6-kDa early secretory antigenic target (ESAT-6), the 10-kDa culture filtrate protein (CFP-10), and TB7.7. The third generation of this assay, named the QuantiFERON-TB Gold In-Tube (QFT) test, has a specificity close to 100% when diagnosing active TB (8, 24). However, the sensitivity of this test was suboptimal, between 55 and 94%, when a majority of patients with active TB were enrolled (8, 17, 24). The sensitivity of LTBI diagnostic tests may be significantly improved by selecting adequate secreted cytokines beyond IFN-γ. For example, the 10-kDa IFN-γ-inducible protein (IP-10) or interleukin-2 (IL-2) may be used as an adjunct biomarker in addition to IFN-γ for in vitro testing of LTBI (5, 21, 22, 27).
The aim of this study was to identify additional biomarkers that can be used with QFT to improve the diagnosis of LTBI among HCW exposed to patients with TB.
MATERIALS AND METHODS
Data collection and participants.
This study was carried out within a large, multicenter study, named QuantiFERON for detection of latent TB in health care workers (QUANTIPS), which assessed the cost-effectiveness of QFT versus that of the TST for the detection of latent TB among exposed HCW. The participants were adults working in medical units of French university hospitals with a high risk of M. tuberculosis exposure. In the present substudy, the population consisted of 70 BCG-vaccinated HCW from the Respiratory Diseases and Infectious Diseases Departments of the Centre Régional Hospitalier Universitaire (CHRU) de Montpellier in Montpellier, France. None of the workers enrolled were infected with human immunodeficiency virus, on anti-TB treatment or had a clinical examination suggesting an active disease. At the baseline, a TST was done using the Mantoux technique unless a previous test was positive before enrolment. All TST results were checked 48 to 72 h later and were considered positive when the induration area was ≥10 mm. Informed consent was obtained from all participants. This study was registered under the identifier NCT00797836 and was approved by the Institutional Review Board of Assistance Publique, Hôpitaux de Paris.
Study design.
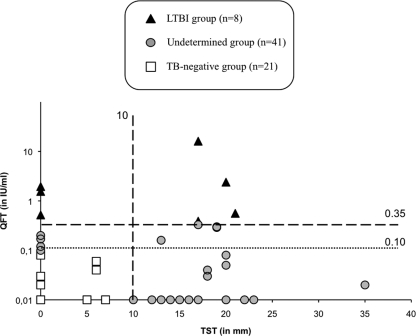
This study was conducted in two steps. During the first step, participants were classified into three groups according to their TB screening results. (i) The LTBI-positive group was composed of HCW positive for QFT using an IFN-γ cutoff of 0.35 IU/ml, independently of the TST. (ii) The LTBI-negative group was composed of HCW with a normal QFT value (<0.1 IU/ml) and a negative TST result (<10 mm). (iii) The undetermined group was composed of HCW with a subpositive QFT result (between 0.1 and 0.35 IU/ml) and/or a positive TST result (≥10 mm) (Fig. 1).
Fig 1.
QFT assay and TST results of 70 HCW. QFT assay values were obtained after ex vivo stimulation with ESAT-6, CFP-10, and TB7.7 antigens, while TST values are the results of tuberculin stimulation. Participants were stratified into three distinct groups according to their QFT assay and TST results. The black lines represent the cutoff value of the commercially available QFT assay of 0.35 IU/ml and the TST of 10 mm, while the dotted line represents the threshold value of a subpositive QFT assay response (i.e., 0.1 IU/ml).
Second, the concentrations of additional cytokines secreted in response to RD1 stimulation during the QFT assays were measured. Cytokines with concentrations that discriminate between the LTBI-positive and LTBI-negative groups were selected using receiver operating characteristic (ROC) curves. The selected cytokines were then used with their positive cutoff values to identify HCW from the undetermined group suspected of having LTBI.
QFT assay.
