Abstract
One of the unique features of Helicobacter pylori is its ability to assimilate free-cholesterol (FC) into its membranes. Via FC assimilation, H. pylori strengthens the membrane lipid barrier and/or evades the host immune system. No previous studies, however, have investigated the FC uptake mechanisms of the H. pylori cell. Phosphatidylethanolamine (PE) is the most prevalent lipid component of bacteria, including H. pylori, but the function of PE remains unclear. We were therefore interested in H. pylori PE (HpPE) and investigated the interaction of its PE with cholesterols. The PE isolated from H. pylori underwent a unique molecular interaction with FC, cholesterol ester (CE), and 2,6-di-O-methyl-β-cyclodextrin (dMβCD), a sterol solubilizer. HpPE interacted not only with the FC molecule, but also with the FC-dMβCD inclusion complex. In contrast, Escherichia coli PE (EcPE), prepared as a reference PE, seemed to bind only FC, and only via a hydrophobic interaction, without binding dMβCD. HpPE was clearly more potent than EcPE in binding FC. Intriguingly, HpPE had a negligible affinity for CE, while EcPE had a high affinity for CE, comparable to its affinity for FC. Further, HpPE interacted with 3β-OH steroids, pregnenolone and dehydroepiandrosterone, in the absence of dMβCD. Gas chromatogram-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS) analyses revealed that the fatty acid compositions of HpPE were quite distinct from those of EcPE, and the C14:0 fatty acid in the HpPE molecule was found to be significant in binding FC selectively. These results indicate that PE is a key candidate of nonesterified steroid-binding lipids in H. pylori.
INTRODUCTION
Helicobacter pylori is a Gram-negative curved rod equipped with polar flagella as motility organs. Pathogenically, H. pylori colonizes the human stomach and induces both gastritis and peptic ulcers (8, 16, 29). Over a longer period of colonization, the pathogen also contributes to the development of gastric cancer and marginal-zone B-cell lymphoma (7, 19, 20, 25, 27).
One of the unique features of H. pylori is its ability to assimilate free-cholesterol (FC) into its membranes. H. pylori cells aggressively ingest FC from FC-supplemented medium or extract FC from the lipid raft of the epithelial cell membrane. The FC absorbed into H. pylori membranes is glucosylated via enzymatic action (14), and the organism cells retain both FC itself and glucosylated FC as membrane lipid components (11, 23). A previous study by our group identified three types of glucosylated FC (9): cholesteryl-α-d-glucopyranoside (CGL), cholesteryl-6-O-tetradecanoyl-α-d-glucopyranoside (CAG), and cholesteryl-6-O-phosphatidyl-α-d-glucopyranoside (CPG). The enzyme involved in CGL synthesis in H. pylori has been identified as a cholesterol α-glucosyltransferase encoded by the HP0421 gene (14). The HP0421 protein catalyzes the glucosylation of not only FC, but also various steroids with a 3β-hydroxyl (OH) group (10). The enzymes involved in the acylation and phosphatidylation of the CGL molecule have yet to be identified.
Though the biological significance of sterol (or steroid) glucosylation in H. pylori has been unclear for many years, Wunder and coworkers have revealed that H. pylori evades host immune systems by glucosylating the FC absorbed into its membranes (28). The glucosylation of FC also has been shown to play an important role in the colonization of H. pylori in the stomachs of mice and gerbils (17, 28). Our group, meanwhile, has demonstrated that H. pylori retains steroids to reinforce the membrane lipid barrier, with or without glucosylation, and thereby acquires resistance against the bacteriolytic action of phosphatidylcholines (PCs) (23). A recent study by another group has shown that H. pylori expresses further resistance against bile salts (including ceragenins) by absorbing FC, even in the HP0421 (cgt) gene knockout mutant (26). These studies, in combination, have provided a good understanding of the roles of steroid absorption and glucosylation in the survival of H. pylori. None of the previous studies, however, have investigated the nonesterified steroid uptake mechanisms of the H. pylori cell.
The H. pylori cell generally divides and proliferates in medium containing 5 or 10% serum under microaerobic conditions. For H. pylori in serum-free medium, the medium is supplemented with 2,6-di-O-methyl-β-cyclodextrin (dMβCD) as a growth-supporting factor (4, 15). While the mechanism by which dMβCD supports H. pylori growth remains unclear, an earlier study by our group demonstrated that dMβCD protects H. pylori from the antimicrobial actions of unsaturated fatty acids and lysophosphatidylcholine (LPC) by inhibiting the binding of those lipophilic compounds to the cells of the organism (23). In sum, one of the functions of dMβCD is to somehow scavenge toxic compounds that affect the survival of H. pylori.
dMβCD is a cyclic oligomer consisting of seven methylated d-glucose molecules linked by α(1→4) glucosidic bonds. dMβCD forms an inclusion complex with various hydrophobic compounds and solubilizes the compounds into water solvent. Hydrophobic compounds, such as flavonoid glycosides (31) and sodium salicylate (12), are thought to bind to the inner ring structure of the dMβCD molecule at a molar ratio of 1:1 via molecular interactions, such as hydrophobic bonding, hydrogen bonding, and van der Waals attraction. Though FC has been identified as one of the most suitable “guest molecules” of dMβCD in forming the inclusion complex (3, 6, 18, 30, 32), the molecular interaction between FC and dMβCD has yet to be understood in detail.
Empirically, our group has demonstrated an enhancement of FC absorption by H. pylori when the organism's cells are cultured with FC in serum-free medium supplemented with dMβCD. Conversely, dMβCD inhibits the absorption of steroid hormones, such as estrone, dehydroepiandrosterone, and epiandrosterone, in H. pylori, even though steroid hormones are typical FC analogues (10). dMβCD also counteracts the bactericidal activity of progesterone and its derivatives against H. pylori (11; see Fig. S1 in the supplemental material). Thus, an important question to investigate is why dMβCD promotes the incorporation of FC only into the cell membranes of H. pylori. However, apart from this question, an analysis of the molecular interactions between the FC-dMβCD inclusion complex and H. pylori cell components may help us identify a nonesterified steroid-binding factor of the H. pylori cell.
Our study in 2004 demonstrated that the glucosyl cholesterol contents hardly change in H. pylori cells undergoing growth phase changes from the logarithmic phase to the decline phase in a serum-supplemented medium; the level of CGL (a basic structure of glucosyl cholesterols) decreased in close correlation with the increases in CAG and CPG levels (22). This indicates that the active absorption of FC is observed only in H. pylori cells in the logarithmic phase. In other words, the decrease in the CGL level reflects the reduction of FC absorption by the H. pylori cell. Interestingly, the phosphatidylethanolamine (PE) content in the membrane lipid composition was found to decrease remarkably from about 66% to about 29% in H. pylori cells undergoing growth phase changes. Further, the decline curve of the PE level almost completely corresponded to that of the CGL level. We therefore assumed that the PE of H. pylori regulates FC absorption by H. pylori cells. Incidentally, the phosphatidylglycerol-cardiolipin (PG-CL) content in the membrane lipid composition was not altered in H. pylori during growth phase changes. To this end, we investigated the binding of cholesterols and 3β-OH steroids to the PE of H. pylori.
MATERIALS AND METHODS
Bacterial strains and cultures.
This study examined an H. pylori strain (NCTC 11638) and an Escherichia coli strain (ATCC 11775). Both H. pylori and E. coli were cultured in a pleuropneumonia-like organism (PPLO) broth (Difco Laboratories, Detroit, MI) without either serum or dMβCD. The culture of H. pylori was carried out with continuous shaking under microaerobic conditions at 37°C in an atmosphere of 10% CO2, 5% O2, and 85% N2. The culture of E. coli was carried out with continuous shaking under aerobic conditions at 37°C.
Cholesterols, 3β-OH steroids, dMβCD, and PEs.
