Abstract
The formation of bacterial biofilms is initiated by cells transitioning from the free-swimming mode of growth to a surface. This review is aimed at highlighting the common themes that have emerged in recent research regarding the key components, signals, and cues that aid in the transition and those involved in establishing a more permanent surface association during initial attachment.
INTRODUCTION
Biofilms are composed of microorganisms attached to a surface (substratum or interface) in moist environments. The community of sessile cells is surrounded by a hydrated polymeric matrix of their own synthesis (27). The formation of these bacterial communities is not random. Based on studies of single-species communities, biofilm formation is initiated with surface attachment by planktonic, free-swimming bacteria that, once attached, will grow into a mature form, which in some bacterial species is characterized by the presence of differentiated, mushroom- or pillar-like structures or microcolonies interspersed with fluid-filled channels. The developmental progression leading to a mature biofilm not only coincides with observable phenotypic changes but also requires alterations in gene expression. It is well recognized that biofilm cells differ from their free-swimming counterparts with respect to gene expression, protein production, and resistance to the immune system and antimicrobial agents (for reviews of biofilm developmental processes and regulation, see references 1, 107, 110, 122, and 147). Biofilm-associated microorganisms have been shown to colonize a wide variety of man-made and medical devices and have been implicated in over 80% of chronic inflammatory and infectious diseases of soft tissues, including chronic otitis media, native valve endocarditis, gastrointestinal ulcers, urinary tract and middle ear infections, and chronic lung infections in cystic fibrosis (CF) patients (reviewed in references 28 and 38).
Considering the growing appreciation for the need to prevent and control biofilms in the medical setting and beyond, this review focuses on the process that commences biofilm formation—bacterial attachment—and is based on studies of single-species communities on primarily inert surfaces. To that end, it addresses the roles in surface attachment of various determinants, including environmental conditions, bacterial cell surface features and polysaccharides, surface contact, and signaling molecules, with a particular emphasis on the underlying regulatory mechanisms. Given the broad nature of this topic, this review will highlight the common themes of attachment as a regulated response to environmental cues by focusing on more-thoroughly characterized systems governing attachment to surfaces.
WHY ATTACH IN THE FIRST PLACE?
Biofilms likely represent the prevalent microbial mode of existence in nature, with estimates suggesting that more than 90% of bacteria exist within biofilms (27, 49). Considering the prevalence of biofilms, one has to wonder what makes the immobilized lifestyle so attractive to bacteria. Biofilms, as coordinated groups analogous to multicellular organisms, display increased rates of gene transfer, cooperation, and stratification. Thus, the sessile lifestyle affords bacteria multiple protective advantages and allows them to remain within a favorable environmental niche or host. Compared to free-swimming bacteria, biofilms are better adapted to withstand nutrient deprivation, pH changes, oxygen radicals, biocides, and antimicrobial agents (reviewed in reference 27).
With respect to attachment, the surface itself provides bacteria an advantage over the liquid above it, as any surface, regardless of chemical or physical properties (reviewed in reference 127), will absorb (macro)molecules. This absorptive layer, referred to as a conditioning film (reviewed in reference 39), not only changes the physicochemical properties of the surface but also contributes to the accumulation of proteins, polysaccharides, and other molecules at the surface that provide a metabolically favorable environment for bacterial cells and serve as nutritional cues to trigger biofilm formation. In Pseudomonas aeruginosa, these cues are integrated by the catabolite repression control protein, which plays a role in the regulation of carbon metabolism and biofilm formation, as a crc mutant formed only a dispersed monolayer of cells devoid of microcolonies which are typical of mature biofilms of the wild-type strain (123). One would therefore assume that an abundant supply of nutrients fosters attachment and biofilm formation. However, bacteria grown under high-nutrient conditions either fail to form biofilms or form loose, floc-like structures at the substratum that are easily disrupted with fluid shear (27, 31, 68, 97). Instead, bacteria tend to form biofilms under low-nutrient or starvation conditions (17, 27, 97, 130). For instance, Dewanti and Wong found that Escherichia coli O157:H7 biofilms developed on stainless steel chipped faster and contained a higher number of adherent cells when grown in low-nutrient media than when grown in tryptic soy broth (36). The attachment-upon-starvation response has also been commonly observed for various bacteria, including pathogenic bacteria as well as those found in soil and the marine environment (4, 27, 65, 80, 81, 97, 134). Increased biofilm formation under low-nutrient conditions is not limited to availability of carbon sources, as limitations in nitrogen and phosphorous were also shown to correlate with increased biofilm formation. However, below certain thresholds, nutrient limitation may serve to impair adherence. Monds et al. (111) demonstrated that biofilm formation by Pseudomonas aureofaciens PA147-2 requires a threshold concentration of extracellular inorganic phosphate (Pi), with attachment being impaired below this threshold in a manner similar to that of mutants defective in Pi transport.
These findings indicate that the decision by free-swimming cells to commit to a surface-attached lifestyle is highly regulated by environmental cues, with different bacteria responding to different signals. For instance, specific environmental factors may provide suitable carbon and energy sources or metabolic products, thus serving as indicators of favorable conditions for growth. Prüß et al. screened E. coli K-12 wild type and mutants cultured in 96-well polystyrene plates for the effect of multiple combinations of environmental conditions and carbon sources on biofilm formation (131). The phenotype microarray experiments illustrated that carbon sources that are metabolized to acetyl coenzyme A, acetyl phosphate, and acetate are particularly supportive of biofilm formation. Moreover, strains inactivated in genes associated with acetate metabolism demonstrated reduced biofilm formation. The findings suggested that acetate metabolism functions as a metabolic sensor, transmitting changes in environmental conditions to mechanisms regulating biofilm biomass and structure (131). Other nutritional cues have been shown to serve as building blocks of adhesins and polysaccharides that are directly perceived as signals that activate particular regulatory cascades. Vibrio cholerae is both an inhabitant of estuarine environments and the etiologic agent of the diarrheal disease cholera. Attachment by V. cholerae is enhanced by the presence of monosaccharides either by supplementation of the growth medium or by degradation of the polysaccharide surface (e.g., the chitinaceous surface of arthropods) to which the cells are attached (76). A lack of monosaccharides impairs V. cholerae biofilm formation at the monolayer stage. These environmental signals have been identified as activators of vps gene transcription that are responsible for synthesis of the Vibrio polysaccharide (VPS) and biofilm formation (76). In contrast, Ca2+ was found to enhance vps-independent biofilm formation, with removal of Ca2+ (freshwater is calcium poor) resulting in the disintegration of V. cholerae biofilms as well as the biofilms of several other Vibrio species (77). The finding suggests that environmental activators of vps-dependent biofilm development are present in freshwater in association with arthropods while environmental activators of vps-independent biofilm development are present in calcium-rich seawater (76). Vibrio vulnificus also exhibits a differential dependency of attachment on environmental conditions, with nutrient and glucose availability but not sodium chloride availability of the marine environment serving as factors altering adherence (101). Additional environmental cues include osmolarity, pH, and divalent ions (Table 1).
Table 1.
Overview of environmental cues known to modulate attachment
| Environmental stimulus | Species | Attachmenta | Regulatory system and mechanism of adhesion modulationb | Reference |
|---|---|---|---|---|
| Nutrient availability | ||||
| Acetate metabolism carbon sources | E. coli | + | 24, 131 | |
| Amino acid starvation | E. coli | + | BarE, sRNAs csrB and csrC | 72 |
| Mannose, N-acetyl-d-galactosamine | E. coli | − | 78 | |
| Monosaccharides | P. aeruginosa | − | 78, 102 | |
| V. cholerae | + | PTS, VPS polysaccharide | 76 | |
| Ca2+ | V. cholerae | + | O-antigen polysaccharide | 76, 77 |
| Mg2+ (low) | P. aeruginosa | + | RetS; upregulation of rsmYZ and pel and psl | 115 |
| Phosphate (high) | Sinorhizobium meliloti | + | EPS I succinoglycan | 103 |
| Phosphate limitation | P. aureofaciens | − | PhoPQ; inactivation of Pho regulon | 111 |
| P. fluorescens | − | RapA/LapD; loss of cell surface LapA adhesin | 109, 118, 119 | |
| Phosphate starvation | S. meliloti | + | PhoBR, EPS II galactoglucan | 7, 168 |
| Iron | P. aeruginosa | + | 141 | |
| P. aeruginosa | − | 11 | ||
| B. cenocepacia | − | 11 | ||
| Iron limitation | Acinetobacter baumannii | + | Chaperone-usher pilus assembly | 72, 153 |
| P. putida | − | TonB | 108 | |
| Host factors | ||||
| Epinephrine, norepinephrine | E. coli | + | 8 | |
| Indole | E. coli | − | SdiA; motility | 8, 90 |
| V. cholerae | + | VPS polysaccharide | 114 | |
| Mucin | P. aeruginosa | + | 88 | |
| H. pylori | − | 25 | ||
| Osmolarity | ||||
| High | E. coli | − | CpxAR; repression of csgD (curli) | 74 |
| High Na+, alkaline pH | E. coli | + | NhaR, poly-β-1,6-N-acetyl-d-glucosamine (PGA) adhesin | 51 |
| Others | ||||
| Surface contact | C. crescentus | + | PleD, polar holdfast adhesin | 93, 94 |
| A. biprosthecum | + | Polar holdfast adhesin | 94 | |
| A. tumefaciens | + | Polar holdfast adhesin | 94 | |
| P. aeruginosa | + | 155, 156 | ||
| E. coli | + | Cpx and NlpE, P pili | 40, 126 | |
| Blue light | C. crescentus | + | LovKR | 132 |
+, positive effect on attachment; −, negative effect on attachment.
PTS, phosphoenolpyruvate phosphotransferase system.
Factors signaling host-specific environments also influence attachment to biotic surfaces, but their effects may vary between different bacteria. For instance, indole, which is prevalent in the gastrointestinal tract, has been proposed to act as an extracellular signal molecule influencing biofilm formation in a range of bacteria. In V. cholerae, indole has been shown to activate genes involved in VPS production that are essential for vps-dependent biofilm development (114). In E. coli O157:H7, indole has the opposite effect of reducing attachment to both abiotic surfaces and epithelial cells (8). While mucin is thought to enhance P. aeruginosa attachment and biofilm formation in the lungs (88), it serves to promote planktonic growth of Helicobacter pylori within the stomach environment (25). Observations such as these underscore the importance of extracellular cues in driving attachment but also caution against making generalizing conclusions regarding the universal impact of particular environmental conditions on adherence capabilities of various bacterial species.