Whole-blood stimulation and quantification of IFN-γ production were performed according to the manufacturer's instructions (Cellestis, Darmstadt, Germany). After the 24-h incubation period, plasma was collected and the IFN-γ concentration was measured. The optical density was read using a 450-nm filter and an enzyme-linked immunosorbent assay plate reader. QFT positivity was first defined on the basis of the IFN-γ threshold recommended by the manufacturer (>0.35 IU/ml). Because using an uncertainty zone around the cutoff could improve the performance of the LTBI diagnostic test of HCW (16, 19), we considered HCW with IFN-γ levels between 0.1 and 0.35 IU/ml to have a subpositive QFT result, suggesting possible LTBI in this population exposed to TB. Remaining plasma samples were stored at −80°C until quantification of cytokines was carried out.
Cytokine profile in cell-free culture supernatant of QFT.
After 24 h of stimulation by antigens from the QFT assay, cytokine secretion was quantitated in cell-free culture supernatants of 69/70 HCW by using a microbead-based multiplex method (cytokine human panel; Invitrogen, Villebon sur Yvette, France) and a Luminex 100 apparatus (Luminex, Oosterhout, The Netherlands) according to the manufacturers' instructions. The sensitivities of detection of these cytokines range from 3 to 30 pg/ml. Data were acquired using 7,800 to 15,200 double discriminator gate settings. Standard curves were established to determine cytokine concentrations, and a minimum of 100 microspheres per analyte were used. Concentrations above the superior point of the curve were given an arbitrary value equal to the superior point of the standard curve for each biomarker.
Statistical analysis.
The median cytokine levels (interquartile ranges [IQR]) of the LTBI-positive and LTBI-negative groups were compared using the nonparametric Mann-Whitney U test. The individual alpha errors for multiple comparisons were corrected by adjusting the P values using the Holm technique (an adjusted P value of <0.05 was considered significant).
ROC curves of the selected biomarkers were then constructed by plotting the true positive samples (sensitivity) against the false-positive samples (1 − specificity) for each possible cutoff point. Areas under the curve (AUC) were calculated along with their 95% confidence intervals (95% CI) using a nonparametric approach. Cutoffs for antigen-specific IL-2, IL-15, IP-10, and the monokine induced by IFN-γ (MIG) were estimated at various sensitivities and specificities. To avoid false-positive results, a cutoff value was retained corresponding to the maximum Youden index (YI), which was defined as sensitivity + specificity − 1. Selected cytokines with their optimal cutoff were applied to the undetermined group to detect additional LTBI.
Finally, the nonparametric Spearman rank correlation coefficient was used to evaluate associations between selected secreted cytokines.
RESULTS
Study participants.
Clinical characteristics of the HCW in this study are shown in Table 1. Forty-one (59%) of the 70 participants had a TST result greater than the positive threshold established at 10 mm (median = 17 mm; IQR, 14 to 20 mm), whereas 29 participants (41%) had a negative TST result. The participants were classified into three groups based on QFT test and TST results. Eight HCW (11%) were positive by the QFT test using the manufacturer cutoff and were considered to have LTBI. In contrast, 21/70 HCW (30%) had a negative QFT test result of <0.1 IU/ml and a TST result of <10 mm. Thus, a majority of the HCW (59%) included were classified into an undetermined group because of subpositive IFN-γ secretion (between 0.1 and 0.35 IU/ml) and/or a TST result of ≥10 mm (Fig. 1).
Table 1.