FC (Wako Pure Chemical Industries Ltd., Tokyo, Japan), cholesterol hexanoate (cholesterol ester [CE]) (Tokyo Kasei Kogyo Co., Ltd., Tokyo, Japan), pregnenolone (PN) (Wako Pure Chemical Industries Ltd.), or dehydroepiandrosterone (dEA) (Wako Pure Chemical Industries Ltd.) was dissolved in chloroform, adjusted to a 5 mM concentration, and stored at −30°C as a stock solution until it was used in experiments. dMβCD (Sigma-Aldrich Inc., St. Louis, MO) was dissolved in 50 mM Tris (pH 7.5) buffer, adjusted to a 50 mM concentration, and stored at 4°C as a stock solution until it was used in experiments. Dimyristoyl PE (dMPE) and dipalmitoyl PE (dPPE), from Sigma-Aldrich Inc., were dissolved in chloroform-methanol (2:1) solvent, adjusted to a 5 mM concentration, and stored at room temperature in the dark as a stock solution until they were used in experiments.
Quantification of FC absorbed into H. pylori cells.
FC beads were prepared by a method described elsewhere (11). H. pylori (approximately 106 CFU/ml) was cultured for 24 h with FC beads (FC concentration, 250 μM) in PPLO broth (30 ml) in the presence or absence of 0.2% dMβCD with shaking under microaerobic conditions. After the FC beads were removed via centrifugation (10 × g; 1 min), the H. pylori cells (approximately 108 CFU) were recovered via centrifugation (8,600 × g; 10 min). Membrane lipids were then purified from the H. pylori cells by the organic solvent distribution method using a chloroform-methanol-water (10:5:3) solvent system (11), and the dry weight of the purified membrane lipids was measured. The FC contained in the membrane lipid compositions was quantified by the ferrous chloride-sulfuric acid method. In brief, a ferrous chloride-sulfuric acid reagent (phosphoric acid-sulfuric acid [2:25] solution containing 0.2% FeCl2-6H2O) (400 μl) was added to the lipid-acetic acid solution (600 μl), vigorously stirred, and incubated for 15 min at room temperature. After color reaction and cooling, the absorbance of the lipid solution (200 μl) mixed with the ferrous chloride-sulfuric acid reagent was measured using a Versa max microplate reader (Molecular Devices Co., CA) at a wavelength of 550 nm. The amounts of FC were quantified based on an FC standard curve, and the FC content was calculated as a ratio to the dry weight of total lipid. Meanwhile, the purified membrane lipids were analyzed by thin-layer chromatography (TLC). In brief, membrane lipids (200 μg) dissolved in chloroform-methanol (2:1) solvent (40 μl) were dotted onto a silica gel G60 plate (Merck, Darmstadt, Germany), and each lipid was developed with a chloroform-methanol-water (70:30:5) solvent system. After the TLC, the silica gel G60 plate was sprayed with a 60% sulfuric acid solution and heated at 180°C to visualize the spots of lipid on the plate surface.
Analysis of absorption of FC and 3β-OH steroids into bacterial cells.
The FC-chloroform solution (20 μl) or chloroform (20 μl) was dotted onto paper disks (8-mm diameter; 1-mm thickness) from Tokyo Roshi Kaisha Ltd. (Tokyo, Japan) and dried at room temperature to vaporize the chloroform solvent. Bacterial cells (109 CFU) were suspended in PPLO broth (5 ml) without dMβCD or in the same broth (5 ml) with a 5 mM concentration of dMβCD (see Fig. S2 in the supplemental material) and added to a single well of a 6-well cell culture plate (Corning Inc., NY). Next, the FC (100 nmol)-fixed paper disks and FC-free paper disks (chloroform-dotted paper disks) were soaked in the bacterial cell suspensions and incubated for 4 h with continuous shaking (95 rpm) at 25°C. H. pylori cells and E. coli cells were incubated under microaerobic conditions and aerobic conditions, respectively. After 4 h of incubation, the bacterial cells were recovered via centrifugation (10,000 × g; 10 min), washed three times with phosphate-buffered saline (PBS) via centrifugation (10,000 × g; 10 min), and subjected to a lipid purification procedure described elsewhere (11). The FC contained in the purified bacterial lipids was quantified by a ferrous chloride-sulfuric acid method. The FC (nmol) in the bacterial lipids was quantified based on a regression line (y axis, A550; x axis, FC amount) calculated using FC standard solutions in each experiment. For greater accuracy in the quantification of the FC absorbed into the bacterial cells (109 CFU), the A550 in bacterial lipids purified from the cells incubated with the FC-free paper disk was subtracted from the A550 in bacterial lipids purified from the cells incubated with the FC-fixed paper disk. In addition, the absorption of PN or dEA into H. pylori cells was assayed using a PN (100 nmol)-fixed paper disk or dEA (100 nmol)-fixed paper disk in place of an FC-fixed paper disk. The only difference in the experimental procedures was that H. pylori cells were incubated at 37°C, not at 25°C. The amounts of PN and dEA assimilated into H. pylori cells were quantified with the respective regression lines using the PN and dEA standard solutions.
Assay of binding of dMβCD to bacterial cells.
The experiments described in “Analysis of absorption of FC and 3β-OH steroids into bacterial cells” above were performed without an FC-fixed paper disk to quantify the amounts of dMβCD bound to bacterial cells. We know that dMβCD is composed of seven methylated d-glucose molecules and is recovered at the chloroform phase in an organic solvent distribution procedure for lipid purification using a chloroform-methanol-water (10:5:3) solvent system. Meanwhile, the membrane lipid composition of H. pylori cultured in a medium without 3β-OH steroids contains no glucosyl steroids; like E. coli, the organism is incapable of synthesizing either cholesteryl glucosides or glucosyl steroids. Incidentally, a lipopolysaccharide (LPS), the most prevalent glycolipid of Gram-negative bacteria (21), remains in the cellular debris after the sonication of bacterial cells with chloroform-methanol (2:1) solution and is not distributed into the chloroform phase. To measure the amount of dMβCD contained in the purified bacterial lipids, we therefore adopted a phenol-sulfuric acid method for the quantification of sugars (see Fig. S3 in the supplemental material). In brief, a 5% phenol solution (100 μl) was added to purified bacterial lipids suspended in 50 mM Tris (pH 7.5) buffer (100 μl) and vigorously stirred. Next, a sulfuric acid solution (500 μl) was added to the phenol-lipid mixed solution, vigorously stirred, and incubated for 20 min at room temperature. After color reaction and cooling, the absorbance of the lipid solution (200 μl) mixed with phenol and sulfuric acid was measured using a Versa max microplate reader at a wavelength of 490 nm. The dMβCD (nmol) in the bacterial lipids was quantified based on a regression line (y axis, A490; x axis, dMβCD amount) calculated using dMβCD standard solutions in each experiment. For greater accuracy in the quantification of the dMβCD bound to the bacterial cells (109 CFU), the A490 in bacterial lipids purified from the cells incubated without dMβCD was subtracted from the A490 in bacterial lipids purified from the cells incubated with dMβCD.
Treatment of heat-killed bacterial cells with dMβCD.
Bacterial cells suspended in PBS were autoclaved for 15 min at 121°C. The heat-killed cells were then stained with Coomassie brilliant blue (CBB) to observe the state of the cell bodies. Next, the heat-killed cell (approximately 109 CFU) suspension was incubated overnight in the presence or absence of dMβCD (5 mM) in PBS (2 ml) at 25°C with continuous shaking (100 rpm), washed three times with PBS, and subjected to the purification of membrane lipids (10). The membrane lipids were then analyzed by TLC with a chloroform-methanol-water (70:30:5) solvent system and detected with a 60% sulfuric acid solution.
Purification of bacterial lipids.
Bacterial cell pellets obtained from the cultures in PPLO broth (2 liters) were suspended in PBS (32 ml) and sufficiently sonicated. After the sonication, chloroform-methanol (2:1) solvent (160 ml) was added to the cell lysate solution, and the mixture was vigorously shaken and incubated overnight at 4°C. After the incubation, the recovered chloroform phase was dried at 60°C with a rotary evaporator (Buchi Rotavapor R 114; Shibata Scientific Technology Ltd., Saitama, Japan), and the dry weight of the bacterial whole lipids from the chloroform phase was measured. The lipids were dissolved in chloroform, adjusted to a 10-mg/ml concentration, and stored at −30°C until they were used in experiments.