THE FIRST CONTACT
Biofilm formation is initiated with surface attachment by planktonic, free-swimming bacteria. Using primarily glass or plastic surfaces, microscopic studies as well as genetic analyses have shown that the initial surface attachment phase by many bacteria proceeds in two distinct steps (2, 20, 104, 124, 140), which are referred to as the reversible and irreversible attachment stages. In the first step, known as reversible attachment, cells are loosely attached via a single pole and may readily detach and return to the planktonic phase. During this phase, bacteria can be rapidly spinning, vibrating, or moving across the substrate surface. Spinning is an indication of flagellar attachment to the substrate (140, 155), with the bacterium rotating around its axis. In addition to Brownian motion, some bacterial cells in contact with the surface exhibit a jerky type of motion called twitching motility, which requires pili. Adhesins, such as pili and flagella, assist in making the first physical contact with the surface, probably by allowing bacteria to overcome the hydrodynamic boundary layer caused by drag and frictional forces at the surface and to overcome the repulsive force due to the overall net negative electrostatic charge of bacterial cells and the surface (reviewed in references 55 and 130) (Fig. 1).
Fig 1.
Overview of events and factors playing a role in enabling bacteria to transition to the surface-associated lifestyle at a solid-liquid interface. To accomplish contact with the surface, bacteria first have to overcome the hydrodynamic boundary layer and repulsive forces as they approach the surface. Factors that accumulate in the conditioning film, compared to those of the bulk liquid, contribute to the decision of whether to commit to the surface or to leave. Reversible attachment is characterized by cells loosely attached via a single pole (resulting in a spinning motion) that may readily detach and return to the planktonic phase. Twitching motility is also observed. Surface contact is sensed via flagellar rotation being impeded due to close proximity and/or surface sensory proteins (e.g., NlpE). Transition to irreversible attachment is indicated by cells attached to the surface along their axis, with the resulting reduction in flagellar rotation triggering increased polysaccharide production and, potentially, c-di-GMP levels. This is followed by cells showing an aggregative behavior indicated by cell-to-cell contact and induction of quorum sensing. While the timing of events during this attachment stage has not been fully explored, it appears that the Lap and the SadBC/BifA systems are essential for the transition to the irreversible attachment stage.
Recent evidence suggests, however, that the flagellum does not simply act as an adhesin, as the presence of flagella or flagellum-mediated swimming alone is not sufficient to explain the role of the flagellar apparatus in biofilm formation. Evidence for this was provided by several research laboratories (92, 155, 156, 163) and is exemplified by findings of Van Dellen et al. (160) demonstrating that while V. cholerae O139 flagellar mutants show reduced attachment, they eventually form a robust monolayer biofilm. In contrast, monolayer formation was severely impaired in the absence of the flagellar motor. The P. aeruginosa genome encodes only one flagellum and one motor but two flagellar stators, MotAB and MotCD, which are the static elements of the bacterial motor, providing energy to turn this appendage and therefore propel the cell through its environment. While either flagellar stator is sufficient for swimming, both are necessary for surface-associated swarming motility (156). Moreover, mutations in either MotAB or MotCD render P. aeruginosa defective in attachment in both static and flow cell systems. While both the MotAB and MotCD stators play a role in initial polar attachment to the surface (reversible attachment), MotAB also participates in the downstream irreversible attachment, with inactivation of motAB, but not motCD, significantly increasing the percentage of cells transitioning to the irreversible attachment stage, as indicated by cells attached along their axis to the surface (155). Both MotAB and MotCD affected the flagellum reversal rate, which is significantly increased upon deletion of the flagellar stators, resulting in increased surface movement (155). Similar observations were made in Listeria monocytogenes by comparing the wild type with flagellum-lacking and paralyzed-flagellum mutants (92). In Vibrio parahaemolyticus, it has been shown that restricting flagellar rotation (by close proximity of the bacterium with a surface) induces a signal transduction cascade that prepares this organism for swarming across a surface (99, 100). The findings demonstrated a role of flagellar rotation in attachment. But why is the rotation of the flagellum rather than the flagellum itself so important for attachment? Taken together with the findings described above, reports establishing a link between flagella and polysaccharide production suggest that the flagellum serves as a mechanosensor for adhesion, with restriction of flagellar rotation (accomplished following contact with the surface) triggering events, including surface-associated motility (e.g., twitching) and the expression of genes involved in polysaccharide biosynthesis, that allow bacteria to enable a more permanent association with the surface. Contributing to the evidence of a link between flagella and polysaccharide production, Hickman and Harwood (60) demonstrated an inverse relationship between flagellum biosynthesis and polysaccharide biosynthesis in P. aeruginosa PAO1, with FleQ, known to activate expression of flagellum biosynthesis genes, repressing the transcription of genes, including the pel operon involved in Pel polysaccharide biosynthesis. Moreover, Garrett et al. (48) observed that in P. aeruginosa, the alternative sigma factor AlgT, essential for the production of the exopolysaccharide alginate, also modulates fliC expression upon surface attachment, probably by activating a negative effector of flagellum synthesis. Similar observations have been made in other biofilm-forming species (14, 94), thus demonstrating that flagellar movement acts on biofilm formation by a feedback mechanism in the regulation of exopolysaccharide production (Fig. 1).
The importance of surface-associated motility has been implicated in contributing to structural differences in biofilm architecture, with limited surface motility resulting in biofilms characterized by increased biomass accumulation and more-extensive three-dimensional architecture.
This is exemplified by work by Kirisits et al. (79), who compared surface-associated behaviors of P. aeruginosa wild-type and sticky (ST) small-colony variants, with ST variants forming biofilms with more biomass and a three-dimensional architecture distinct from that of wild-type biofilms. In this work, the movements of 70 randomly selected, newly adherent wild-type and ST cells on a surface were tracked using time-lapse microscopy. The analysis demonstrated that wild-type cells were very mobile and that many progeny were released into the bulk liquid (34% ramblers [daughter cells showing extensive movement], 51% flyers [daughter cells that are released into the bulk liquid]) while the ST cells tended to remain close to the point of initial attachment (71% squatters [daughter cells that remained adherent to the surface and showed limited movement]). The predominant ST squatter behavior was attributed to increased expression of genes related to type IV pilus biosynthesis and exopolysaccharide production (psl and pel) (79). This work also exemplifies the events that take place once bacteria have made contact with the surface and the potential fates of cells following the transition: remaining surface attached, detaching from the surface, growing, or rejoining the surface. Overall, it appears that during the initial attachment stage of surface colonization, the rate of attachment is greater than the rate of growth or the rate of detachment, with the rate of growth on the surface being quite slow (89). Moreover, Klausen et al. (82) demonstrated that the development of mushroom-like multicellular structures in P. aeruginosa biofilms is dependent on type IV pili, providing additional evidence for surface motility contributing not only to attachment but also to the biofilm architecture.
BEING STICKY IS THE KEY
Biofilm cells are embedded in an extracellular polymeric substance (EPS) matrix composed of polysaccharide, extracellular DNA (eDNA), lipids, and proteins (reviewed in references 46, 47, and 139). Although EPS matrices are a large component of bacterial biofilms, their contribution to the stickiness of bacteria during attachment and to biofilm structure and function has been examined for only a few organisms. While less is known about lipids and proteins, it is now well established that eDNA assists in attachment. Whitchurch et al. (166) first demonstrated that P. aeruginosa PAO1 biofilm formation was significantly reduced under flowing conditions in the presence of DNase. eDNA has been subsequently shown to contribute to biofilm formation by clinical P. aeruginosa isolates as well as by a variety of bacterial species, including Staphylococcus epidermidis, through analyses of biofilm formation by lysis-defective mutants and of DNA removal from the biofilm matrix (67, 117, 133, 146, 150). The contribution of eDNA to attachment and biofilm formation, however, appears to be temporal: experiments utilizing DNase I have suggested that cells in young PAO1 biofilms are held together by eDNA, whereas the cells in more-mature PAO1 biofilms are held together primarily by components other than eDNA (98, 166). Subsequent microscopic investigations of mature P. aeruginosa biofilms stained with nucleic acid stains suggested a structural function of eDNA specific to the formation of mushroom-shaped microcolonies (5).
Exopolysaccharides can vary in their chemical and physical properties not only between bacterial species but between strains of a single species as well. P. aeruginosa is known to produce at least three different polysaccharides, namely, alginate, Psl, and Pel (the structure of Pel is unknown). Early reporter studies by Davies and Geesey (33) demonstrated that the expression of algC, a gene required for alginate and lipopolysaccharide (LPS) biosynthesis, is activated as early as 15 min after the bacterial cell attaches to either a Teflon or a glass substratum. The surface-dependent induction of algC expression correlated with stability of attachment, with cells that did not undergo algC upregulation demonstrating reduced ability to remain attached to the surface relative to that of cells with activated expression (33). While these findings suggested increased alginate production upon attachment, with alginate production contributing to biofilm resistance and being inversely linked to both flagellum-driven motility and twitching motility (48, 139, 165), it is now apparent that alginate is neither the major matrix polysaccharide nor required for biofilm development by nonmucoid P. aeruginosa strains, which are the first to colonize CF patients (121, 144, 167). Instead, recent chemical and genetic studies have demonstrated that the major polysaccharides produced by P. aeruginosa strains PAO1 and PA14 are Psl and Pel, with the roles of these two polysaccharides differing with respect to attachment and biofilm formation in a strain-specific manner. In strain PAO1, while Pel plays no role in attachment or biofilm development, Psl contributes to attachment to glass and mucin-coated surfaces and was demonstrated by Colvin et al. (26) to be the primary structural polysaccharide essential for biofilm maturation. Strain PA14, which does not produce Psl, does not require Pel for surface attachment (26). Instead, the Pel polysaccharide plays a role only once cells have made initial contact with the surface, as a loss of Pel was shown to not affect biofilm initiation but to impair biofilm formation at the monolayer stage (26). Time-lapse microscopy demonstrated that the absence of cell aggregates was likely due to Δpel mutants detaching from the substratum at a significantly increased rate compared to that of the wild type. Using an elegant assay involving optical tweezers to trap bacteria on a glass surface (in a confined space), followed by release after 5, 20, and 45 min, Colvin et al. (26) were able to demonstrate that under the conditions tested, wild-type cells readily aggregated while Δpel mutants failed to do so even after extended trapping time. The findings suggested that Pel serves as a primary structural scaffold for PA14 biofilms. The disparate roles of Pel and Psl polysaccharides are further made apparent by the difference in the expression profiles of the respective genes. While psl biosynthetic genes appear to be constitutively expressed, pel genes were found to be induced upon attachment and biofilm formation (26). These findings of the diverse roles of polysaccharides in attachment by P. aeruginosa are typical of other biofilm-forming bacteria, such as E. coli, in which adherence capabilities and biofilm formation are differentially dependent on colanic acid and β-1,6-N-acetyl-d-glucosamine (PGA). Using a genetic approach, Danese et al. (30) demonstrated that the production of the polysaccharide colanic acid is not required for E. coli K-12 attachment to abiotic surfaces. Rather, colanic acid was found to be critical for the formation of complex three-dimensional structures. In contrast, PGA was shown to be required for permanent attachments and cell-to-cell interactions, as determined using a gravity displacement assay combined with a fast Fourier transform analysis (3).