Clinical characteristics of the 70 HCW included in this study
| Characteristic or group | Values |
|---|---|
| Median age in yr (IQR) | 44 (36–50) |
| No. of females/total (%) | 59/70 (84.3) |
| Working groups | |
| No. of doctors/total (%) | 8/70 (11) |
| No. of nurses/total (%) | 28/70 (40) |
| No. of auxiliary nurses/total (%) | 24/70 (34) |
| No. of paramedical staff members/total (%) | 1/70 (1) |
| No. of other hospital workers/total (%) | 9/70 (13) |
| Hospital departments | |
| No. in tropical and infectious diseases/total (%) | 45/70 (64) |
| No. in pneumology/total (%) | 25/70 (36) |
| QFT results | |
| No. negative/total (%) | 62/70 (89) |
| No. positive/total (%) | 8/70 (11) |
| TST (mm) | |
| No. negative (<10 mm)/total (%) | 29/70 (41) |
| No. positive (≥10 mm)/total (%) [median, IQR] | 41/70 (59) [17-14-20] |
| No. with abnormal chest X-ray/total (%) | 4/70 (6) |
Identification of four additional biomarkers discriminating LTBI-positive from LTBI-negative HCW.
Besides IFN-γ, 22 cytokines were analyzed after overnight T-cell-specific stimulation by RD1 peptides contained in QFT tubes. Higher concentrations of IL-2, IL-15, IP-10, and MIG were secreted by the LTBI-positive group than by the LTBI-negative group of HCW after QFT-specific stimulation (P < 0.05) (Table 2).
Table 2.
Concentrations of cytokines secreted by peripheral blood mononuclear cells from HCW according to QFT results
| Marker | Median concn (IQR) |
P valuea | |
|---|---|---|---|
| HCW with LTBI (n = 8) | LTBI-negative HCW (n = 17) | ||
| IL-1RA | 8,491 (6,966–9,885) | 5,837 (5,528–6,869) | 0.140 |
| IL-2 | 87 (69–140) | 10 (9–12) | <0.001 |
| IL-2R | 1,751 (1,696–1,817) | 1,714 (1,678–1,751) | 0.242 |
| IL-4 | 271 (267–282) | 263 (261–269) | 0.126 |
| IL-5 | 15 (14–23) | 13 (13–15) | 0.323 |
| IL-6 | 656 (430–800) | 546 (335–937) | 0.852 |
| IL-7 | 133 (133–134) | 133 (131–133) | 0.435 |
| IL-10 | 8 (6–15) | 7 (6–10) | 0.448 |
| IL-12p40/70 | 848 (783–894) | 857 (823–892) | 0.764 |
| IL-13 | 31 (24–56) | 28 (22–31) | 0.263 |
| IL-15 | 205 (200–211) | 50 (39–200) | 0.021 |
| IL-17 | 208 (206–208) | 208 (208–208) | 0.545 |
| TNF-α | 167 (151–195) | 161 (152–220) | 0.815 |
| GM-CSF | 262 (210–266) | 261 (54–263) | 0.300 |
| MIP-1α | 2,436 (1,607–2,728) | 2,193 (1,078–4,506) | 0.966 |
| MIP-1β | 4,037 (2,286–4,869) | 1,658 (768–1,796) | 0.061 |
| IP-10 | 3,903 (3,004–5,080) | 225 (76–668) | 0.004 |
| MIG | 515 (419-684) | 358 (358–361) | 0.003 |
| Eotaxin | 117 (106–130) | 106 (89–145) | 0.618 |
| RANTES | 16,760 (15,483–16,760) | 13,402 (11,651–16,760) | 0.216 |
| MCP-1 | 15,197 (5,395–22,172) | 5,449 (5,131–7,138) | 0.107 |
| IFN-α | 109 (108–110) | 108 (108–109) | 0.181 |
Mann-Whitney test comparing the two groups, with a P value of <0.05 considered statistically significant.
Performance of IL-2, IL-15, IP-10, or MIG testing in HCW positive or negative for LTBI.