Purification of PE and PG-CL from bacterial lipid constituents.
Total bacterial lipids (10 mg) dissolved in chloroform (1 ml) were applied to a column (1-cm diameter; 5-cm height) filled with chloroform-activated Iatrobeads 6RS-8060 (Mitsubishi Kagaku Iatron Inc., Tokyo, Japan), and the column was washed with chloroform (15 ml) and acetone (15 ml). Each lipid was eluted from the column sequentially with acetone-methanol (7:3) solution (10 ml), acetone-methanol (4:6) solution (10 ml), and acetone-methanol (2:8) solution (10 ml). PE and PG-CL eluted with the acetone-methanol (2:8) solution and acetone-methanol (7:3) solution, respectively, was dried with a rotary evaporator and weighed. PE and PG-CL were dissolved in chloroform, adjusted to a concentration of 10 mg/ml, and stored at −30°C until they were used in experiments.
Assay of binding of cholesterols, 3β-OH steroids, and dMβCD to PE and PG-CL.
FC (100 nmol) and PE (100, 200, or 300 μg) dissolved in chloroform (20 μl) were dotted on a pair of paper disks; then, the disks were dried at room temperature to vaporize the solvent. A chloroform-dotted paper disk (FC-free paper disk) was prepared as a negative control. Both the FC-fixed paper disk and the PE-fixed paper disk were soaked in 50 mM Tris (pH 7.5) buffer (2 ml) without dMβCD or in the same buffer (2 ml) with 5 mM dMβCD and incubated for 4 h at 25°C using a single well of a 12-well cell culture plate (Sumitomo Bakelite Co., Ltd., Tokyo, Japan) on a shaker (100 rpm). After the incubation, the PE-fixed paper disk was transferred into a single well of a new 12-well cell culture plate, washed six times with distilled water by shaking (100 rpm; 10 min), and dried using a centrifugal concentrator (Tomy Seiko Co., Ltd., Tokyo, Japan). The PE-fixed paper disk was then soaked in chloroform (600 μl) and vigorously shaken to elute the lipid components. After the chloroform solvent was vaporized, the FC (nmol) contained in the PE-fixed paper disk was quantified based on a regression line (y axis, A550; x axis, FC amount) by the ferrous chloride-sulfuric acid method described in “Quantification of FC absorbed into H. pylori cells” above. To improve the accuracy of the FC quantification, the A550 in the PE-fixed paper disk incubated with the FC-free paper disk was subtracted from the A550 in the PE-fixed paper disk incubated with the FC-fixed paper disk. The dMβCD in the PE-fixed paper disk was also quantified based on a regression line (y axis, A490; x axis, dMβCD amount) by the phenol-sulfuric acid method described in “Assay of binding of dMβCD to bacterial cells” above. To more accurately calculate the amount of dMβCD bound to the PE, the A490 in the PE-fixed paper disk incubated without dMβCD was subtracted from the A490 in the PE-fixed paper disk incubated with dMβCD. As with the PE-fixed paper disk, a paper disk dotted with PG-CL (300 μg) was also assayed to examine the binding of FC to its glycerophospholipids with the same experimental procedure described above. The binding of CE (cholesterol hexanoate), PN, or dEA to PE was assayed using a CE (50 nmol)-fixed paper disk, a PN (100 nmol)-fixed paper disk, or a dEA (100 nmol)-fixed paper disk in place of an FC-fixed paper disk. The assay method was performed using the same experimental procedures described above. The amounts of CE, PN, and dEA bound to PE were quantified with the respective regression lines using the CE, PN, and dEA standard solutions. In addition, the binding of FC and CE to dMPE and dPPE was assayed using a dMPE-fixed paper disk and a dPPE-fixed paper disk.
TLC analysis of PE and PG-CL contained in paper disks after incubation with dMβCD.
A PE (100 μg)-fixed paper disk or a PG-CL (100 μg)-fixed paper disk was soaked in 50 mM Tris (pH 7.5) buffer (2 ml) containing dMβCD (5 mM) and incubated for 4 h at 25°C with continuous shaking (100 rpm). After the incubation, the paper disks were washed 6 times with distilled water with shaking and treated with chloroform (600 μl) to elute the remaining PE and PG-CL in the paper disks, and then the chloroform solution was recovered. After the chloroform solvent was vaporized, the PE and PG-CL specimens were dissolved in chloroform-methanol (2:1) solvent (40 μl) and then analyzed by TLC as described in “Quantification of FC absorbed into H. pylori cells” above.
TLC analysis of the FC and CE bound to PE.
After the binding of FC and CE to PE was assayed using a paper disk dotted with the sterols of FC (50 nmol) and CE (50 nmol) via the same method described in “Assay of binding of cholesterols, 3β-OH steroids, and dMβCD to PE and PG-CL” above, the FC and CE contained in the PE (300 μg)-fixed paper disk were analyzed by TLC with a chloroform-acetone-acetic acid (9:1:1) solvent system. After the TLC, the silica gel G60 plate was sprayed with a sulfuric acid-acetic acid (1:1) solution and heated at 90°C in order to visualize the spots of FC and CE on the plate surface.
Analysis of the fatty acid composition of PE molecules.
After the purified PE (20 μg) was treated for 30 min with methanol solution (1 ml) containing 3% acetyl chloride at 70°C, the methanolyzed PE specimen was dried, and hexane (1 ml) was then added to prepare a fatty acid methyl ester solution. Next, the fatty acid-hexane solution (1 μl) was applied to a GCMS-QP2010 device (Shimadzu Techno-Research Inc., Kyoto, Japan) to analyze the fatty acid compositions of the PE by gas chromatogram-mass spectrometry (GC-MS). The fatty acids attached to the PE were identified by comparing the mass spectra of the fatty acid methyl ester molecules registered in the computer database library. The ratios of the fatty acid components of the PE molecules were calculated based on the magnitude of the peak area of each fatty acid spectrum.
Analysis of PE molecular species.
After the purified PE (100 μg/ml) was dissolved in the solvent of chloroform-methanol (1:1), the PE solution was diluted to a 1-μg/ml concentration with methanol. The diluted PE solution (5 μl) was then applied to an Asahipak ODP-40 2D column (2.0 by 150 mm) of a LC-20A system at 40°C (Shimadzu Techno-Research Inc.) for analysis by high-performance liquid chromatography (HPLC). After the HPLC, the mass spectra of the PE molecular species were detected by a negative mode of electrospray ionization mass spectrometry using an API3000 mass spectrometry device (AB Sciex Co., CA). The total carbon numbers and lack of hydrogen atoms from the two fatty acid molecules constituting the acyl groups in the PE molecules were calculated based on the molecular weights of various fatty acids (including unsaturated fatty acids and cyclopropane fatty acids). The predominant PE molecular species were determined by calculating the magnitude of the peak area of each PE mass spectrum.
Analysis of the phosphoethanolamine moiety in the PE molecule.
The purified PE (29 mg) was dissolved in a deuterated-chloroform–deuterated-methanol (3.6:1) solvent (800 μl) containing a small amount of tetramethylsilane. As a reference PE, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (4 mg; Sigma-Aldrich Inc.) was dissolved in the same solvent (800 μl) described above. The H. pylori PE and reference PE were then subjected to a proton nuclear magnetic resonance (1H-NMR) analysis using an Avance 400 spectrometer (Bruker BioSpin KK, Kanagawa, Japan). The following conditions were adopted for the 1H-NMR analysis: resonant frequency, 400.1 MHz; flip angle, 45°; data acquisition time, 4 s; pulse repetition time, 10 s; and temperature, 23°C (Mitsubishi Chemical Analytech Co., Ltd., Tokyo, Japan). The tetramethylsilane was used as the baseline (0.0 ppm) of the chemical shifts. The 1H-NMR spectrum patterns of H. pylori PE and the reference PE were compared.