Numerous other polysaccharides have been identified to contribute to attachment and biofilm formation. It is of interest to note, however, that not all polysaccharides help biofilms stick to surfaces. In fact, a surprising number of polysaccharides in Vibrio spp. appear to have negative roles. For example, loss of the group 1 capsular polysaccharide (CPS) in V. vulnificus is associated with increased attachment and, subsequently, reduced biofilm formation (23, 73). V. cholerae O139 contains a locus with genes for CPS and lipopolysaccharide (LPS) O antigen biosynthesis that also plays a negative role in biofilm formation (77). A third example is a putative O antigen CPS locus of V. parahaemolyticus, VP0214-VP0237, whose loss increases attachment (43).
MODULATION OF CYCLIC DI-GMP
Induced expression of genes involved in polysaccharide production is indicative of bacteria transitioning to the irreversible attachment stage, which is also marked by a reduction in surface-associated swarming behavior. It has become apparent that these changes are linked to the ubiquitous intracellular messenger signaling molecule bis-(3′-5′)-cyclic di-GMP (c-di-GMP) (reviewed in references 29 and 58). First described to control extracellular cellulose biosynthesis in Acetobacter xylinum, high c-di-GMP levels are now known to correlate with the motile-sessile transition in several microorganisms (32, 69, 112, 136–138, 142, 152). Modulation of c-di-GMP has furthermore been linked to biofilm dispersion (9, 50, 112, 152), a mechanism used by biofilm bacteria to successfully transition to the planktonic growth state (140). C-di-GMP production and degradation are controlled by diguanylate cyclases (DGCs) and phosphodiesterases (PDEs), respectively, with overexpression of these enzymes generally causing global effects.
The genome of P. aeruginosa PAO1 encodes 17 different proteins with a DGC domain, 5 with a PDE domain, and 16 that contain both of these domains (86). While the majority of these were not found to have an effect on biofilm formation under static conditions during a mariner transposon mutant screen, insertions in a number of genes encoding DGCs reduced (PA1107, PA1181, PA1433, PA1727, and PA3702) or abolished (PA0169, PA1120, PA4959, and PA5487) attachment (86). In contrast, increased attachment coinciding with pellicle formation was observed for potential DGC-PDE mutants (PA0861, PA3311, PA4367, and PA5017). Insertions in genes encoding only PDE domains, with the exception of pvrR, did not cause a biofilm-related phenotype under the conditions tested (86). Among the identified DGCs playing a role in attachment was WspR (PA3702). WspR is part of the Wsp chemosensory signal transduction system, which operates to produce c-di-GMP as its effector output function. The Wsp system is homologous to chemotaxis systems and includes a membrane-bound receptor protein, WspA, and a response regulator GGDEF protein, WspR, that catalyzes c-di-GMP synthesis when phosphorylated and upon contact with surfaces (61). In addition to controlling the adhesiveness of P. aeruginosa upon surface contact, WspR is also linked to regulation of the chaperone usher pathway (cup) genes that encode putative fimbrial adhesins. These genes have been demonstrated to be required for biofilm formation in P. aeruginosa (158, 159) and to be inversely controlled by the EAL-containing PvrR response regulator (106). Mutations in a gene called wspF, part of the Wsp chemosensory signal-transduction operon, have been shown to result in cell aggregation and altered colony morphology, a phenotype which correlated with increased cellular levels of c-di-GMP and increased biofilm formation. Transcriptomic analysis suggested that the wspF mutant phenotype can be attributed to increased expression of the pel and psl operons, which were among at least 560 genes with affected expression in this strain (61). Expression of a protein predicted to catalyze degradation of c-di-GMP (e.g., PvrR) reversed the phenotype of a ΔwspF mutant and inhibited biofilm initiation by wild-type cells, indicating that the presence of c-di-GMP is necessary for biofilm formation (42, 61). A mutant screen in Yersinia pestis resulted in the identification of three c-di-GMP-modulating proteins playing a role in attachment and biofilm formation, including the DGC HmsT and the PDE HmsP, which inversely regulate biofilm formation by controlling the synthesis of the polysaccharide poly-β-1,6-N-acetylglucosamine, both in vitro and in the foregut of its flea vector, as well as y3730, encoding a functional DGC (13, 148). While c-di-GMP is an important factor in driving cell-surface and cell-cell interactions, Starkey et al. (145) demonstrated that elevated c-di-GMP levels alone are not sufficient to alter the expression of all of the genes affected following wspF inactivation. Using a ΔwspF single mutant and a nonaggregative ΔwspF Δpel Δpsl triple mutant, they demonstrated that the regulation of multiple genes is actually dependent on the aggregative phenotype rather than c-di-GMP overproduction.
Effects of c-di-GMP on cellular behaviors are likely accomplished through its binding, triggering posttranslational modulation of the functions of certain regulatory proteins. Many c-di-GMP-binding proteins playing a role in attachment harbor PilZ, FleQ, PelD, and PleD domains (reviewed in reference 58). PilZ domains have been discovered in sequences of polysaccharide-related proteins, including bacterial cellulose synthases and alginate biosynthesis protein Alg44, as well as proteins involved in flagellar motility, including the enterobacterial YcgR and firmicute YpfA protein families (58). In E. coli, the c-di-GMP receptor YcgR has been shown to bind to the FliG subunit of the flagellum switch complex, with high levels of c-di-GMP inducing the counterclockwise bias in E. coli flagellar rotation, resulting in smooth swimming (44). In P. aeruginosa PAO1, expression of flagellum biosynthesis genes is under the control of the c-di-GMP-binding protein FleQ. While activating flagellum gene expression, FleQ represses pel transcription at low c-di-GMP levels (under planktonic, free-swimming growth conditions), with its inhibitory effect on pel transcription negated when intracellular c-di-GMP levels are high (60). Pel polysaccharide biosynthesis is furthermore regulated in P. aeruginosa by the c-di-GMP receptor protein PelD (91). Recently, a novel c-di-GMP protein that triggers attachment by inducing polysaccharide production was identified in V. cholerae (83). This work demonstrated that the transcriptional regulator VpsT directly senses c-di-GMP to inversely control Vps polysaccharide production and motility. The effect on VpsT, the master regulator for biofilm formation, was found to be due to a change in oligomerization upon c-di-GMP binding rather than phosphorylation (83).
While c-di-GMP has been emerging as an important contributor to attachment and biofilm formation, the inducing signals and the timing of c-di-GMP level modulation are poorly explored. What induces a change in c-di-GMP levels? Is the increase in c-di-GMP levels triggered upon surface contact (reversible or irreversible attachment) or a prerequisite for attachment? Considering the link between c-di-GMP, flagellum biosynthesis, and polysaccharide production, it is likely that c-di-GMP levels are increased upon restriction of flagellar rotation (accomplished following contact with the surface). And if so, what would be the signal in nonmotile bacteria? The answers to these questions await future investigations.
LET'S MAKE IT PERMANENT: TRANSITION TO IRREVERSIBLE ATTACHMENT
The function of c-di-GMP and the proteins regulating its level expands beyond initial attachment, as a number of c-di-GMP-associated proteins have been identified as being required for more-permanent associations with surfaces. In P. aeruginosa, the proteins SadB, SadC, and BifA are required for the transition from reversible to irreversible attachment by regulating several surface-associated behaviors, including swarming, EPS production, and modulation of flagellar reversal rates via the chemotaxis cluster IV in a c-di-GMP-dependent manner. Merritt et al. (104) demonstrated that deletion of sadC, encoding an inner membrane-localized DGC, results in a hyperswarming strain defective in biofilm formation. The mutant was also deficient in polysaccharide production and displayed altered reversal patterns while swimming in high-viscosity medium, two behaviors proposed to influence biofilm formation and swarming motility. Similarly, SadB was found to inversely regulate biofilm formation and swarming motility via its ability to both modulate flagellar reversals in a viscosity-dependent fashion and influence the production of the Pel exopolysaccharide (19, 20). The role of SadB in the transition to irreversible attachment was further confirmed by microscopic examination that revealed an increased number of sadB mutant cells adhering via the cell pole. Inactivation of bifA, encoding a c-di-GMP PDE, was shown by Kuchma et al. (84) to have the opposite effect, with ΔbifA mutants demonstrating hyper-biofilm formation and severe swarming defects. The three proteins act in concert: SadC and BifA inversely modulate the respective intracellular pool(s) of c-di-GMP upon receiving the proper environmental cues (such as nutritional cues or contact with an appropriate surface) via SadC, with SadB being predicted to transmit this signal to the Pel machinery and components of the CheIV chemotaxis-like cluster. The CheIV-like cluster is one of five chemotaxis-like gene clusters in P. aeruginosa and is involved in twitching motility (45).