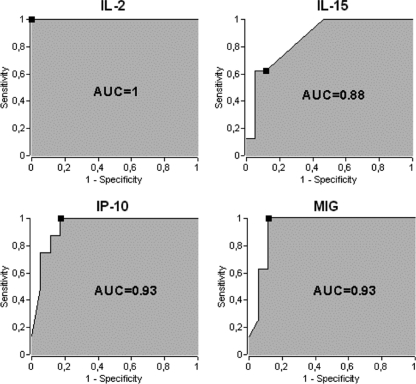
Using ROC curves, the ability of IL-2, IL-15, IP-10, and MIG testing to discriminate LTBI-positive from LTBI-negative HCW was evaluated (Fig. 2). The most discriminating cytokines were IL-2 (threshold, 66 pg/ml; AUC = 1; 95% CI, 1 to 1), IL-15 (threshold, 200 pg/ml; AUC = 0.88; 95% CI, 0.68 to 0.96), IP-10 (threshold, 1,259 pg/ml; AUC = 0.93; 95% CI, 0.75 to 0.98), and MIG (threshold, 392 pg/ml; AUC = 0.93; 95% CI, 0.67 to 0.99). These four cytokines detect all HCW from the LTBI-positive group. Regarding the non-TB group, 7 HCW were misclassified for IL-15, 3 for IP-10 and MIG, and none for IL-2, suggesting that increasing the sensitivity to 100% will lead to a concomitant reduction of specificity.
Fig 2.
Determination of additional biomarkers for the diagnosis of HCW with LTBI. IL-2, IL-15, IP-10, and MIG secretion levels were quantified in QFT supernatants, and ROC curves display sensitivity versus specificity for each biomarker in differentiating the LTBI-positive group from the LTBI-negative group. The black squares correspond to the maximum YI. The AUC is indicated in each graph.
LTBI detection using IL-2, IL-15, IP-10, or MIG in HCW undetermined for TB infection.
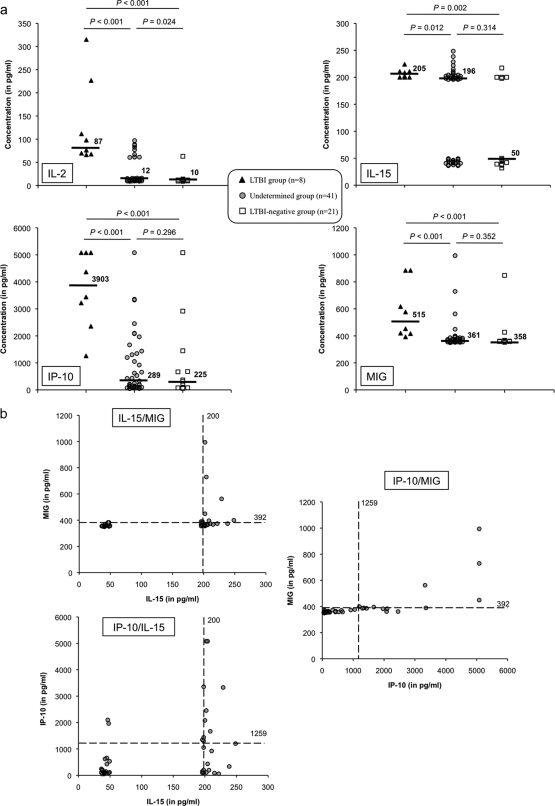
We investigated TB infection in HCW from the undetermined group using the cytokines previously selected (Fig. 3a). Fifteen of 41 HCW were positive for IL-15, 13 were positive for IP-10, and 6 were positive for MIG and IL-2 (Fig. 3a).
Fig 3.
Identification of additional biomarkers for improved LTBI detection in HCW in the undetermined group. Individual IL-2, IL-15, IP-10, and MIG concentrations are shown for the LTBI-positive (black triangles), undetermined (gray circles), and LTBI-negative (white squares) groups of HCW in panel a, while two-by-two combinations of these cytokines are shown for the undetermined group of HCW only in panel b. The dotted line represents the cutoff value of each cytokine as previously determined with ROC curves. The median concentration of each biomarker for each group is shown in each panel. A P value of <0.05 indicates a significant difference between groups using the adjusted Mann-Whitney U test.
