Abstract
In the northern part of Western Europe, Echinococcus multilocularis is primarily detected in and spreading among foxes. The present case marks E. multilocularis as an emerging pathogen for humans, as it describes the first human case of probably locally acquired E. multilocularis in The Netherlands, with various interesting clinical aspects.
CASE REPORT
A55-year-old female with a history of myxoid liposarcoma in the left leg (1993) and metastases in the spinal column (2001 and 2007) presented at our hospital with cervical pain. As new metastases of the myxoid liposarcoma were suspected, a diagnostic workup was initiated. Fluorodeoxyglucose positron emission tomography (FDG-PET) was negative, but computed tomography (CT), performed simultaneously with FDG-PET, and magnetic resonance imaging showed seven lesions in the liver, with a maximum size of 1.7 cm (Fig. 1). These lesions were not detected by an abdominal CT performed 6 months earlier. The patient's laboratory results were as follows: alkaline phosphatase, 89 U/liter; gamma-glutamyl transferase, 28 U/liter; aspartate aminotransferase, 19 U/liter; alanine aminotransferase, 23 U/liter; lactate dehydrogenase, 346 U/liter; bilirubin, 10.1 μmol/liter; white blood cells, 4.9 × 109/liter with 62% neutrophils, 22% lymphocytes, 15% monocytes, 1% eosinophils, and 0% basophils. Without pathological confirmation of the presumed metastases of her myxoid liposarcoma, neoadjuvant chemotherapy was started. As this was bilobar liver disease, radical surgery with curative intent was possible only by performing two separate operations in order to prevent liver failure. Therefore, after chemotherapy, partial left hepatectomy and contralateral portal ligation were performed to allow liver regeneration, leaving four lesions in situ in the right liver lobe.
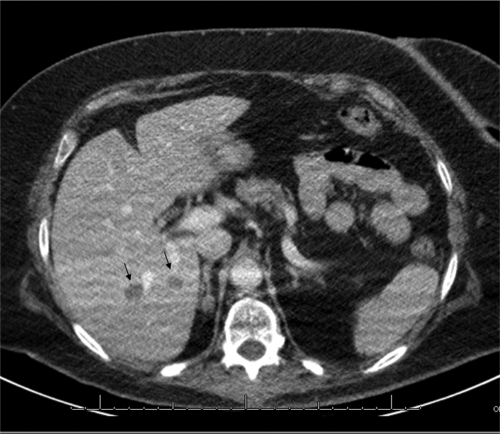
Fig 1.
Computed tomogram of the liver after intravenous administration of iodine-containing contrast medium, scanned in the portovenous phase. In this transverse plane of the liver, two hypodense lesions are visible (arrows).
Pathological examination of the liver tissue revealed three circumscribed intact nodules with central necrosis and a peripheral wall of histiocytes with focal giant cells. Central cysts and budding daughter cysts with a trilayered membrane wall were seen, consistent with echinococcosis. No protoscolices, hooklets, or calcareous corpuscles were noticed. Protoscolices, however, are often absent in Echinococcus multilocularis lesions (5). Hence, the findings were compatible with echinococcosis (Fig. 2 and 3). There was no evidence of metastases of the liposarcoma. Subsequently, the serum of this patient was sent to a Dutch reference center and Echinococcus granulosus serology came back weakly positive with an IgG titer of 1:80 (in-house enzyme-linked immunosorbent assay; cutoff, 1:40) and immunoblot IgG1 positive and IgG4 negative (in-house immunoblot assay [9]). The result of an E. multilocularis-specific enzyme-linked immunosorbent assay (Em2plus) was negative (1, 8).
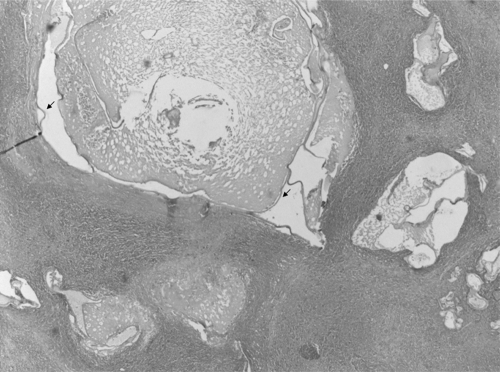
Fig 2.
Histopathology of liver lesion. One central cyst and budding daughter cysts with a trilayered membrane wall (arrows) are visible. No protoscolices, hooklets, or calcareous corpuscles are visible; however, the image is still compatible with echinococcosis. Magnification, ×100; hematoxylin and eosin stain.