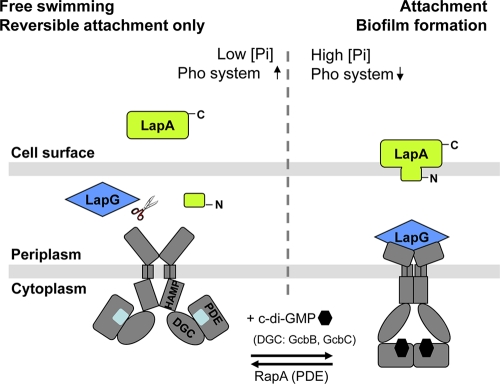
C-di-GMP controls biofilm formation by Pseudomonas fluorescens strains Pf0-1 and WCS365 by promoting the cell surface localization of a large adhesive protein, LapA, that is exported out of the cell via an ATP-binding cassette (ABC) transporter composed of LapE, LapB, and LapC (Fig. 2) (62). A similar system has recently been identified in Pseudomonas putida (50). Time-lapse microscopy studies determined that ΔlapA mutants (as well as other lap mutants) are unable to progress from reversible to irreversible attachment on abiotic surfaces that these organisms likely encounter in the environment (62). Localization of the adhesin LapA is regulated posttranslationally by a c-di-GMP effector system consisting of LapD and LapG. lapD mutants are unable to retain LapA on the cell surface once secreted (118) (Fig. 2). The inner membrane protein LapD contains enzymatically inactive DGC and PDE (EAL) domains, with c-di-GMP binding to the EAL domain serving as a signal that is communicated across the cytoplasmic membrane to the periplasmic domain of LapD via a HAMP relay (HAMP is a conserved domain present in histidine kinases, adenyl cyclases, methyl-accepting proteins, and phosphatases) (118). This inside-out signaling mechanism by LapD triggers sequestration of the periplasmic protease LapG, thus preventing cleavage of the surface adhesin LapA (116, 118) (Fig. 2). In contrast, the intracellular module of LapD assumes an inactive conformation in the absence of c-di-GMP, with the consequence that LapA is lost from the bacterial cell surface. The inside-out mechanism is further supported by the finding that a lack of LapD cannot be overcome by artificially elevating intracellular c-di-GMP levels through DGC overexpression. The findings strongly support observations by Merritt et al. (105) that changes in total levels of c-di-GMP cannot solely account for the specific surface-related phenotypes. This raises the question of which protein produces the c-di-GMP that is sensed by LapD. Using a transposon mutant screen, Newell et al. (119) showed that out of 28 DGCs encoded in the P. fluorescens genome, only two DGCs, GcbB and GcbC, appear to specifically produce the c-di-GMP pools that are sensed by LapD to promote LapA-dependent attachment. The P. fluorescens Lap system plays a role in the environmental modulation by inorganic phosphate of intracellular c-di-GMP levels and, thus, surface commitment (118). Phosphate limitation results in conditional expression (via the pho regulon) of the PDE RapA, which serves as an inhibitor of biofilm formation by lowering c-di-GMP levels and consequently inactivating LapD and inhibiting the secretion of LapA (109, 118) (Fig. 2). It is of interest to note that the Lap system is conserved in soil but not pathogenic pseudomonads and that it has not been linked to polysaccharide production or surface-associated motility.
Fig 2.
Model for inorganic-phosphate (Pi)- and Lap-mediated control of biofilm formation in soil pseudomonads. In high Pi conditions (right), c-di-GMP accumulates in the cell, probably via the DGCs GcbB and GcbC. LapD binds c-di-GMP via its EAL domain and sequesters LapG at the inner membrane, promoting the maintenance of the LapA adhesin on the cell surface and, thus, fostering biofilm formation. When low extracellular Pi is sensed (left) by the Pho system, activated PhoR promotes rapA transcription via PhoB. The PDE activity of RapA depletes cellular c-di-GMP and leads to the dissociation of c-di-GMP from LapD and a conformational change of LapD. In the absence of bound c-di-GMP, LapD is unable to interact with the protease LapG, and LapG, in turn, cleaves the N terminus of LapA in the periplasm, promoting its loss from the cell surface. Release of LapA from attached cells promotes their detachment from the substratum and bacteria returning to the free-swimming mode of growth. The model is based on structural and functional analyses (50, 62, 109, 116, 118).
In Caulobacter crescentus, transition between reversible and polysaccharide-mediated irreversible attachment is stimulated through the interplay between pili and flagellum rotation (94). Reversible attachment of C. crescentus is mediated by motile cells bearing pili, with surface contact resulting in the rapid pilus-dependent arrest of flagellum rotation and the concurrent stimulation of polar holdfast adhesive polysaccharide. The holdfast is required for irreversible surface anchoring, with its formation depending on the DGC PleD and posttranslational regulation (94). Similar stimulation of polar adhesin production by surface contact occurs in Asticcacaulis biprosthecum and Agrobacterium tumefaciens, indicating that single bacterial cells respond to their initial contact with surfaces by triggering just-in-time adhesin production (94).
The finding of C. crescentus surface contact resulting in flagellum rotation arrest and subsequent induction of just-in-time adhesin production to allow irreversible surface attachment (94) demonstrates that bacterial adhesion to surfaces is not simply mediated by extracellular structures but that it also results in the induction of gene expression (reviewed in reference 85). The requirement for novel genes for attachment was first demonstrated by O'Toole and Kolter (125), who showed that initial attachment by P. fluorescens WCS365 was significantly reduced when cells were pretreated with tetracycline at sublethal concentration or exposed to tetracycline present in the growth medium during attachment. Indications of bacteria being able to sense contact with a surface and, in response, alter gene expression to promote stable cell-surface interactions comes from studies with holdfast proteins in various bacterial species (Table 1) as well as the E. coli signaling system that responds to membrane stress, Cpx (Table 1). Cpx is a two-component signaling pathway composed of the sensor kinase CpxA and the response regulator CpxR that responds to membrane stress (126). Consistent with its role in surface sensing, the Cpx pathway is activated upon surface interaction, and both the ΔcpxR and ΔcpxA mutants form less stable cell-surface interactions and biofilms with less biomass compared to those of the wild type when grown in microtiter plates (40, 126). The transduction of the signals through this pathway is dependent on the outer membrane proteins OmpA and NlpE, with the latter likely being the direct sensor of contact with a surface, resulting in differential expression of genes involved in cellulose and P-pilus biosynthesis (96, 126).
LET'S TALK: INTERCELLULAR SIGNALING
Permanent associations with the surface have been linked to quorum sensing. Quorum sensing is a cell-cell communication process in which bacteria use the production and detection of extracellular chemicals called autoinducers to monitor cell population density. In general, Gram-negative bacteria use acylated homoserine lactones (AHLs) as autoinducers and Gram-positive bacteria use processed oligopeptides to communicate and to synchronize the gene expression and behaviors of the group (reviewed in references 21 and 120). These processes include symbiosis, virulence, competence, conjugation, antibiotic production, motility, sporulation, and biofilm formation. The role of quorum sensing in biofilm formation (and its inhibition) has been the subject of numerous research publications and reviews (for examples, see references 10, 12, 21, and 120), with quorum sensing playing a role as early as the attachment stage (Fig. 1). This was first demonstrated by Davies et al. (35) by showing that a P. aeruginosa ΔlasI mutant formed only flat, undifferentiated biofilms (monolayers) that, unlike wild-type biofilms, are sensitive to the biocide sodium dodecyl sulfate (SDS). Addition of the AHL synthesized by LasI restored wild-type biofilm formation and SDS resistance, indicating that the defect was a result of AHL absence. The Las quorum-sensing system was demonstrated to be activated upon transition to the irreversible attachment stage, as determined by the onset of reporter activity for the lasB gene (140).
Considering that both quorum sensing and c-di-GMP play critical roles in attachment and biofilm formation, it is not surprising that the two signaling processes converge. In describing the P. aeruginosa tyrosine phosphatase TbpA, Ueda and Wood (157) recently connected the two signaling processes with the synthesis of Pel polysaccharide and biofilm formation. The study showed a tbpA mutant to have increased biofilm biomass, correlating with decreased swimming and abolished swarming but increased attachment, aggregation, pellicle formation, Pel polysaccharide production, and c-di-GMP levels. TbpA, which promoted c-di-GMP production and pel gene expression, was found to be positively regulated by Las-mediated quorum sensing.
Quorum-sensing signaling also participates in the regulation of V. cholerae attachment to chitinous surfaces and intestinal substrates. Levels of the secreted attachment factor GlcNAc binding protein A (GbpA), necessary for adherence to these surfaces, are dependent on the central V. cholerae quorum-sensing regulator HapR, which activates the expression of genes encoding the secreted proteases HapA and PrtV that are involved in GbpA degradation (75). Quorum sensing is also required for normal attachment and biofilm formation by the aquatic bacterium V. vulnificus (101). Interestingly, in some species, such as Serratia marcescens, the requirement for quorum-sensing regulation of attachment depends on the nature of the surface, with adherence to abiotic but not biotic surfaces being modulated by quorum sensing (87). Overall, the importance of quorum sensing in the regulation of cell-surface and cell-cell attachment has been repeatedly reported for a variety of other bacterial species, including E. coli, L. monocytogenes, Burkholderia cenocepacia, and Burkholderia cepacia (22, 37, 52, 64, 154).
With quorum sensing playing a role in the motile-sessile transition, it is not surprising that intercellular signaling also modulates the reverse sessile-motile transition. The fatty acid messenger cis-2-decenoic acid (CDA) has recently been described to play a role in self-induced dispersion by P. aeruginosa biofilms (34). This molecule is functionally and structurally related to the class of short-chain fatty acid signaling molecules that includes the diffusible signal factor DSF, which acts as a cell-to-cell communication molecule in Xanthomonas campestris (41). Active at nanomolar concentrations, CDA was demonstrated to also induce dispersion of biofilms formed by a variety of Gram-negative and -positive bacteria as well as by Candida albicans. Davies and Marques (34) further demonstrated that CDA impaired the formation of biofilms at the monolayer stage. While the mechanism of CDA function was not explored, CDA is produced by planktonic and biofilm cells alike. The findings of CDA accumulation preventing biofilm formation may have important implications for biofilms grown in batch-like conditions (e.g., microtiter plate assays) and explain why one does not readily observe biofilms forming in batch culture flasks (following extended incubation times) but can easily spot biofilms forming on the walls of a chemostat.
NONCODING RNAS MATTER
How do bacteria perceive their environment at the surface? Bacteria use two-component system (TCS) signaling pathways to translate external signals into adaptive responses at the level of transcriptional or posttranscriptional regulation (reviewed in reference 107). The E. coli carbon storage regulator system (Csr), with the RNA-binding protein CsrA as the central player, exemplifies such an adaptive regulatory cascade. Csr is a global regulatory system that controls bacterial gene expression posttranscriptionally. Originally identified as a system to regulate glycogen biosynthesis, CsrA represses numerous genes associated with the stationary phase of growth but activates certain exponential-phase metabolic pathways. In addition, virulence, motility, cell surface properties, and adherence are modulated by CsrA in E. coli (reviewed in references 6 and 135), with CsrA acting as a repressor of biofilm formation (68). Gene expression is controlled by CsrA binding to leader segments of target mRNAs, affecting their translation and stability. In the case of flhDC mRNA, which is required for flagellum biosynthesis, binding of CsrA to the leader of flhDC stabilizes the respective mRNA, with overexpression of csrA resulting in increased motility (164). In addition, CsrA functions as a translational repressor capable of destabilizing mRNA transcripts, such as those associated with glycogen and PGA synthesis (6, 135) as well as those encoding NhaR, the transcriptional regulator involved in pga operon expression (128). CsrA is antagonized by the small regulatory RNAs (sRNAs) CsrB and CsrC, which are transcriptionally modulated by the membrane-bound TCS BarA/UvrY. BarA/UvrY, also modulated by autoinducer-2 (AI-2) quorum sensing (120), activates sRNA transcription in response to weak acids, such as formate and acetate, as well as perturbations in the levels of Krebs cycle intermediates (24).