There was a positive correlation between IL-15 and IP-10 secretion (r = 0.57, P < 0.001), between IL-15 and MIG secretion (r = 0.69, P < 0.001), and between IP-10 and MIG secretion (r = 0.78, P < 0.001) (Table 3). IL-2 secretion was also correlated with IL-15 (r = 0.55, P < 0.001), IP-10 (r = 0.39, P = 0.001), and MIG secretion (r = 0.49, P < 0.001) (Table 3).
Table 3.
Spearman coefficient of correlation between two-by-two combinations of IL-2, IL-15 IP-10, and MIG secretion levels after QFT in 69 HCW
| Marker | Spearman coefficient (P valuea) |
|||
|---|---|---|---|---|
| IL-2 | IL-15 | IP-10 | MIG | |
| IL-2 | 0.55 (<0.001) | 0.39 (0.001) | 0.49 (<0.001) | |
| IL-15 | 0.55 (<0.001) | 0.57 (<0.001) | 0.69 (<0.001) | |
| IP-10 | 0.39 (0.001) | 0.57 (<0.001) | 0.78 (<0.001) | |
| MIG | 0.49 (<0.001) | 0.69 (<0.001) | 0.78 (<0.001) | |
Mann-Whitney test, with a P value of <0.05 considered statistically significant.
Improving specificity by using a combination of biomarkers.
IL-2 appeared to be a perfect marker in our sample, with a specificity and a sensitivity of 100%. Although IL-2 is certainly a marker of interest (5), such perfect diagnostic performance could be pure luck; its true sensitivity is between 63% and 100%, and its true specificity lies between 80% and 100%. As a result, we decided to consider IL-2 by itself for further analyses. We also investigated in parallel whether an approach combining the other biomarkers could increase the specificity of the LTBI diagnostic test, which was 58.8%, 82.4%, or 88.2% when IL-15, IP-10, or MIG was used individually (Fig. 3b). The sensitivity was still 100% and the specificity increased to 94.1% when HCW had both IL-15 at ≥200 pg/ml and MIG at ≥392 pg/ml. In contrast, the MIG–IP-10 (sensitivity = 100%, specificity = 88.2%), IL-15–IP-10 (sensitivity = 100%, specificity = 88.2%), and IL-15–IP-10/MIG (sensitivity = 100%, specificity = 94.1%) combinations did not provide better results than MIG–IL-15 (sensitivity = 100%, specificity = 94.1%), which is the best combination for discriminating LTBI-positive from LTBI-negative subjects.
Using IL-15 and MIG cutoffs, six of the undetermined HCW could have been considered to have LTBI after subjected to a second-look LTBI diagnosis (Fig. 4).
Fig 4.
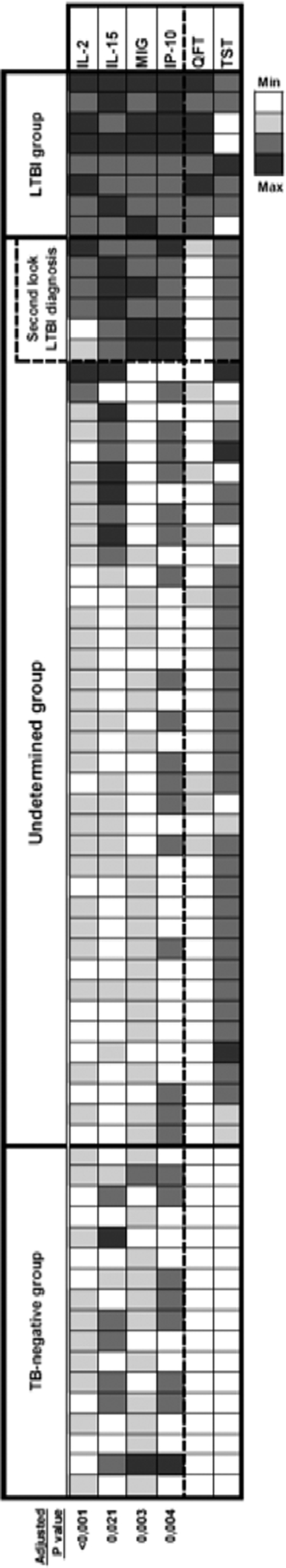
Positivity for additional biomarkers for the diagnosis of LTBI in 69 HCW after RD1 stimulation. Cytokine secretion is shown as a heat map, with each row representing the cytokine concentrations in individual HCW. Only cytokines for which a significant difference was observed between the LTBI-positive and LTBI-negative groups after adjustment for multiple comparisons are represented. Adjusted P values are shown below the heat map. HCW are ranked according to cytokine concentrations and TST and QFT test results. For each parameter, an appropriate color was assigned ranging from white, representing the lowest values (TST, ≤5 mm; QFT, <0.1 IU/ml; cytokine concentrations less than the median of the LTBI-negative group), to black, symbolizing the highest values (TST, >20 mm; QFT, greater than or equal to the median of those of the LTBI-positive group, i.e., 1.055 IU/ml; cytokine concentrations greater than or equal to the median of those of the LTBI-positive group), passing by light gray (TST [5 to 10] mm, QFT [0.1 to 0.35] IU/ml, and cytokine concentrations [the median in the LTBI-negative group to the cytokine thresholds]) and dark gray (TST [10 to 20] mm, QFT [0.35 to the median in the LTBI-positive group, i.e., 1.055 [IU/ml, and cytokine concentrations [the cytokine thresholds to the median in the LTBI-positive group]). The second-look LTBI diagnosis was done using IL-15 and MIG measurements after the QFT assay and previously defined thresholds.