RESULTS
Enhancement of FC absorption of H. pylori by dMβCD.
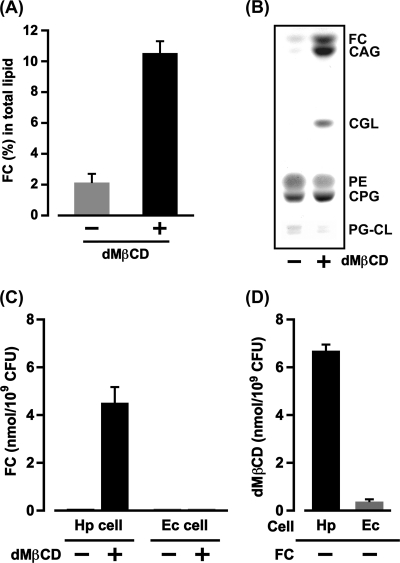
Our first experiment was to examine the effect of dMβCD on the FC absorption of H. pylori cells. When H. pylori cells were cultured with FC beads in the presence or absence of dMβCD, the FC absorption of H. pylori was enhanced remarkably by dMβCD, and the FC content of the H. pylori cells cultured in the presence of dMβCD was 5-fold greater than the FC content of the H. pylori cells cultured in the absence of dMβCD (Fig. 1A). The TLC analysis also revealed obvious differences in the FC content between H. pylori cultured in the presence and absence of dMβCD: the spots of FC and glucosylated FCs (CGL, CAG, and CPG) detected in the membrane lipid constituents of H. pylori cultured in the presence of dMβCD had tremendously high densities (Fig. 1B). In the membrane lipid constituents of H. pylori cultured in the absence of dMβCD, only one spot, namely, CPG, was detected at high density, and the spots of FC, CGL, and CAG were detected at negligible levels. These results demonstrate that dMβCD promotes the FC absorption of H. pylori via certain mechanisms.
Fig 1.
dMβCD as an FC carrier molecule to the H. pylori cell. (A) After H. pylori (106 CFU) was cultured for 24 h with FC beads (FC concentration, 250 μM) in PPLO broth (30 ml) in the presence or absence of 0.2% dMβCD, the H. pylori cells (108 CFU) were recovered to purify membrane lipids, and then the amounts of FC absorbed into the membranes were quantified by the ferrous chloride-sulfuric acid method. The results are indicated as the mean percent FC and standard deviation (SD) (a ratio to the dry weight of total lipid) obtained from three independent experiments. (B) After the same experimental procedures described for panel A were performed, the membrane lipids (200 μg/lane) were analyzed by TLC and detected with a 60% sulfuric acid solution. (C) H. pylori (Hp) or E. coli (Ec) cells (109 CFU) were incubated for 4 h with an FC (100 nmol)-fixed paper disk in the presence or absence of dMβCD (5 mM) in PPLO broth (5 ml). After the cells were recovered and washed to extract the membrane lipids, the FC in the membrane lipids was quantified by the ferrous chloride-sulfuric acid method. The results are indicated as the mean FC and SD obtained from three independent experiments. (D) H. pylori or E. coli cells (109 CFU) were incubated for 4 h in the presence of dMβCD (5 mM) in PPLO broth (5 ml). After the cells were recovered and washed to extract the membrane lipids, the dMβCD in the membrane lipids was quantified by the phenol-sulfuric acid method. The results are indicated as the mean dMβCD and SD obtained from three independent experiments.
FC absorption of H. pylori cells mediated by dMβCD.
Next, we investigated the mechanisms of dMβCD that enhance FC absorption by H. pylori. H. pylori cells (109 CFU) were incubated with an FC (100 nmol)-fixed paper disk in PPLO broth (5 ml) either without or with dMβCD (5 mM). No FC was detected in the purified membrane lipids from the cells incubated in the absence of dMβCD (Fig. 1C). Meanwhile, significant amounts of FC were detected in the purified membrane lipids from the H. pylori cells incubated with dMβCD. In contrast, no FC whatsoever was detected in the purified membrane lipids from E. coli cells (109 CFU) incubated with or without dMβCD. These results indicate that the H. pylori cells used for this experiment were incapable of absorbing the FC in the paper disk without the mediation of dMβCD.
Next, we assayed the interaction of dMβCD with H. pylori cells by comparing it with the interaction of dMβCD with E. coli cells. H. pylori cells (109 CFU) or E. coli cells (109 CFU) were incubated with dMβCD (5 mM) in PPLO broth (5 ml), and the amount of dMβCD bound to the bacterial cells was measured (Fig. 1D). The H. pylori cells efficiently bound dMβCD, and the amount of dMβCD detected in the purified membrane lipids from H. pylori cells was noticeably higher than that detected in the purified membrane lipids from E. coli cells. In sum, dMβCD exhibited a much higher affinity for H. pylori cells than for E. coli cells. Judging from these results, together with the results of the FC absorption of H. pylori cells (Fig. 1C), we conclude that dMβCD carries FC molecules to H. pylori cells and that H. pylori cells can bind the FC-dMβCD inclusion complex via certain cell components.
Elution of PE from dead H. pylori cells induced by dMβCD.
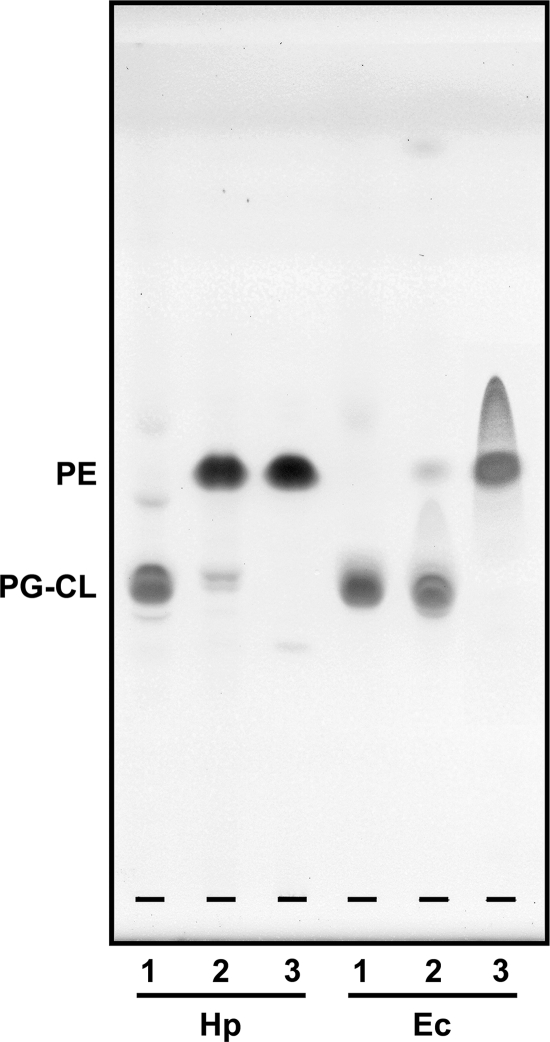
As shown in Fig. 1D, viable H. pylori cells efficiently retained dMβCD. Next, we examined the effect of dMβCD on dead H. pylori cells. We prepared heat-killed bacterial cells via autoclaving and microscopically observed the bacterial cell bodies. The H. pylori and E. coli cell bodies remained intact even when the cells were heated for 15 min at 121°C (Fig. 2A and B). When the heat-killed H. pylori cells were incubated with dMβCD, intriguingly, the TLC analysis revealed that the level of PE detected in the heat-killed H. pylori cells incubated in the presence of dMβCD was remarkably low in comparison with that of PE detected in the heat-killed H. pylori cells incubated in the absence of dMβCD (Fig. 2C). In sum, dMβCD induced the elution of PE from the dead H. pylori cells. Given that dMβCD makes its first contact with the bacterial cell at the outermost layer of the outer membrane and given that conspicuous elution of PE is induced via the action of dMβCD, we can assume that the outermost layer of the outer membrane of H. pylori contains PE in large amounts. These results, in combination with the results obtained from the viable cells (Fig. 1C and D), suggest that H. pylori binds the FC-dMβCD inclusion complex and dMβCD itself via the mediation of PE in the outermost layer of the outer membrane. In contrast, the elution of glycerophospholipids from the dead E. coli cells induced by dMβCD was more conspicuous in PG-CL than in PE: the level of PG-CL detected in the heat-killed E. coli cells incubated with dMβCD was notably lower than that of PG-CL detected in the heat-killed E. coli cells incubated without dMβCD in the TLC analysis (Fig. 2C). These results tell us that the outermost layer of the outer membrane of E. coli contains more PG-CL than PE.