Many pathogenic bacteria, including P. aeruginosa, Salmonella enterica, H. pylori, Legionella pneumophila, and V. cholerae, rely on Csr-like systems to posttranscriptionally modulate diverse transcripts in order to control virulence mechanisms and group behaviors. Among the systems regulating attachment in P. aeruginosa, few have received more attention than the Csr system homolog—the LadS/RetS/Gac/Rsm signal transduction network. This intricate signaling system has been implicated as a switch between acute/planktonic and chronic/biofilm modes of growth by reciprocally regulating gene expression associated with type III secretion or type VI secretion and exopolysaccharide production. Ultimately, this system controls attachment and virulence via the CsrA homolog RsmA (18). While RsmA is thought to elevate type III secretion system (TTSS) transcript abundance, binding of RsmA to the 5′ untranslated region of psl mRNA was recently shown to prevent ribosome access and protein translation (66). In P. aeruginosa, RsmA function is antagonized by the sRNAs rsmZ and rsmY, whose expression is directly controlled by GacA/GacS, the TCS homolog of the E. coli BarA/UvrY system (reviewed in reference 107). GacA/GacS function is, in turn, inversely controlled by the two-component hybrids RetS and LadS (53, 161). RetS negatively controls rsmY and rsmZ gene expression, while LadS positively controls sRNA levels. Inactivation of retS was shown to result in hyperattachment with elevated Psl exopolysaccharide gene expression and suppression of the TTSS, with the phenotype abolished by a secondary mutation in gacS (53). In contrast, LadS inactivation was found to result in decreased attachment, reduced Psl production, and elevated TTSS expression, suggesting that LadS may function to counteract RetS. While the mode of LadS function remains uncharacterized, RetS has been shown to reduce sRNA expression by interfering with GacS autophosphorylation through the formation of RetS/GacS heterodimers (54). Although the reciprocal LadS/RetS regulation of rsmYZ levels via GacAS provides an elegant model of the regulation of the motile-sessile switch by P. aeruginosa, increasing evidence is suggesting that this system is far more complex. Additional components were recently identified as HptB and SagS.
Inactivation of hptB was observed to enhance attachment in P. aeruginosa PAK but not PAO1 parental strain backgrounds, indicating potential strain-specific differences in the fine-tuning of the Gac/Rsm signaling (16, 129). HptB is proposed to regulate rsmY levels in a GacA-dependent manner via as-yet-unknown factors (16). This, however, is not true of SagS, a sensor-regulator hybrid that participates in a phosphotransfer event with HptB (63, 95) and is involved in the regulation of attachment and biofilm formation (129). SagS suppresses sRNA levels predominantly under planktonic growth conditions, with inactivation of sagS resulting in a temporary enhancement of attachment but a defect in the later stages of biofilm formation (129). Moreover, the ΔsagS phenotype superseded those of both the gacA and rsmYZ mutants, suggesting that SagS represents a novel level of Gac/Rsm regulation of attachment (129).
Considering that the multicomponent Csr and Gac/Rsm systems inversely control attachment (and polysaccharide production and adhesiveness) and virulence, it has been suggested that they act as a genetic switch that reciprocally regulates free-swimming and surface-attached lifestyles as well as acute and chronic infections (6, 107, 135). Unfortunately, the signals perceived by the Gac/Rsm system or SagS are not known. Sequence analysis of LadS and RetS suggested that the two hybrid sensors harbor a signaling domain consisting of a 7-transmembrane region (7TMR) and a periplasmic sensor domain (diverse intracellular signaling module extracellular 2 [DISMED2]). The latter domain is believed to act as a receptor for carbohydrates and their derivatives. The structure of the periplasmic RetS sensory domain has recently been solved and was found to be similar to carbohydrate-binding modules found in other proteins (162). This observation suggests that RetS, and probably also LadS, responds to carbohydrate-like structures.
The Gac/Rsm system and c-di-GMP signaling are probably two of the best-characterized intracellular regulatory mechanisms driving adherence in pathogenic bacteria. Yet the relationship between the two has remained elusive, especially in P. aeruginosa. The first indication of an association between a Csr system and c-di-GMP levels came with the characterization of the GGDEF and EAL domain-containing protein CsrD, which targets the sRNAs csrB/csrC for degradation by RNase E (149). While CsrD is not directly involved in c-di-GMP synthesis or turnover, it requires both the GGDEF and the EAL domains for its function (149). The domain requirement is reminiscent of LapD, and it is likely that c-di-GMP-driven conformational change (but not an inside-out mechanism) is associated with CsrD targeting sRNAs for RNase E-mediated degradation (149). The relationship between Csr/Rsm regulation and c-di-GMP signaling has been strengthened by the findings of CsrA regulating the expression of proteins containing the GGDEF, GGDEF-EAL, and EAL domains in E. coli and Salmonella enterica serovar Typhimurium via direct binding to the mRNA leaders of the respective genes (70, 71). Although a link between Gac/Rsm regulation and cyclic-di-GMP has recently been established in P. aeruginosa, the relationship remains poorly understood in this organism. Moscoso et al. (113) demonstrated that the type III to type VI secretion virulence switch mediated by RetS is dependent on and can be mimicked by changes in c-di-GMP levels. While this study did not directly assess effects on adherence, given the mode of RsmA regulation of targeted secretion and exopolysaccharide transcripts, it is likely that Gac/Rsm regulation of attachment is also dependent on c-di-GMP signaling.
IS ATTACHMENT AN INDICATOR FOR BIOFILM FORMATION?
Biofilms are thought to be the underlying cause of many chronic infections, including those in wounds and in the lungs of patients with cystic fibrosis. The switch from the free-swimming to the surface-associated mode of growth has been linked to global shifts from acute virulence to chronic infections. It is thus not surprising that attachment is considered an important step for controlling biofilm development and, thus, chronic and persistent infections. It follows that attachment is biofilms' Achilles' heel that is essential for biofilm formation and crucial pathogenic functions. But is attachment (or the lack thereof) indeed an indicator for biofilm formation? Does reduced attachment indicate impaired biofilm development and vice versa? The answer is a clear “maybe,” as some genetic determinants appear to play only a temporary role. For example, loss of flagella has been shown in numerous bacteria to significantly reduce attachment, while continued incubation led to the formation of a robust monolayer and biofilms (160). Although P. aeruginosa ΔretS mutants exhibit a hyperattachment phenotype, they are impaired in biofilm development upon continued incubation under flowing conditions. In fact, all components of the Gac/Rsm system appear to have opposite effects on attachment and biofilm formation, including GacA, GacS, RetS, RsmY, and RsmZ. A similar disconnect between attachment and biofilm development was found for SagS. Inactivation of sagS coincided with hyperattachment and the planktonic-stage-specific increase in rsmYZ levels and Psl polysaccharide production (129). However, these phenotypes were only temporary, with the difference in attachment diminishing upon continued surface exposure. Using microscopy-based and proteomic approaches, the ΔsagS mutant was found to be arrested in the transition to the irreversible attachment stage (129).
Additional factors have been shown to not affect attachment but to impair further biofilm development once the monolayer stage has been reached. Bomchil et al. (15) reported on the identification of MbaA, a member of a family of regulatory proteins containing GGDEF and EAL domains. Although the absence of mbaA did not significantly affect the initial attachment of V. cholerae cells to the surface, it led to the formation of biofilms that lack the typical structure, including the pillars of cells separated by fluid-filled channels that are evident in many mature wild-type biofilms, including those of V. cholerae. Microscopic analysis indicated that monolayer formation of the ΔmbaA mutant correlated with increased amounts of extracellular matrix material in the mutant biofilms. Similarly, a lack of the Pel polysaccharide in P. aeruginosa strain PA14 was shown to not affect attachment but to impair biofilm formation at the monolayer stage (26). In contrast, an alginate-overproducing P. aeruginosa strain was found to be impaired in attachment but demonstrated significantly enhanced biofilm and microcolony formation (56).
There are also some inconsistencies in the role of other factors in attachment. For instance, the sigma factor RpoS is a positive regulator of psl gene expression in P. aeruginosa (66), yet inactivation of rpoS has been correlated with more-substantial biofilm biomass accumulation compared to that of the wild type (59). Similarly, P. aeruginosa rpoN mutants are capable of forming biofilms but are impaired in attachment while demonstrating increased production of sadB, which is essential for attachment (20, 57, 143, 151). SadC has been demonstrated to contribute to attachment. However, while inactivation of sadC by transposon mutagenesis in P. aeruginosa PA14 was shown to result in increased attachment and pellicle formation (86), deletion of sadC in the same parental strain rendered the mutant impaired in biofilm formation (104). It is likely that such inconsistencies arise from differences in parental strains or in the manner in which mutants were generated or from differences in growth conditions and experimental setup or that it may simply be a question of when over the course of the biofilm developmental process the biofilm formation capabilities were analyzed. However, these inconsistencies also underscore the finding that biofilms form in a complex and regulated manner in a process that one may call a developmental progression (110).
CONCLUDING REMARKS
This review, aimed at giving an overview of factors involved in attachment, is by no means complete and may occasionally present conflicting information. It is apparent from the above-reviewed literature that the mechanism of initial attachment to surfaces is a complex process involving several factors. While inactivation of a single factor may impact the transition from the free-swimming to the surface-attached mode of growth, each factor can be considered a part of a larger regulatory network to accomplish bacterial initial attachment. For instance, while bacterial flagella play a role in making the first contact, their rotation (or inability to do so) at a surface is providing the signal in many species to induce expression of polysaccharide genes to accomplish a more permanent surface interaction. Polysaccharides, which are regulated by intracellular c-di-GMP levels and the Csr/Rsm systems, in turn, create a negative feedback loop modulating flagellum-driven motility. Considering that bacteria first interact with the surface as single cells (rather than with each other), it is not surprising that the function of some components (e.g., flagella) is restricted to the attachment stage. In conclusion, future work needs to focus on the integration of the known signals triggering attachment and the factors required for adherence into regulatory cascades that translate specific cues and signals into cellular processes driving the motile-sessile switch.