DISCUSSION
Currently, the QFT assay stands as the most specific immunoassay dedicated to LTBI screening. However, the sensitivity of IGRAs to LTBI is questionable, as these tests were evaluated mainly against active TB defined by a positive microbiological test (either microscopic detection of acid-fast bacilli in sputum or sputum culture). Recently, 75% sensitivity and 37% specificity have been reported for the QFT assay with samples from smear-negative subjects, suggesting that the performance of IGRAs could be worse among smear-negative patients than among smear-positive subjects, even in a high-burden country (13). This insufficient sensitivity of the QFT assay cannot be overcome by simply reducing the positive cutoff, since that would have an impact on specificity.
The combination of the TST and the QFT test could be an alternative way of improving diagnostic testing for LTBI (1, 9, 18, 25). However, the TST itself has a poor sensitivity of 80% (17) and poor specificity among individuals vaccinated with BCG, which is the case for most HCW exposed to TB. Our results confirm that this test is unlikely to be useful in this context unless it is used in combination with an IGRA. In particular, 3/8 HCW with a positive QFT have a negative TST result. This is in line with a recent review indicating that use of the QFT test, rather than the TST, is currently recommended for contact tracing and as a screening test for HCW with LTBI in low-incidence countries and for BCG-vaccinated individuals (6). In addition, for HCW, QFT screening only is more cost-effective than TST screening (7, 12).
Our approach to improving the diagnosis of LTBI consisted of increasing the performance of the current IGRA based on the detection of cytokines in addition to IFN-γ. HCW undetermined for LTBI, i.e., with a possible diagnosis of LTBI, had a TST result of ≥10 mm and/or a QFT value (0.1 to 0.35 IU/ml) above the range generally observed in healthy controls, suggesting that effector memory T cells directed against RD1 antigens may be present in these exposed subjects. This group of HCW is of interest because considerable within-subject variability in the IFN-γ response measured during QFT has been revealed by serial testing (16, 19). Therefore, using an uncertainty zone around the cutoff QFT, such as 0.1 to 0.35 IU/ml, could improve the discrimination between healthy HCW and LTBI-positive HCW with a subpositive QFT response. Along with IFN-γ, IL-2, IL-15, MIG, and IP-10 could be useful biomarkers for differentiating subjects with LTBI from those without LTBI. Our results suggest that up to 15 QFT-negative individuals have a positive response to these cytokines and may have LTBI, which is consistent with the “expected” 20% false-negative tests (i.e., 14/70 people), given the 80% sensitivity of QFT. Notably, this result was obtained without substantially weakening the test's specificity. Instead of serial testing, we propose the use of a combination of additional biomarkers, such as IL-15 and MIG, as a second-line diagnostic tool before offering preventive chemotherapy to HCW with borderline negative results in a low-incidence country.