Fig 2.
Effect of dMβCD on the elution of glycerophospholipids from dead bacterial cells. (A and B) A bacterial cell suspension (approximately 109 CFU/ml) was autoclaved for 15 min at 121°C, stained with CBB, and observed microscopically. Bars, 10 μm. (C) The heat-killed cells (109 CFU) of H. pylori and E. coli were incubated overnight in PBS (2 ml) in the presence or absence of dMβCD (5 mM) at 25°C with shaking, and then the membrane lipids from the cells (109 CFU) were purified to be analyzed by TLC. The spots of lipid on the plate surface were detected with a 60% sulfuric acid solution.
Bacterial lipid profiles separated via Iatrobead column chromatography.
Next, we isolated PEs from the membrane lipids of H. pylori and E. coli by Iatrobead column chromatography. The TLC analysis confirmed the presence of high-purity PEs from H. pylori and E. coli cells in the acetone-methanol (2:8) eluate after Iatrobead column chromatography (Fig. 3). Meanwhile, PG-CLs from H. pylori and E. coli cells were detected in the acetone-methanol (7:3) eluate in the TLC analysis. Although the purities of PG-CL isolated from the membrane lipids of H. pylori and E. coli were somewhat lower than those of PE isolated from the membrane lipids of the two bacterial species, we used the acetone-methanol (2:8) and acetone-methanol (7:3) fractions as the PE and PG-CL preparations, respectively.
Fig 3.
TLC analysis of membrane lipid fractions obtained from H. pylori and E. coli separated by Iatrobead column chromatography. Lanes 1, 2, and 3 are lipid profiles in eluates of acetone-methanol (7:3), acetone-methanol (4:6), and acetone-methanol (2:8), respectively. The spots of lipids were detected with a 60% sulfuric acid solution after TLC with a chloroform-methanol-water (70:30:5) solvent system. The amount of lipid placed on the silica gel plate was 100 μg/lane.
Significant binding of FC to H. pylori PE.
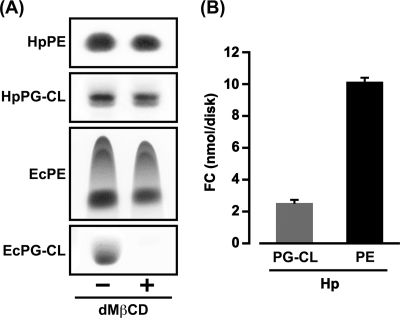
First, we examined whether PE and PG-CL eluted from the paper disks dotted with those phospholipids in the presence of dMβCD. When a PE (100 μg)-fixed paper disk or a PG-CL (100 μg)-fixed paper disk was incubated in the presence or absence of dMβCD (5 mM), the PE and PG-CL of H. pylori were negligibly eluted from the paper disks even in the presence of dMβCD; the PE and PG-CL in the paper disks incubated with dMβCD were detected at somewhat lower densities than those phospholipids in the paper disks incubated without dMβCD in the TLC analysis (Fig. 4A). Similarly, the elution of E. coli PE from the paper disk was also negligible in the presence of dMβCD; the level of PE detected in the paper disk incubated with dMβCD was somewhat lower than that of PE detected in the paper disk incubated without dMβCD in the TLC analysis. In contrast, dMβCD induced conspicuous elution of E. coli PG-CL from the paper disk; the PG-CL in the paper disk incubated with dMβCD was undetectable in the TLC analysis. These results, together with the results shown in Fig. 2C, indicate that dMβCD strongly interacts with E. coli PG-CL and somehow solubilizes its phospholipid into the water solvent and tell us that the PG-CL of E. coli is unsuitable for this paper disk experiment system. Based on these results, the PE and PG-CL of H. pylori and the PE of E. coli were used in the subsequent experiments.
Fig 4.
Solubilization of E. coli PG-CL by the action of dMβCD and significant binding of FC to H. pylori PE. (A) A paper disk dotted with PE (100 μg) or PG-CL (100 μg) from H. pylori and E. coli cells was incubated for 4 h in the presence or absence of dMβCD (5 mM) in a Tris buffer (2 ml) at 25°C with continuous shaking, and then PE and PG-CL in the paper disks were detected on a silica gel plate surface by visualizing them with a 60% sulfuric acid solution after TLC. (B) A paper disk dotted with PE (300 μg) or PG-CL (300 μg) from H. pylori cells was incubated for 4 h with an FC (100 nmol)-fixed paper disk in the presence of dMβCD (5 mM) in a Tris buffer (2 ml) at 25°C with continuous shaking, and then the amounts of FC in the PE-fixed paper disk and the PG-CL-fixed paper disk were quantified by the ferrous chloride-sulfuric acid method. The results are indicated as the mean FC and SD obtained from three independent experiments.
To estimate the potent ability of H. pylori PE to bind FC, we compared the amounts of FC contained in a PE-fixed paper disk and the PG-CL-fixed paper disk recovered after incubation with an FC-fixed paper disk in the presence of dMβCD. In the glycerophospholipids of H. pylori, PE was obviously more potent than PG-CL in binding FC; the level of FC detected in the paper disk dotted with PE (300 μg) was higher than that of FC detected in the paper disk dotted with the same amount of PG-CL (Fig. 4B). In sum, FC was found to exhibit much higher affinity for H. pylori PE than for its PG-CL. On this basis, we progressed to the next steps in experiments using PEs isolated from H. pylori and E. coli cells.
Binding of FC and dMβCD to H. pylori PE.
Paper disks dotted with various amounts of H. pylori PE or E. coli PE were incubated with an FC (100 nmol)-fixed paper disk in the presence of dMβCD (5 mM), and then the FC in the PE-fixed paper disks was quantified by the ferrous chloride-sulfuric acid method. H. pylori PE was clearly more potent than E. coli PE in binding FC; the levels of FC detected in the paper disks dotted with H. pylori PE (200 μg and 300 μg) were consistently higher than the levels of FC detected in the paper disks dotted with the same amounts of E. coli PE (Fig. 5A). When the dMβCD contained in the PE-fixed paper disks was measured at that time, much larger amounts of dMβCD were detected in the disks dotted with H. pylori PE (100 μg to 300 μg) than in the disks dotted with E. coli PE (100 μg to 300 μg) (Fig. 5B). Incidentally, H. pylori PE also had a potent ability to bind the dMβCD molecule by itself in the absence of FC (see Fig. S4 in the supplemental material). These results, together with those in Fig. 5A and Fig. S4 in the supplemental material, tell us that H. pylori PE has a strong ability to bind both the FC and dMβCD molecules via various molecular interactions. E. coli PE, meanwhile, seemed to bind only the FC molecule, and only via the hydrophobic bond.
Fig 5.