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (R01 A107525701 and R01 AI080710).
Footnotes
Published ahead of print 2 March 2012
REFERENCES
- 1. Abee T, Kovacs AT, Kuipers OP, van der Veen S. 2011. Biofilm formation and dispersal in Gram-positive bacteria. Curr. Opin. Biotechnol. 22:172–179 [DOI] [PubMed] [Google Scholar]
- 2. Agladze K, Jackson D, Romeo T. 2003. Periodicity of cell attachment patterns during Escherichia coli biofilm development. J. Bacteriol. 185:5632–5638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agladze K, Wang X, Romeo T. 2005. Spatial periodicity of Escherichia coli K-12 biofilm microstructure initiates during a reversible, polar attachment phase of development and requires the polysaccharide adhesin PGA. J. Bacteriol. 187:8237–8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allegrucci M, et al. 2006. Phenotypic characterization of Streptococcus pneumoniae biofilm development. J. Bacteriol. 188:2325–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allesen-Holm M, et al. 2006. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 59:1114–1128 [DOI] [PubMed] [Google Scholar]
- 6. Babitzke P, Romeo T. 2007. CsrB sRNA family: sequestration of RNA-binding regulatory proteins. Curr. Opin. Microbiol. 10:156–163 [DOI] [PubMed] [Google Scholar]
- 7. Bahlawane C, Baumgarth B, Serrania J, Ruberg S, Becker A. 2008. Fine-tuning of galactoglucan biosynthesis in Sinorhizobium meliloti by differential WggR (ExpG)-, PhoB-, and MucR-dependent regulation of two promoters. J. Bacteriol. 190:3456–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bansal T, et al. 2007. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect. Immun. 75:4597–4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barraud N, et al. 2009. Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J. Bacteriol. 191:7333–7342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bassler BL. 1999. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 2:582–587 [DOI] [PubMed] [Google Scholar]
- 11. Berlutti F, et al. 2005. Iron availability influences aggregation, biofilm, adhesion and invasion of Pseudomonas aeruginosa and Burkholderia cenocepacia. Int. J. Immunopathol. Pharmacol. 18:661–670 [DOI] [PubMed] [Google Scholar]
- 12. Bjarnsholt T, Tolker-Nielsen T, Høiby N, Givskov M. 2010. Interference of Pseudomonas aeruginosa signalling and biofilm formation for infection control. Expert Rev. Mol. Med. 12:e11. [DOI] [PubMed] [Google Scholar]
- 13. Bobrov AG, et al. 2011. Systematic analysis of cyclic di-GMP signalling enzymes and their role in biofilm formation and virulence in Yersinia pestis. Mol. Microbiol. 79:533–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boles BR, McCarter LL. 2002. Vibrio parahaemolyticus scrABC, a novel operon affecting swarming and capsular polysaccharide regulation. J. Bacteriol. 184:5946–5954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bomchil N, Watnick P, Kolter R. 2003. Identification and characterization of a Vibrio cholerae gene, mbaA, involved in maintenance of biofilm architecture. J. Bacteriol. 185:1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bordi C, et al. 2010. Regulatory RNAs and the HptB/RetS signalling pathways fine-tune Pseudomonas aeruginosa pathogenesis. Mol. Microbiol. 76:1427–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bowden GHW, Li YH. 1997. Nutritional influences on biofilm development. Adv. Dent. Res. 11:81–99 [DOI] [PubMed] [Google Scholar]
- 18. Brencic A, Lory S. 2009. Determination of the regulon and identification of novel mRNA targets of Pseudomonas aeruginosa RsmA. Mol. Microbiol. 72:612–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caiazza NC, Merritt JH, Brothers KM, O'Toole GA. 2007. Inverse regulation of biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J. Bacteriol. 189:3603–3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caiazza NC, O'Toole GA. 2004. SadB is required for the transition from reversible to irreversible attachment during biofilm formation by Pseudomonas aeruginosa PA14. J. Bacteriol. 186:4476–4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Camilli A, Bassler BL. 2006. Bacterial small-molecule signaling pathways. Science 311:1113–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Challan Belval S, et al. 2006. Assessment of the roles of LuxS, S-ribosyl homocysteine, and autoinducer 2 in cell attachment during biofilm formation by Listeria monocytogenes EGD-e. Appl. Environ. Microbiol. 72:2644–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chatzidaki-Livanis M, Jones MK, Wright AC. 2006. Genetic variation in the Vibrio vulnificus group 1 capsular polysaccharide operon. J. Bacteriol. 188:1987–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chavez RG, Alvarez AF, Romeo T, Georgellis D. 2010. The physiological stimulus for the BarA sensor kinase. J. Bacteriol. 192:2009–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cole SP, Harwood J, Lee R, She R, Guiney DG. 2004. Characterization of monospecies biofilm formation by Helicobacter pylori. J. Bacteriol. 186:3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Colvin KM, et al. 2011. The Pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 7:e1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711–745 [DOI] [PubMed] [Google Scholar]
- 28. Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 29. Cotter PA, Stibitz S. 2007. c-di-GMP-mediated regulation of virulence and biofilm formation. Curr. Opin. Microbiol. 10:17–23 [DOI] [PubMed] [Google Scholar]
- 30. Danese PN, Pratt LA, Kolter R. 2000. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 182:3593–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Danhorn T, Hentzer M, Givskov M, Parsek MR, Fuqua C. 2004. Phosphorus limitation enhances biofilm formation of the plant pathogen Agrobacterium tumefaciens through the PhoR-PhoB regulatory system. J. Bacteriol. 186:4492–4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. D'Argenio DA, Miller SI. 2004. Cyclic di-GMP as a bacterial second messenger. Microbiology 150:2497–2502 [DOI] [PubMed] [Google Scholar]
- 33. Davies DG, Geesey GG. 1995. Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl. Environ. Microbiol. 61:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davies DG, Marques CNH. 2009. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol. 191:1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davies DG, et al. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295–298 [DOI] [PubMed] [Google Scholar]
- 36. Dewanti R, Wong ACL. 1995. Influence of culture conditions on biofilm formation by Escherichia coli O157:H7. Int. J. Food Microbiol. 26:147–164 [DOI] [PubMed] [Google Scholar]
- 37. Domka J, Lee J, Wood TK. 2006. YliH (BssR) and YceP (BssS) regulate Escherichia coli K-12 biofilm formation by influencing cell signaling. Appl. Environ. Microbiol. 72:2449–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Donlan RM. 2001. Biofilms and device-associated infections. Emerg. Infect. Dis. 7:277–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Donlan RM. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dorel C, Vidal O, Prigent-Combaret C, Vallet I, Lejeune P. 1999. Involvement of the Cpx signal transduction pathway of E. coli in biofilm formation. FEMS Microbiol. Lett. 178:169–175 [DOI] [PubMed] [Google Scholar]
- 41. Dow JM, et al. 2003. Biofilm dispersal in Xanthomonas campestris is controlled by cell-cell signaling and is required for full virulence to plants. Proc. Natl. Acad. Sci. U. S. A. 100:10995–11000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Drenkard E, Ausubel FM. 2002. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416:740–743 [DOI] [PubMed] [Google Scholar]
- 43. Enos-Berlage JL, Guvener ZT, Keenan CE, McCarter LL. 2005. Genetic determinants of biofilm development of opaque and translucent Vibrio parahaemolyticus. Mol. Microbiol. 55:1160–1182 [DOI] [PubMed] [Google Scholar]
- 44. Fang X, Gomelsky M. 2010. A post-translational, c-di-GMP-dependent mechanism regulating flagellar motility. Mol. Microbiol. 76:1295–1305 [DOI] [PubMed] [Google Scholar]
- 45. Ferrández A, Hawkins AC, Summerfield DT, Harwood CS. 2002. Cluster II che genes from Pseudomonas aeruginosa are required for an optimal chemotactic response. J. Bacteriol. 184:4374–4383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Flemming H-C, Neu TR, Wozniak DJ. 2007. The EPS matrix: the “house of biofilm cells.” J. Bacteriol. 189:7945–7947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Flemming HC, Wingender J. 2010. The biofilm matrix. Nat. Rev. Microbiol. 8:623–633 [DOI] [PubMed] [Google Scholar]
- 48. Garrett ES, Perlegas D, Wozniak DJ. 1999. Negative control of flagellum synthesis in Pseudomonas aeruginosa is modulated by the alternative sigma factor AlgT (AlgU). J. Bacteriol. 181:7401–7404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Geesey GG, Richardson WT, Yeomans HG, Irvin RT, Costerton JW. 1977. Microscopic examination of natural sessile bacterial populations from an alpine stream. Can. J. Microbiol. 23:1733–1736 [DOI] [PubMed] [Google Scholar]
- 50. Gjermansen M, Nilsson M, Yang L, Tolker-Nielsen T. 2010. Characterization of starvation-induced dispersion in Pseudomonas putida biofilms: genetic elements and molecular mechanisms. Mol. Microbiol. 75:815–826 [DOI] [PubMed] [Google Scholar]
- 51. Goller C, Wang X, Itoh Y, Romeo T. 2006. The cation-responsive protein NhaR of Escherichia coli activates pgaABCD transcription, required for production of the biofilm adhesin poly-β-1,6-N-acetyl-d-glucosamine. J. Bacteriol. 188:8022–8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gonzalez Barrios AF, et al. 2006. Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum-sensing regulator (MqsR, B3022). J. Bacteriol. 188:305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Goodman AL, et al. 2004. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev. Cell 7:745–754 [DOI] [PubMed] [Google Scholar]
- 54. Goodman AL, et al. 2009. Direct interaction between sensor kinase proteins mediates acute and chronic disease phenotypes in a bacterial pathogen. Genes Dev. 23:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goulter RM, Gentle IR, Dykes GA. 2009. Issues in determining factors influencing bacterial attachment: a review using the attachment of Escherichia coli to abiotic surfaces as an example. Lett. Appl. Microbiol. 49:1–7 [DOI] [PubMed] [Google Scholar]
- 56. Hay ID, Gatland K, Campisano A, Jordens JZ, Rehm BHA. 2009. Impact of alginate overproduction on attachment and biofilm architecture of a supermucoid Pseudomonas aeruginosa strain. Appl. Environ. Microbiol. 75:6022–6025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hendrickson EL, Plotnikova J, Mahajan-Miklos S, Rahme LG, Ausubel FM. 2001. Differential roles of the Pseudomonas aeruginosa PA14 rpoN gene in pathogenicity in plants, nematodes, insects, and mice. J. Bacteriol. 183:7126–7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hengge R. 2009. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 7:263–273 [DOI] [PubMed] [Google Scholar]