Like IFN-γ, IL-2, MIG, IP-10, and IL-15 are involved in the T helper type 1 (Th1) cellular immune response, which is pivotal in the clearance of M. tuberculosis. Our results are consistent with recent findings indicating that the measurement of T cells directed against TB and secreting IP-10 may be useful for TB diagnosis (20–22, 27). In addition, MIG is expressed by IFN-γ-stimulated cells in TB (2) and was detected in the bronchial epithelium, which is consistent with the recruitment of activated T cells during TB (23). IL-15 has many biological properties in common with IL-2 despite having no sequence homology (26). Unlike IP-10, MIG and IL-15 have not yet been well documented in the case of LTBI diagnosis. To our knowledge, only one recent report suggested that IL-15 may be a reliable cytokine for the detection of TB (10). Testing of several parameters may limit false-negative results due to interindividual variations in the immune response during TB infection.
In conclusion, adding at least MIG and IL-15 to IFN-γ could markedly improve the performance of IGRAs when detecting LTBI. These data should be confirmed in larger studies. Among HCW, a QFT assay could be used first to screen for LTBI-positive individuals and then the QFT supernatants of patients (i) with IFN-γ responses between 0.1 and 0.35 IU/ml or (ii) with a QFT result of <0.1 IU/ml but with a TST result of >10 mm should be tested for additional cytokines to detect supplemental LTBI-positive cases. Further studies, including cost-effectiveness analyses, are needed to provide evidence for the reliability of such a diagnostic strategy for LTBI in HCW in a low-incidence country.
ACKNOWLEDGMENTS
We thank all of the subjects who provided blood samples. We also thank Cara-Chan Manville for carefully reviewing the English language of the manuscript. We are grateful to the French Agency for AIDS Research (director, Jean-François Delfraissy) for constant support and encouragement.
This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM) and the CHRU.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 7 March 2012
REFERENCES
- 1. Abdalhamid B, et al. 2010. Utilization of the QuantiFERON-TB Gold test in a two-step process with the tuberculin skin test to evaluate health care workers for latent tuberculosis. J. Clin. Microbiol. 48:2955–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abramo C, et al. 2006. Monokine induced by interferon gamma and IFN-gamma response to a fusion protein of Mycobacterium tuberculosis ESAT-6 and CFP-10 in Brazilian tuberculosis patients. Microbes Infect. 8:45–51 [DOI] [PubMed] [Google Scholar]
- 3. Andersen P, Munk ME, Pollock JM, Doherty TM. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099–1104 [DOI] [PubMed] [Google Scholar]
- 4. Anonymous 2006. Test de detection de la production d'interféron-gamma pour le diagnostic des infections tuberculeuses. Haute Autorité de Santé, Saint-Denis La Plaine, France: http://www.has-sante.fr/portail/upload/docs/application/pdf/synthese_detection_de_linterferon-gamma.pdf [Google Scholar]
- 5. Biselli R, et al. 2010. Detection of interleukin-2 in addition to interferon-gamma discriminates active tuberculosis patients, latently infected individuals, and controls. Clin. Microbiol. Infect. 16:1282–1284 [DOI] [PubMed] [Google Scholar]
- 6. Denkinger CM, Dheda K, Pai M. 2011. Guidelines on interferon-gamma release assays for tuberculosis infection: concordance, discordance or confusion? Clin. Microbiol. Infect. 17:806–814 [DOI] [PubMed] [Google Scholar]
- 7. de Perio MA, Tsevat J, Roselle GA, Kralovic SM, Eckman MH. 2009. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch. Intern. Med. 169:179–187 [DOI] [PubMed] [Google Scholar]
- 8. Diel R, et al. 2011. Interferon-gamma release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur. Respir. J. 37:88–99 [DOI] [PubMed] [Google Scholar]