Characterization of H. pylori PE in interaction with FC, dMβCD, and CE. (A) An FC (100 nmol)-fixed paper disk was incubated for 4 h with a PE-fixed paper disk (the amounts of PE are indicated) in the presence of dMβCD (5 mM) in Tris buffer (2 ml). After the PE-fixed paper disk was recovered and washed, the FC in the disk was quantified by the ferrous chloride-sulfuric acid method. The results are indicated as the mean FC ± SD obtained from three independent experiments. (B) The amounts of dMβCD in PE-fixed paper disks obtained after the same experimental procedure described for panel A were quantified by the phenol-sulfuric acid method. The results are indicated as the mean dMβCD ± SD obtained from three independent experiments. (C) The same experiment described for panel A was carried out, but with a CE (50 nmol)-fixed paper disk used in place of an FC-fixed paper disk. The CE in the PE-fixed paper disks (the amounts of PE are indicated) was then quantified. The results are indicated as the mean CE ± SD obtained from three independent experiments. (D) The same experiment described for panel A was carried out, but with a paper disk dotted with the sterols of both FC (50 nmol) and CE (50 nmol) in place of an FC-fixed paper disk. The FC and CE in the PE (300 μg)-fixed paper disk were then detected by TLC analysis. −, levels of FC and CE detected in the paper disk without PE (negative control); RS, reference sterols of FC (10 nmol) and CE (10 nmol).
Selective binding of FC, but not CE, to H. pylori PE.
We next examined whether H. pylori PE binds CE as efficiently as it binds FC. PE-fixed paper disks were incubated with a CE (50 nmol)-fixed paper disk in the presence of dMβCD (5 mM), and then the CE in the PE-fixed paper disks was quantified by the ferrous chloride-sulfuric acid method. Surprisingly, H. pylori PE had a remarkably low affinity for CE; the amount of CE detected was very small compared to the amount of FC detected, even in the paper disks dotted with the largest amount of PE (300 μg) (Fig. 5A and C). In contrast, E. coli PE had a stronger ability to bind CE than H. pylori PE; conspicuously larger amounts of CE were detected in the paper disks dotted with E. coli PE (100 μg to 300 μg) than in the paper disks dotted with H. pylori PE in the same amounts (Fig. 5C).
As with the experiments to quantify FC and CE by the ferrous chloride-sulfuric acid method, the TLC analysis also revealed the selective binding of FC to H. pylori PE (Fig. 5D). When an H. pylori PE (300 μg)-fixed paper disk or E. coli PE (300 μg)-fixed paper disk was incubated with a paper disk dotted with the sterols of both FC (50 nmol) and CE (50 nmol) in the presence of dMβCD (5 mM), the H. pylori PE bound only a negligible amount of CE, and conspicuously less CE was detected in the H. pylori PE-fixed paper disk than in the E. coli PE-fixed paper disk. In contrast, H. pylori PE efficiently bound FC, and more FC was detected in the H. pylori PE-fixed paper disk than in the E. coli PE-fixed paper disk. The spot of CE was of somewhat lower density than the spot of FC on the same E. coli PE-fixed paper disk in the TLC analysis, but this result corresponded well with the FC and CE amounts quantified via the ferrous chloride-sulfuric acid method (Fig. 5A and C). These results tell us that H. pylori PE has a more selective affinity for FC than for CE and that H. pylori PE may serve an important function in the assimilation of FC into the cell membranes. In contrast, E. coli PE exhibited similar affinities for both CE and FC and appeared to bind cholesterols only via hydrophobic interaction.
Interaction of 3β-OH steroids with H. pylori cells and PE.
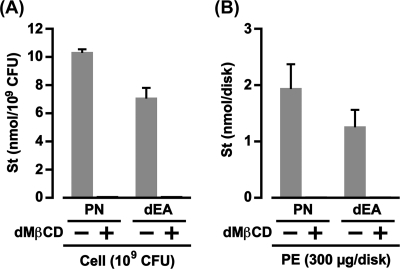
A study by our group in 2009 described, without showing the data, that the absorption of 3β-OH steroids, such as PN and dEA, into H. pylori cell membranes is inhibited by a high concentration of dMβCD (10). Therefore, we first showed the influence of dMβCD on the absorption of PN and dEA by H. pylori (Fig. 6A). The H. pylori cells (109 CFU) absorbed approximately 10 nmol of PN from the paper disk dotted with 100 nmol of its 3β-OH steroid only in the absence of dMβCD. Absorption of dEA into the H. pylori cells was also observed in the absence of dMβCD, although the amount of dEA absorbed into the H. pylori cells was somewhat smaller than that of PN. In contrast, the H. pylori cells (109 CFU) in the presence of dMβCD (5 mM) absorbed neither PN nor dEA. These results indicate that dMβCD somehow obstructs the interaction between 3β-OH steroids and the cell surface components of H. pylori.
Fig 6.
Obstruction of the interaction of 3β-OH steroids with H. pylori cells and PE by dMβCD. St, steroid. (A) The same experimental procedures described for Fig. 1C were carried out using a PN (100 nmol)-fixed paper disk and a dEA (100 nmol)-fixed paper disk in place of an FC-fixed paper disk. The results are shown as the mean steroid and SD obtained from three independent experiments. (B) The same experimental procedures described for Fig. 5A were carried out using steroid (100 nmol)-fixed paper disks in place of an FC-fixed paper disk. The results are shown as the mean steroid and SD obtained from three independent experiments.
Next, we examined the influence of dMβCD on the binding of PN and dEA to H. pylori PE (Fig. 6B). When the H. pylori PE (300 μg)-fixed paper disk was incubated with the paper disk dotted with 100 nmol of PN or dEA in the presence or absence of dMβCD (5 mM), the PE bound PN and dEA only in the absence of dMβCD. In sum, dMβCD obstructed the molecular interaction between 3β-OH steroids and H. pylori PE. These results completely correspond with the results observed in the binding of PN and dEA to H. pylori cells and strongly suggest that PE of H. pylori contributes to the assimilation, not only of FC, but also of 3β-OH steroids into the cell membranes.
Fatty acid compositions of H. pylori PE.
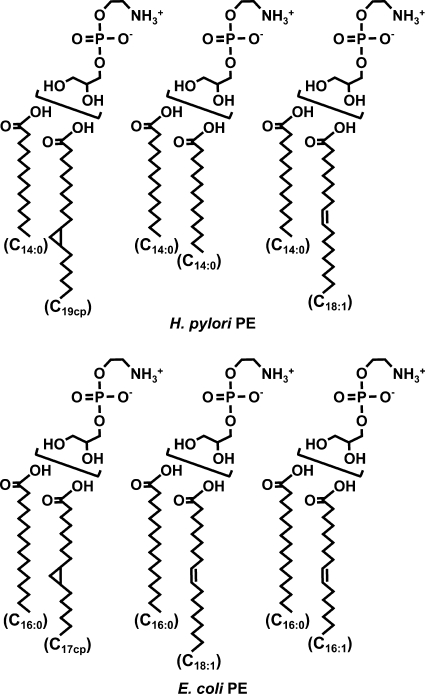
GC-MS analysis revealed obvious differences between the fatty acid compositions of H. pylori PE and E. coli PE (Table 1). Most of the acyl groups attached to the glycerophosphorylethanolamines of H. pylori were C14:0 and C19 cyclopropane fatty acids, whereas most of the acyl groups attached to the glycerophosphorylethanolamines of E. coli were C16:0, C18:1, and C17 cyclopropane fatty acids.
Table 1.
Fatty acid compositions of PE molecules of H. pylori and E. coli
| Fatty acida | Fatty acid composition (%) of PEb |
|
|---|---|---|
| H. pylori | E. coli | |
| C14:0 | 33.9 | 1.0 |
| C15:0 | UD | 2.7 |
| C16:0 | 0.9 | 36.3 |
| C16:1 | UD | 5.0 |
| C17:0 | UD | 7.0 |
| C17cp | UD | 12.8 |
| C18:0 | 2.6 | 1.0 |
| C18:1 | 9.0 | 31.5 |
| C19cp | 52.4 | 2.7 |
| UI | 1.2 | UD |
C17cp, C17 cyclopropane; C19cp, C19 cyclopropane; UI, unidentified fatty acid.
UD, undetected.
Analysis of PE molecular species of H. pylori.