- 59. Heydorn A, et al. 2002. Statistical analysis of Pseudomonas aeruginosa biofilm development: impact of mutations in genes involved in twitching motility, cell-to-cell signaling, and stationary-phase sigma factor expression. Appl. Environ. Microbiol. 68:2008–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hickman JW, Harwood CS. 2008. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol. Microbiol. 69:376–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hickman JW, Tifrea DF, Harwood CS. 2005. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc. Natl. Acad. Sci. U. S. A. 102:14422–14427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hinsa SM, Espinosa-Urgel M, Ramos JL, O'Toole GA. 2003. Transition from reversible to irreversible attachment during biofilm formation by Pseudomonas fluorescens WCS365 requires an ABC transporter and a large secreted protein. Mol. Microbiol. 49:905–918 [DOI] [PubMed] [Google Scholar]
- 63. Hsu JL, Chen HC, Peng HL, Chang HY. 2008. Characterization of the histidine-containing phosphotransfer protein B-mediated multistep phosphorelay system in Pseudomonas aeruginosa PAO1. J. Biol. Chem. 283:9933–9944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huber B, et al. 2001. The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility. Microbiology 147:2517–2528 [DOI] [PubMed] [Google Scholar]
- 65. Humphrey B, Kjelleberg S, Marshall KC. 1983. Responses of marine bacteria under starvation conditions at a solid-water interface. Appl. Environ. Microbiol. 45:43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Irie Y, et al. 2010. Pseudomonas aeruginosa biofilm matrix polysaccharide Psl is regulated transcriptionally by RpoS and post-transcriptionally by RsmA. Mol. Microbiol. 78:158–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Izano EA, Amarante MA, Kher WB, Kaplan JB. 2008. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl. Environ. Microbiol. 74:470–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jackson DW, et al. 2002. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 184:290–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jenal U. 2004. Cyclic di-guanosine-monophosphate comes of age: a novel secondary messenger involved in modulating cell surface structures in bacteria? Curr. Opin. Microbiol. 7:185–191 [DOI] [PubMed] [Google Scholar]
- 70. Jonas K, et al. 2010. Complex regulatory network encompassing the Csr, c-di-GMP and motility systems of Salmonella typhimurium. Environ. Microbiol. 12:524–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jonas K, et al. 2008. The RNA binding protein CsrA controls cyclic di-GMP metabolism by directly regulating the expression of GGDEF proteins. Mol. Microbiol. 70:236–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jonas K, Melefors Ö. 2009. The Escherichia coli CsrB and CsrC small RNAs are strongly induced during growth in nutrient-poor medium. FEMS Microbiol. Lett. 297:80–86 [DOI] [PubMed] [Google Scholar]
- 73. Joseph LA, Wright AC. 2004. Expression of Vibrio vulnificus capsular polysaccharide inhibits biofilm formation. J. Bacteriol. 186:889–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jubelin G, et al. 2005. CpxR/OmpR interplay regulates curli gene expression in response to osmolarity in Escherichia coli. J. Bacteriol. 187:2038–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jude BA, Martinez RM, Skorupski K, Taylor RK. 2009. Levels of the secreted Vibrio cholerae attachment factor GbpA are modulated by quorum-sensing-induced proteolysis. J. Bacteriol. 191:6911–6917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kierek K, Watnick PI. 2003. Environmental determinants of Vibrio cholerae biofilm development. Appl. Environ. Microbiol. 69:5079–5088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kierek K, Watnick PI. 2003. The Vibrio cholerae O139 O-antigen polysaccharide is essential for Ca2+-dependent biofilm development in sea water. Proc. Natl. Acad. Sci. U. S. A. 100:14357–14362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. King SS, Young DA, Nequin LG, Carnevale EM. 2000. Use of specific sugars to inhibit bacterial adherence to equine endometrium in vitro. Am. J. Vet. Res. 61:446–449 [DOI] [PubMed] [Google Scholar]
- 79. Kirisits MJ, Prost L, Starkey M, Parsek MR. 2005. Characterization of colony morphology variants isolated from Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 71:4809–4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kjelleberg S, Humphrey BA, Marshall KC. 1982. Effect of interfaces on small, starved marine bacteria. Appl. Environ. Microbiol. 43:1166–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kjelleberg S, Humphrey BA, Marshall KC. 1983. Initial phases of starvation and activity of bacteria at surfaces. Appl. Environ. Microbiol. 46:978–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Klausen M, Aaes-Jørgensen A, Molin S, Tolker-Nielsen T. 2003. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol. Microbiol. 50:61–68 [DOI] [PubMed] [Google Scholar]
- 83. Krasteva PV, et al. 2010. Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science 327:866–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kuchma SL, et al. 2007. BifA, a c-di-GMP phosphodiesterase, inversely regulates biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J. Bacteriol. 189:8165–8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kuchma SL, O'Toole GA. 2000. Surface-induced and biofilm-induced changes in gene expression. Curr. Opin. Biotechnol. 11:429–433 [DOI] [PubMed] [Google Scholar]
- 86. Kulasekara H, et al. 2006. Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3′-5′)-cyclic-GMP in virulence. Proc. Natl. Acad. Sci. U. S. A. 103:2839–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Labbate M, et al. 2007. Quorum-sensing regulation of adhesion in Serratia marcescens MG1 is surface dependent. J. Bacteriol. 189:2702–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Landry RM, An D, Hupp JT, Singh PK, Parsek MR. 2006. Mucin-Pseudomonas aeruginosa interactions promote biofilm formation and antibiotic resistance. Mol. Microbiol. 59:142–151 [DOI] [PubMed] [Google Scholar]
- 89. Lappin-Scott HM, Bass C. 2001. Biofilm formation: attachment, growth, and detachment of microbes from surfaces. Am. J. Infect. Control 29:250–251 [DOI] [PubMed] [Google Scholar]
- 90. Lee J, Jayaraman A, Wood TK. 2007. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol. 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee VT, et al. 2007. A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol. Microbiol. 65:1474–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lemon KP, Higgins DE, Kolter R. 2007. Flagellar motility is critical for Listeria monocytogenes biofilm formation. J. Bacteriol. 189:4418–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Levi A, Jenal U. 2006. Holdfast formation in motile swarmer cells optimizes surface attachment during Caulobacter crescentus development. J. Bacteriol. 188:5315–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li G, et al. 2012. Surface contact stimulates the just-in-time deployment of bacterial adhesins. Mol. Microbiol. 83:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lin C-T, et al. 2006. Identification of an HptB-mediated multi-step phosphorelay in Pseudomonas aeruginosa PAO1. Res. Microbiol. 157:169–175 [DOI] [PubMed] [Google Scholar]
- 96. Ma Q, Wood TK. 2009. OmpA influences Escherichia coli biofilm formation by repressing cellulose production through the CpxRA two-component system. Environ. Microbiol. 11:2735–2746 [DOI] [PubMed] [Google Scholar]
- 97. Marshall JC. 1988. Adhesion and growth of bacteria at surfaces in oligotrophic habitats. Can. J. Microbiol. 34:503–506 [Google Scholar]
- 98. Matsukawa M, Greenberg EP. 2004. Putative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm development. J. Bacteriol. 186:4449–4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. McCarter L, Hilmen M, Silverman M. 1988. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell 54:345–351 [DOI] [PubMed] [Google Scholar]
- 100. McCarter L, Silverman M. 1990. Surface-induced swarmer cell differentiation of Vibrio parahaemolyticus. Mol. Microbiol. 4:1057–1062 [DOI] [PubMed] [Google Scholar]
- 101. McDougald D, Lin WH, Rice SA, Kjelleberg S. 2006. The role of quorum sensing and the effect of environmental conditions on biofilm formation by strains of Vibrio vulnificus. Biofouling 22:133–144 [DOI] [PubMed] [Google Scholar]
- 102. McEwan NA, Rème CA, Gatto H, Nuttall TJ. 2008. Monosaccharide inhibition of adherence by Pseudomonas aeruginosa to canine corneocytes. Vet. Dermatol. 19:221–225 [DOI] [PubMed] [Google Scholar]
- 103. Mendrygal KE, Gonzalez JE. 2000. Environmental regulation of exopolysaccharide production in Sinorhizobium meliloti. J. Bacteriol. 182:599–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Merritt JH, Brothers KM, Kuchma SL, O'Toole GA. 2007. SadC reciprocally influences biofilm formation and swarming motility via modulation of exopolysaccharide production and flagellar function. J. Bacteriol. 189:8154–8164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Merritt JH, et al. 2010. Specific control of Pseudomonas aeruginosa surface-associated behaviors by two c-di-GMP diguanylate cyclases. mBio 1(4):e00183–10 doi:10.1128/mBio.00183-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mikkelsen H, Ball G, Giraud C, Filloux A. 2009. Expression of Pseudomonas aeruginosa cupD fimbrial genes is antagonistically controlled by RcsB and the EAL-containing PvrR response regulators. PLoS One 4:e6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mikkelsen H, Sivaneson M, Filloux A. 2011. Key two-component regulatory systems that control biofilm formation in Pseudomonas aeruginosa. Environ. Microbiol. 13:1666–1681 [DOI] [PubMed] [Google Scholar]
- 108. Molina MA, et al. 2005. Role of iron and the TonB system in colonization of corn seeds and roots by Pseudomonas putida KT2440. Environ. Microbiol. 7:443–449 [DOI] [PubMed] [Google Scholar]
- 109. Monds RD, Newell PD, Gross RH, O'Toole GA. 2007. Phosphate-dependent modulation of c-di-GMP levels regulates Pseudomonas fluorescens Pf0-1 biofilm formation by controlling secretion of the adhesin LapA. Mol. Microbiol. 63:656–679 [DOI] [PubMed] [Google Scholar]
- 110. Monds RD, O'Toole GA. 2009. The developmental model of microbial biofilms: ten years of a paradigm up for review. Trends Microbiol. 17:73–87 [DOI] [PubMed] [Google Scholar]
- 111. Monds RD, Silby MW, Mahanty HK. 2001. Expression of the Pho regulon negatively regulates biofilm formation by Pseudomonas aureofaciens PA147-2. Mol. Microbiol. 42:415–426 [DOI] [PubMed] [Google Scholar]