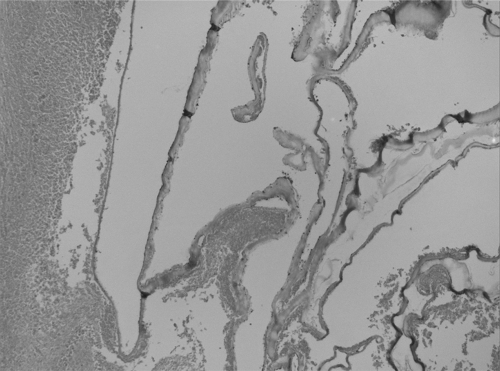
Fig 3.
Histopathology of liver lesion. Separated membranes in a cyst wall with peripheral necrosis are visible. Magnification, ×400; hematoxylin and eosin stain.
To finally confirm the diagnosis, isolated DNA from unpreserved liver material was sent to the Center for Infectious Disease Control (Bilthoven, The Netherlands). DNA from our patient's lesions was compared with DNA isolated from E. multilocularis samples from infected foxes originating from Limburg Province (The Netherlands); this was the province where the patient lived. A PCR amplifying DNA sequences of two mitochondrial targets, cytochrome c oxidase subunit 1 (cox1) and NADH dehydrogenase (nad1), was used to identify E. multilocularis and to distinguish the organism from E. granulosus and other taeniid tapeworms (2, 3). The DNA sequences were compared to DNA sequences from the same target from other E. multilocularis and E. granulosus strains in GenBank. DNA sequencing of the PCR amplicons of the nad1 (520 bp) and cox1 (420 bp) genes showed 99 to 100% similarity to the European E. multilocularis strains, including four E. multilocularis fox strains, and only 86 to 93% similarity to E. granulosus.
After this diagnosis, our patient started with albendazole at 400 mg twice daily. Subsequent abdominal CT scans at 4 and 7 months postresection revealed neither progression of the lesions nor a change in aspect. Informed consent was obtained, and this report was approved by the human investigations committee of the Maastricht University Medical Center.
Four lessons can be learned from this case report. First, E. multilocularis lesions can be rapidly progressive: multiple lesions with a maximum size of 1.7 cm developed within a period of 6 months in our patient. The incubation period after ingestion is estimated to be 5 to 15 years (5). Liver cysts grow up to 1 cm in the first 6 months and 2 to 3 cm/year thereafter, but in our patient one lesion was already 1.7 cm in less than 6 months (12). When left untreated, the mortality rate is almost 100% within 15 years after diagnosis (5). Recently, a very progressive E. multilocularis infection has been described in a renal transplant patient who was severely immunocompromised (7). Our patient, however, did not use immunosuppressive drugs in the period before presentation.
Second, E. multilocularis lesions can be mistaken for metastatic lesions, as E. multilocularis lesions are usually revealed by CT with irregular borders and various densities, although variation exists (3). Calcifications are present in 82% and are hyperdense, while necrotic areas are hypodense (4). “Hot spots” are often seen with FDG-PET, although lesions can be metabolically inactive as well, as in our case (4, 6, 13). The FDG-PET-negative, multiple circumscript hypodense lesions in the liver shown by regular CT in our patient were not classical for E. multilocularis and did resemble liver metastases (15); the initial presumptive diagnosis therefore seemed much more likely.
Third, E. multilocularis lesions can develop in patients without reported behavior associated with the ingestion of E. multilocularis eggs (10). Our patient's lifetime travel history revealed three short vacations to areas where the disease is endemic (Switzerland, Italy, and Austria in 2006 and 2007) (14), but she had never lived in an area where the disease is endemic. She was not extensively exposed to a forest environment, did not hunt, did not consume forest fruits, had no contact with (domestic) animals, did not work in her garden, and bought her fruits and vegetables in regular supermarkets.
Lastly, serological results do not necessarily correspond to disease. E. multilocularis is known to cross-react with E. granulosus in serological assays (5), and E. granulosus assays are therefore used in the E. multilocularis workup. Our patient's serology results were weakly positive, illustrating that imaging is essential to exclude E. multilocularis infection. We do not have an explanation for the weak serology results; they might be due to a difference in the E. multilocularis strain in our patient. There is no serological evidence of widespread human contact with E. multilocularis in The Netherlands yet (11), but these results should be interpreted with caution.
Although E. multilocularis is prevalent in foxes in the southern part of The Netherlands, no human E. multilocularis case acquired in The Netherlands has been described so far. Recently, three E. multilocularis cases have been described in a part of Belgium that borders the southern part of The Netherlands, where our patient resides (16). Sequencing results could not define the exact geographic location of acquisition of the E. multilocularis strain in our patient, but The Netherlands belongs to the possibilities. Although due to the long incubation period (5 to 15 years), the time and place of infection are hard to determine retrospectively, considering the duration of exposure of this patient, domestically acquired E. multilocularis seems most likely.