Next, we carried out an LC-MS analysis of the PE molecular species of H. pylori and E. coli to determine which were predominant. The total carbon numbers of predominant fatty acids constituting the acyl groups in PE molecules of H. pylori were C28 (28.9%), C32 (15.0%), and C33 (34.5%). One of the two fatty acid molecules composed of 32 carbon atoms (C32) lacked two hydrogen atoms. Similarly, one of the two fatty acid molecules composed of 33 carbon atoms (C33) lacked two hydrogen atoms. In contrast, the total carbon numbers of predominant fatty acids constituting the acyl groups in PE molecules of E. coli were C32 (12.0%), C33 (25.7%), and C34 (14.7%). One of the two fatty acid molecules composed of these total carbon numbers lacked two hydrogen atoms.
Analysis of phosphoethanolamine moiety in the PE molecule of H. pylori.
The 1H-NMR analysis did not detect the differences in the spectrum patterns of chemical shift between the phosphoethanolamine moieties of the H. pylori PE and reference PE (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine). This means that H. pylori PE attaches a universal phosphoethanolamine molecule without chemical modification.
The binding of cholesterols to dMPE and dPPE.
As shown in Table 1, a significant difference between H. pylori PE and E. coli PE was a saturated fatty acid composition attached to the PE molecules: the most prevalent saturated fatty acid molecule of H. pylori PE was a myristic acid (C14:0), whereas the most prevalent saturated fatty acid molecule of E. coli PE was a palmitic acid (C16:0). Therefore, we conducted the next experiments using a dMPE and a dPPE to examine whether the myristic acid (C14:0) attached to the PE molecule is responsible for the selective binding of FC. When an FC (100 nmol)-fixed paper disk was incubated with the paper disks dotted with various amounts of dMPE, the amounts of FC bound to the dMPE increased linearly along with the increases in the amounts of dMPE dotted onto the paper disks (Fig. 7A). Intriguingly, the binding of CE to dMPE was almost not observed, even when a CE (50 nmol)-fixed paper disk was incubated with the paper disk dotted with the largest amount of dMPE (300 nmol). These results tell us that PE carrying myristic acid (C14:0) molecules selectively interacts with FC, but not with CE. In contrast, dPPE interacted with both FC and CE; the amounts of FC and CE bound to dPPE exhibited linear increases that were dependent on larger amounts of dPPE dotted onto the paper disks (Fig. 7B). These results indicate that the myristic acid (C14:0) attached to the PE molecule plays an important role in the selective binding of FC.
Fig 7.
Selective interaction of dMPE with FC, but not with CE. After an FC (100 nmol)-fixed paper disk or CE (50 nmol)-fixed paper disk was incubated for 4 h with paper disks dotted with dMPE or dPPE (the amounts of dMPE and dPPE are indicated) in Tris buffer (2 ml), the amounts of FC and CE bound to the respective PEs were quantified by the ferrous chloride-sulfuric acid method. The results are shown as the mean sterol ± SD obtained from three independent experiments.
DISCUSSION
This study demonstrated that H. pylori cells potently retain dMβCD and that the PE in the outer membrane of the cells plays an important role in this dMβCD retention. A study by our group in 2009 revealed that dMβCD protects H. pylori cells from the bactericidal action of unsaturated fatty acids and LPC by inhibiting the absorption of those lipophilic compounds into the cell membranes (23). This suggests that the outer membrane of the H. pylori cell may incorporate dMβCD by binding to PE and that the dMβCD so incorporated may prevent the absorption of unsaturated fatty acids and LPC into the H. pylori cell by weakening the hydrophobic interaction between those lipophilic compounds and the outer membrane. In sum, dMβCD may serve to strengthen the hydrophilicity of H. pylori cell surfaces and repel the binding of unsaturated fatty acids and LPC to the cells. Thus, a goal for future investigations will be to elucidate the assimilation of dMβCD into H. pylori cell membranes.
A recent study by another group demonstrated that a phosphatidylcholine carrying a palmitic acid molecule and an oleic acid molecule (POPC) binds the four molecules of methyl-β-cyclodextrin (MβCD, but not dMβCD) and has further suggested that each fatty acid chain of the POPC molecule is embedded inside the ring structure of one or two molecules of MβCD (2). No earlier investigations, however, have revealed the molecular interaction between βCDs (including MβCD and dMβCD) and other glycerophospholipids, such as PE and PG-CL. This study revealed that dMβCD induces conspicuous elution of PE and PG-CL from heat-killed H. pylori and E. coli cells, respectively. Especially, dMβCD exhibited strong affinity for E. coli PG-CL and solubilized its PG-CL into the water solvent. We could not, however, clarify why dMβCD exhibits much higher affinity for E. coli PG-CL than for the other glycerophospholipids investigated in this study. To resolve this question, further investigations will be necessary.
This study revealed that the FC-dMβCD inclusion complex efficiently interacts with H. pylori PE. Conversely, no PN-dMβCD or dEA-dMβCD inclusion complexes interacted with H. pylori PE. In addition to the difference in chemical structures among FC, PN, and dEA, the molecular weights of PN and dEA are smaller than the molecular weight of FC. On this basis, we can assume that the PN and dEA molecules are more deeply embedded than the FC molecule inside the ring structure of dMβCD, and thus, the crucial parts of PN and dEA involved in binding to H. pylori PE may be masked by dMβCD. Conformation analyses in future studies will be necessary to clarify the molecular interaction between dMβCD and those 3β-OH steroids (including FC).
A number of studies by other groups have demonstrated that the most prevalent saturated fatty acid component of PE found in typical Gram-negative bacteria, such as E. coli, Klebsiella pneumoniae, Salmonella enterica serovar Typhimurium, and Pseudomonas aeruginosa, is a palmitic acid (C16:0) (1, 5, 13, 24). The most prevalent saturated fatty acid component of the PE in E. coli analyzed in this study was also C16:0. In contrast, a previous study by our group revealed that H. pylori PE predominantly carried a myristic acid (C14:0) (9), as with the PE of H. pylori analyzed in this study. Moreover, we revealed that the myristic acid (C14:0) molecule attached to PE is responsible for the selective binding of FC. In sum, our findings indicate that the C14:0 saturated fatty acid in the PE molecule of H. pylori plays an important role in more selectively binding FC rather than CE.
In our structural analyses of purified PE by GC-MS, LC-MS, and 1H-NMR, the predominant PE molecular species of H. pylori were surmised to be glycerophosphorylethanolamines acylated by the C14:0 and C19 cyclopropane fatty acid molecules (total carbon numbers, 33), the two C14:0 fatty acid molecules (total carbon numbers, 28), and the C14:0 and C18:1 fatty acid molecules (total carbon numbers, 32) (Fig. 8). Meanwhile, the predominant PE molecular species of E. coli were surmised to be glycerophosphorylethanolamines acylated by the C16:0 and C17 cyclopropane fatty acid molecules (total carbon numbers, 33), the C16:0 and C18:1 fatty acid molecules (total carbon numbers, 34), and the C16:0 and C16:1 fatty acid molecules (total carbon numbers, 32).
Fig 8.
Predominant PE molecular species of H. pylori and E. coli in this study. C19cp, C19 cyclopropane; C17cp, C17 cyclopropane.
In this study, E. coli PE bound FC via hydrophobic interaction, but no FC was incorporated into the E. coli cell membranes. However, apart from this, we demonstrated that the PG-CL content is higher than that of PE in the outermost layer of the outer membrane of E. coli. In addition, we revealed that the binding of FC to H. pylori PG-CL is obviously weak in comparison with the binding of FC to H. pylori PE. In sum, the PG-CL of E. coli is also considered to have only low potency to bind FC, as with H. pylori PG-CL. This circumstantial evidence may explain why E. coli is incapable of absorbing FC into the membranes. H. pylori PE, meanwhile, was found to be contained in large amounts in the outermost layer of the outer membrane and to exhibit strong affinity for FC. In addition, H. pylori PE was capable of binding the 3β-OH steroids, namely, PN and dEA. On this basis, we conclude that PE in the outer membrane of H. pylori functions as a nonesterified steroid-binding lipid in assimilating FC and 3β-OH steroids into the cell membranes. Our recent study has suggested the possibility that progesterone nonreversibly binds to H. pylori cell membranes (11). It will thus be necessary in future studies to examine whether PE is also involved in the binding of progesterone to H. pylori cells. Further, our study in 2009 revealed that PC variants carrying either a linoleic acid molecule or an arachidonic acid molecule bind to H. pylori cells without steroid retention and induce bacteriolysis (23). Thus, another aim for future research will be to ascertain the interaction of PC with H. pylori PE.