- 112. Morgan R, Kohn S, Hwang S-H, Hassett DJ, Sauer K. 2006. BdlA, a chemotaxis regulator essential for biofilm dispersion in Pseudomonas aeruginosa. J. Bacteriol. 188:7335–7343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Moscoso JA, Mikkelsen H, Heeb S, Williams P, Filloux A. 2011. The Pseudomonas aeruginosa sensor RetS switches type III and type VI secretion via c-di-GMP signalling. Environ. Microbiol. 13:3128–3138 [DOI] [PubMed] [Google Scholar]
- 114. Mueller RS, Beyhan S, Saini SG, Yildiz FH, Bartlett DH. 2009. Indole acts as an extracellular cue regulating gene expression in Vibrio cholerae. J. Bacteriol. 191:3504–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mulcahy H, Lewenza S. 2011. Magnesium limitation is an environmental trigger of the Pseudomonas aeruginosa biofilm lifestyle. PLoS One 6:e23307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Navarro MVAS, et al. 2011. Structural basis for c-di-GMP-mediated inside-out signaling controlling periplasmic proteolysis. PLoS Biol. 9:e1000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Nemoto K, et al. 2003. Effect of Varidase (streptodornase) on biofilm formed by Pseudomonas aeruginosa. Chemotherapy 49:121–125 [DOI] [PubMed] [Google Scholar]
- 118. Newell PD, Monds RD, O'Toole GA. 2009. LapD is a bis-(3′,5′)-cyclic dimeric GMP-binding protein that regulates surface attachment by Pseudomonas fluorescens Pf0-1. Proc. Natl. Acad. Sci. U. S. A. 106:3461–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Newell PD, Yoshioka S, Hvorecny KL, Monds RD, O'Toole GA. 2011. Systematic analysis of diguanylate cyclases that promote biofilm formation by Pseudomonas fluorescens Pf0-1. J. Bacteriol. 193:4685–4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ng W-L, Bassler BL. 2009. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 43:197–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Nivens DE, Ohman DE, Williams J, Franklin MJ. 2001. Role of alginate and its O acetylation in formation of Pseudomonas aeruginosa microcolonies and biofilms. J. Bacteriol. 183:1047–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. O'Toole G, Kaplan HB, Kolter R. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49–79 [DOI] [PubMed] [Google Scholar]
- 123. O'Toole GA, Gibbs KA, Hager PW, Phibbs PV, Jr, Kolter R. 2000. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 182:425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. O'Toole GA, Kolter R. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295–304 [DOI] [PubMed] [Google Scholar]
- 125. O'Toole GA, Kolter R. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449–461 [DOI] [PubMed] [Google Scholar]
- 126. Otto K, Silhavy TJ. 2002. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 99:2287–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Palmer J, Flint S, Brooks J. 2007. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol. 34:577–588 [DOI] [PubMed] [Google Scholar]
- 128. Pannuri A, et al. 2012. Translational repression of NhaR, a novel pathway for multi-tier regulation of biofilm circuitry by CsrA. J. Bacteriol. 194:79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Petrova OE, Sauer K. 2011. SagS contributes to the motile-sessile switch and acts in concert with BfiSR to enable Pseudomonas aeruginosa biofilm formation. J. Bacteriol. 193:6614–6628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Pratt LA, Kolter R. 1999. Genetic analyses of bacterial biofilm formation. Curr. Opin. Microbiol. 2:598–603 [DOI] [PubMed] [Google Scholar]
- 131. Prüß B, et al. 2010. Environmental and genetic factors that contribute to Escherichia coli K-12 biofilm formation. Arch. Microbiol. 192:715–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Purcell EB, Siegal-Gaskins D, Rawling DC, Fiebig A, Crosson S. 2007. A photosensory two-component system regulates bacterial cell attachment. Proc. Natl. Acad. Sci. U. S. A. 104:18241–18246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Qin Z, et al. 2007. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology 153:2083–2092 [DOI] [PubMed] [Google Scholar]
- 134. Rochex A, Lebeault JM. 2007. Effects of nutrients on biofilm formation and detachment of a Pseudomonas putida strain isolated from a paper machine. Water Res. 41:2885–2892 [DOI] [PubMed] [Google Scholar]
- 135. Romby P, Vandenesch F, Wagner EGH. 2006. The role of RNAs in the regulation of virulence-gene expression. Curr. Opin. Microbiol. 9:229–236 [DOI] [PubMed] [Google Scholar]
- 136. Römling U, Amikam D. 2006. Cyclic di-GMP as a second messenger. Curr. Opin. Microbiol. 9:218–228 [DOI] [PubMed] [Google Scholar]
- 137. Ross P, et al. 1990. The cyclic diguanylic acid regulatory system of cellulose synthesis in Acetobacter xylinum. Chemical synthesis and biological activity of cyclic nucleotide dimer, trimer, and phosphothioate derivatives. J. Biol. Chem. 265:18933–18943 [PubMed] [Google Scholar]
- 138. Ross P, et al. 1987. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325:279–281 [DOI] [PubMed] [Google Scholar]
- 139. Ryder C, Byrd M, Wozniak DJ. 2007. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr. Opin. Microbiol. 10:644–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Scharfman A, et al. 1996. Adhesion of Pseudomonas aeruginosa to respiratory mucins and expression of mucin-binding proteins are increased by limiting iron during growth. Infect. Immun. 64:5417–5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Simm R, Morr M, Kader A, Nimtz M, Romling U. 2004. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 53:1123–1134 [DOI] [PubMed] [Google Scholar]
- 143. Simpson DA, Ramphal R, Lory S. 1992. Genetic analysis of Pseudomonas aeruginosa adherence: distinct genetic loci control attachment to epithelial cells and mucins. Infect. Immun. 60:3771–3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Stapper AP, et al. 2004. Alginate production affects Pseudomonas aeruginosa biofilm development and architecture, but is not essential for biofilm formation. J. Med. Microbiol. 53:679–690 [DOI] [PubMed] [Google Scholar]
- 145. Starkey M, et al. 2009. Pseudomonas aeruginosa rugose small-colony variants have adaptations that likely promote persistence in the cystic fibrosis lung. J. Bacteriol. 191:3492–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Steinberger RE, Holden PA. 2005. Extracellular DNA in single- and multiple-species unsaturated biofilms. Appl. Environ. Microbiol. 71:5404–5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Stoodley P, Sauer K, Davies DG, Costerton JW. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187–209 [DOI] [PubMed] [Google Scholar]
- 148. Sun Y-C, et al. 2011. Differential control of Yersinia pestis biofilm formation in vitro and in the flea vector by two c-di-GMP diguanylate cyclases. PLoS One 6:e19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Suzuki K, Babitzke P, Kushner SR, Romeo T. 2006. Identification of a novel regulatory protein (CsrD) that targets the global regulatory RNAs CsrB and CsrC for degradation by RNase E. Genes Dev. 20:2605–2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Thomas VC, et al. 2009. A fratricidal mechanism is responsible for eDNA release and contributes to biofilm development of Enterococcus faecalis. Mol. Microbiol. 72:1022–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Thompson LS, Webb JS, Rice SA, Kjelleberg S. 2003. The alternative sigma factor RpoN regulates the quorum sensing gene rhlI in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 220:187–195 [DOI] [PubMed] [Google Scholar]
- 152. Thormann KM, et al. 2006. Control of formation and cellular detachment from Shewanella oneidensis MR-1 biofilms by cyclic di-GMP. J. Bacteriol. 188:2681–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Tomaras AP, Dorsey CW, Edelmann RE, Actis LA. 2003. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology 149:3473–3484 [DOI] [PubMed] [Google Scholar]
- 154. Tomlin KL, et al. 2005. Quorum-sensing mutations affect attachment and stability of Burkholderia cenocepacia biofilms. Appl. Environ. Microbiol. 71:5208–5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Toutain CM, Caizza NC, Zegans ME, O'Toole GA. 2007. Roles for flagellar stators in biofilm formation by Pseudomonas aeruginosa. Res. Microbiol. 158:471–477 [DOI] [PubMed] [Google Scholar]
- 156. Toutain CM, Zegans ME, O'Toole GA. 2005. Evidence for two flagellar stators and their role in the motility of Pseudomonas aeruginosa. J. Bacteriol. 187:771–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Ueda A, Wood TK. 2009. Connecting quorum sensing, c-di-GMP, Pel polysaccharide, and biofilm formation in Pseudomonas aeruginosa through tyrosine phosphatase TpbA (PA3885). PLoS Pathog. 5:e1000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Vallet I, et al. 2004. Biofilm formation in Pseudomonas aeruginosa: fimbrial cup gene clusters are controlled by the transcriptional regulator MvaT. J. Bacteriol. 186:2880–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Vallet I, Olson JW, Lory S, Lazdunski A, Filloux A. 2001. The chaperone/usher pathways of Pseudomonas aeruginosa: identification of fimbrial gene clusters (cup) and their involvement in biofilm formation. Proc. Natl. Acad. Sci. U. S. A. 98:6911–6916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Van Dellen KL, Houot L, Watnick PI. 2008. Genetic analysis of Vibrio cholerae monolayer formation reveals a key role for ΔΨ in the transition to permanent attachment. J. Bacteriol. 190:8185–8196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ventre I, et al. 2006. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc. Natl. Acad. Sci. U. S. A. 103:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Vincent F, et al. 2010. Distinct oligomeric forms of the Pseudomonas aeruginosa RetS sensor domain modulate accessibility to the ligand binding site. Environ. Microbiol. 12:1775–1786 [DOI] [PubMed] [Google Scholar]
- 163. Watnick PI, Lauriano CM, Klose KE, Croal L, Kolter R. 2001. The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Mol. Microbiol. 39:223–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wei BL, et al. 2001. Positive regulation of motility and flhDC expression by the RNA-binding protein CsrA of Escherichia coli. Mol. Microbiol. 40:245–256 [DOI] [PubMed] [Google Scholar]
- 165. Whitchurch CB, et al. 2002. Phosphorylation of the Pseudomonas aeruginosa response regulator AlgR is essential for type IV fimbria-mediated twitching motility. J. Bacteriol. 184:4544–4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. 2002. Extracellular DNA required for bacterial biofilm formation. Science 295:1487. [DOI] [PubMed] [Google Scholar]
- 167. Wozniak DJ, et al. 2003. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. U. S. A. 100:7907–7912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Zhan HJ, Lee CC, Leigh JA. 1991. Induction of the second exopolysaccharide (EPSb) in Rhizobium meliloti SU47 by low phosphate concentrations. J. Bacteriol. 173:7391–7394 [DOI] [PMC free article] [PubMed] [Google Scholar]