- 9. Fox BD, et al. 2009. The QuantiFERON-TB-GOLD assay for tuberculosis screening in healthcare workers: a cost-comparison analysis. Lung 187:413–419 [DOI] [PubMed] [Google Scholar]
- 10. Frahm M, et al. 2011. Discriminating between latent and active tuberculosis with multiple biomarker responses. Tuberculosis (Edinb.) 91:250–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joshi R, Reingold AL, Menzies D, Pai M. 2006. Tuberculosis among health-care workers in low- and middle-income countries: a systematic review. PLoS Med. 3:e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Linas BP, Wong AY, Freedberg KA, Horsburgh CR., Jr 2011. Priorities for screening and treatment of latent tuberculosis infection in the United States. Am. J. Respir. Crit. Care Med. 184:590–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ling DI, et al. 2011. Are interferon-γ release assays useful for diagnosing active tuberculosis in a high-burden setting? Eur. Respir. J. 38:649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mazurek GH, et al. 2010. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection. MMWR Morb. Mortal. Wkly. Rep. 59(RR-5):1–25 [PubMed] [Google Scholar]
- 15. Menzies D, Joshi R, Pai M. 2007. Risk of tuberculosis infection and disease associated with work in health care settings. Int. J. Tuberc. Lung Dis. 11:593–605 [PubMed] [Google Scholar]
- 16. Pai M, et al. 2006. Serial testing of health care workers for tuberculosis using interferon-gamma assay. Am. J. Respir. Crit. Care Med. 174:349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pai M, Zwerling A, Menzies D. 2008. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann. Intern. Med. 149:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pooran A, et al. 2010. Different screening strategies (single or dual) for the diagnosis of suspected latent tuberculosis: a cost effectiveness analysis. BMC Pulm. Med. 10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ringshausen FC, et al. 2011. Within-subject variability of Mycobacterium tuberculosis-specific gamma interferon responses in German health care workers. Clin. Vaccine Immunol. 18:1176–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruhwald M, et al. 2008. Evaluating the potential of IP-10 and MCP-2 as biomarkers for the diagnosis of tuberculosis. Eur. Respir. J. 32:1607–1615 [DOI] [PubMed] [Google Scholar]
- 21. Ruhwald M, et al. 2011. A multicentre evaluation of the accuracy and performance of IP-10 for the diagnosis of infection with M. tuberculosis. Tuberculosis (Edinb.) 91:260–267 [DOI] [PubMed] [Google Scholar]
- 22. Ruhwald M, et al. 2008. Improving T-cell assays for the diagnosis of latent TB infection: potential of a diagnostic test based on IP-10. PLoS One 3:e2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sauty A, et al. 1999. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J. Immunol. 162:3549–3558 [PubMed] [Google Scholar]
- 24. Sester M, et al. 2011. Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur. Respir. J. 37:100–111 [DOI] [PubMed] [Google Scholar]
- 25. Tsiouris SJ, et al. 2006. Sensitivity analysis and potential uses of a novel gamma interferon release assay for diagnosis of tuberculosis. J. Clin. Microbiol. 44:2844–2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waldmann T, Tagaya Y, Bamford R. 1998. Interleukin-2, interleukin-15, and their receptors. Int. Rev. Immunol. 16:205–226 [DOI] [PubMed] [Google Scholar]
- 27. Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. 2011. Immunological biomarkers of tuberculosis. Nat. Rev. Immunol. 11:343–354 [DOI] [PubMed] [Google Scholar]
- 28. Zwerling A, et al. 2012. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax 67:62–70 [DOI] [PubMed] [Google Scholar]