Supplementary Material
Footnotes
Published ahead of print 9 March 2012
Supplemental material for this article may be found at http://jb.asm.org.
REFERENCES
- 1. Amine A, Saloua KS, Mouadh M, Alya EIM, Ahmed L. 2011. The absence of the “GATC-binding protein SeqA” affects DNA replication in Salmonella enterica serovar Typhimurium. In Kusic-Tisma J. (ed), DNA replication and related cellular processes. InTech, Rijeka, Croatia: http://www.intechopen.com/books/dna-replication-and-related-cellular-processes/the-absence-of-the-gatc-binding-protein-seqa-affects-dna-replication-in-salmonella-enterica-serovar-. [Google Scholar]
- 2. Anderson TG, Tan A, Ganz P, Seelig J. 2004. Calorimetric measurement of phospholipid interaction with methyl-β-cyclodextrin. Biochemistry 43:2251–2261 [DOI] [PubMed] [Google Scholar]
- 3. Arima H, Yunomae K, Morikawa T, Hirayama F, Uekama K. 2004. Contribution of cholesterol and phospholipids to inhibitory effect of dimethyl-β-cyclodextrin on efflux function of P-glycoprotein and multidrug resistance-associated protein 2 in vinblastine-resistant Caco-2 cell monolayers. Pharm. Res. 21:625–634 [DOI] [PubMed] [Google Scholar]
- 4. Douraghi M, et al. 2010. Comparative evaluation of three supplements for Helicobacter pylori growth in liquid culture. Curr. Microbiol. 60:254–262 [DOI] [PubMed] [Google Scholar]
- 5. Dunnick JK, O'Leary WM. 1970. Correlation of bacterial lipid composition with antibiotic resistance. J. Bacteriol. 101:892–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fukasawa M, Nishijima M, Itabe H, Takano T, Hanada K. 2000. Reduction of sphingomyelin level without accumulation of ceramide in Chinese hamster ovary cells affects detergent-resistant membrane domains and enhances cellular cholesterol efflux to methyl-beta-cyclodextrin. J. Biol. Chem. 275:34028–34034 [DOI] [PubMed] [Google Scholar]
- 7. Fukase K, et al. 2008. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 372:392–397 [DOI] [PubMed] [Google Scholar]
- 8. Graham DY. 1991. Helicobacter pylori: its epidemiology and its role in duodenal ulcer disease. J. Gastroenterol. Hepatol. 6:105–113 [DOI] [PubMed] [Google Scholar]
- 9. Hirai Y, et al. 1995. Unique cholesteryl glucosides in Helicobacter pylori: composition and structural analysis. J. Bacteriol. 177:5327–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hosoda K, et al. 2009. Anabolic utilization of steroid hormones in Helicobacter pylori. FEMS Microbiol. Lett. 297:173–179 [DOI] [PubMed] [Google Scholar]
- 11. Hosoda K, Shimomura H, Hayashi S, Yokota K, Hirai Y. 2011. Steroid hormones as bactericidal agents to Helicobacter pylori. FEMS Microbiol. Lett. 318:68–75 [DOI] [PubMed] [Google Scholar]
- 12. Junquera E, Peña L, Aicart E. 1998. Binding of sodium salicylate by β-cyclodextrin or 2,6-di-O-methyl-β-cyclodextrin in aqueous solution. J. Pharm. Sci. 87:86–90 [DOI] [PubMed] [Google Scholar]
- 13. Kearns DB, Robinson J, Shimkets LJ. 2001. Pseudomonas aeruginosa exhibits directed twitching motility up phosphatidylethanolamine gradients. J. Bacteriol. 183:763–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lebrun A, et al. 2006. Cloning of a cholesterol-α-glucosyltransferase from Helicobacter pylori. J. Biol. Chem. 281:27765–27772 [DOI] [PubMed] [Google Scholar]
- 15. Marchini A, et al. 1995. Cyclodextrins for growth of Helicobacter pylori and production of vacuolating cytotoxin. Arch. Microbiol. 164:290–293 [DOI] [PubMed] [Google Scholar]
- 16. Marshall B, Warren JR. 1983. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet i:1273–1274 [PubMed] [Google Scholar]
- 17. McGee DJ, et al. 2011. Cholesterol enhances Helicobacter pylori resistance to antibiotics and LL-37. Antimicrob. Agents Chemother. 55:2897–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ohkubo S, Kotani A, Nakahata N. 2002. Lipid raft domains in NG108-15 cells; the role in P2Y2 receptor-mediated intracellular Ca2+ mobilization. Pharmacologist 44:A68 [Google Scholar]
- 19. Peek RM, Jr, Blaser MJ. 2002. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2:28–37 [DOI] [PubMed] [Google Scholar]
- 20. Peek RM, Jr, Crabtree JE. 2006. Helicobacter infection and gastric neoplasia. J. Pathol. 208:233–248 [DOI] [PubMed] [Google Scholar]
- 21. Rietschel ET, et al. 1994. Bacterial endotoxin: molecular relationship of structure to activity and function. FASEB J. 8:217–225 [DOI] [PubMed] [Google Scholar]
- 22. Shimomura H, Hayashi S, Yokota K, Oguma K, Hirai Y. 2004. Alteration in the composition of cholesteryl glucosides and other lipids in Helicobacter pylori undergoing morphological change from spiral to coccoid form. FEMS Microbiol. Lett. 237:407–413 [DOI] [PubMed] [Google Scholar]
- 23. Shimomura H, et al. 2009. Steroids mediate resistance to the bactericidal effect of phosphatidylcholines against Helicobacter pylori. FEMS Microbiol. Lett. 301:84–94 [DOI] [PubMed] [Google Scholar]
- 24. Shokri A, Larsson G. 2004. Characterisation of the Escherichia coli membrane structure and function during fed batch cultivation. Microb. Cell Fact. 3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stolte M, et al. 2002. Helicobacter and gastric MALT lymphoma. Gut 50:III19–III24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trainor EA, Horton KE, Savage PE, Testerman TL, McGee DJ. 2011. Role of the HefC efflux pump in Helicobacter pylori cholesterol-dependent resistance to ceragenins and bile salts. Infect. Immun. 79:88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uemura N, et al. 2001. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345:784–789 [DOI] [PubMed] [Google Scholar]
- 28. Wunder C, et al. 2006. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat. Med. 12:1030–1038 [DOI] [PubMed] [Google Scholar]
- 29. Wyatt JI, Dixon MF. 1988. Chronic gastritis—a pathogenetic approach. J. Pathol. 154:113–124 [DOI] [PubMed] [Google Scholar]
- 30. Yamashita T, Yamaguchi T, Murakami K, Nagasawa S. 2001. Detergent-resistant membrane domains are required for mast cell activation but dispensable for tyrosine phosphorylation upon aggregation of the high affinity receptor for IgE. J. Biochem. 129:861–868 [DOI] [PubMed] [Google Scholar]
- 31. Yu Z, et al. 2007. Investigation of heptakis (2,6-di-O-methyl)-β-cyclodextrin inclusion complexes with flavonoid glucosides by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 21:683–690 [DOI] [PubMed] [Google Scholar]
- 32. Yunomae K, Arima H, Hirayama F, Uekama K. 2003. Involvement of cholesterol in the inhibitory effect of dimethyl-β-cyclodextrin on P-glycoprotein and MRP2 function in Caco-2 cells. FEBS Lett. 536:225–231 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.