This patient represents the first reported case of E. multilocularis acquired in The Netherlands. Surveillance data originating from foxes in the southern part of The Netherlands have shown that E. multilocularis spreads by 2.7 km/year in a northern direction and based on the spreading of E. multilocularis in foxes in this area of The Netherlands (16), the human risk in Limburg Province is estimated to be three human cases by 2018 (17). We therefore believe that E. multilocularis is an emerging pathogen in Western Europe and clinicians should consider this diagnosis when confronted with (asymptomatic) liver lesions.
Footnotes
Published ahead of print 22 February 2012
REFERENCES
- 1. Bart JM, et al. 2007. Comparison of several commercial serologic kits and Em18 serology for detection of human alveolar echinococcosis. Diagn. Microbiol. Infect. Dis. 59:93–95 [DOI] [PubMed] [Google Scholar]
- 2. Bowles J, Blair D, McManus DP. 1992. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 54:165–173 [DOI] [PubMed] [Google Scholar]
- 3. Bowles J, McManus DP. 1993. NADH dehydrogenase 1 gene sequences compared for species and strains of the genus Echinococcus. Int. J. Parasitol. 23:969–972 [DOI] [PubMed] [Google Scholar]
- 4. Bresson-Hadni S, et al. 2006. Imaging aspects and non-surgical interventional treatment in human alveolar echinococcosis. Parasitol. Int. 55(Suppl.):S267–S272 [DOI] [PubMed] [Google Scholar]
- 5. Eckert J, Deplazes P. 2004. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 17:107–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ehrhardt AR, et al. 2007. Assessment of disease activity in alveolar echinococcosis: a comparison of contrast enhanced ultrasound, three-phase helical CT and [(18)F] fluorodeoxyglucose positron emission tomography. Abdom. Imaging 32:730–736 [DOI] [PubMed] [Google Scholar]
- 7. Geyer M, et al. 2011. Rapidly progressive hepatic alveolar echinococcosis in an ABO-incompatible renal transplant recipient. Transpl. Infect. Dis. 13:278–284 [DOI] [PubMed] [Google Scholar]
- 8. Gottstein B, Jacquier P, Bresson-Hadni S, Eckert J. 1993. Improved primary immunodiagnosis of alveolar echinococcosis in humans by an enzyme-linked immunosorbent assay using the Em2plus antigen. J. Clin. Microbiol. 31:373–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herremans T, et al. 2010. Decline of echinococcosis in The Netherlands, 1997-2008. Ned. Tijdschr. Geneeskd. 154:A2297. [PubMed] [Google Scholar]
- 10. Kern P, et al. 2004. Risk factors for alveolar echinococcosis in humans. Emerg. Infect. Dis. 10:2088–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kortbeek LM, Harms M, van Pelt W, van der Giessen J, Pinelli Ortiz E. 2011. Echinococcus multilocularis in The Netherlands; what about humans?, poster P859. 21st Eur. Congr. Microbiol. Infect. Dis., Milan, Italy http://www.poster-submission.com/search/sresult [Google Scholar]
- 12. Pedrosa I, Saiz A, Arrazola J, Ferreiros J, Pedrosa CS. 2000. Hydatid disease: radiologic and pathologic features and complications. Radiographics 20:795–817 [DOI] [PubMed] [Google Scholar]
- 13. Reuter S, et al. 1999. Pericystic metabolic activity in alveolar echinococcosis: assessment and follow-up by positron emission tomography. Clin. Infect. Dis. 29:1157–1163 [DOI] [PubMed] [Google Scholar]
- 14. Romig T, Dinkel A, Mackenstedt U. 2006. The present situation of echinococcosis in Europe. Parasitol. Int. 55(Suppl.):S187–S191 [DOI] [PubMed] [Google Scholar]
- 15. Sheah K, et al. 2008. Metastatic myxoid liposarcomas: imaging and histopathologic findings. Skeletal Radiol. 37:251–258 [DOI] [PubMed] [Google Scholar]
- 16. Takumi K, et al. 2008. Evidence for an increasing presence of Echinococcus multilocularis in foxes in The Netherlands. Int. J. Parasitol. 38:571–578 [DOI] [PubMed] [Google Scholar]
- 17. Takumi K, et al. 2011. Mapping the increasing risk of human alveolar echinococcosis in Limburg, The Netherlands. Epidemiol. Infect. 7:1–5 [DOI] [PubMed] [Google Scholar]