Abstract
RpoS, the master sigma factor during stationary phase and under a variety of stress conditions, is regulated at multiple levels, including regulated degradation. Degradation is dependent upon ClpXP and the RssB adaptor protein. H-NS, a nucleoid-associated protein, affects the regulated degradation of RpoS; in the absence of H-NS, RpoS is stable. The mechanisms involved in this regulation were not known. We have found that H-NS inhibits the expression of iraD and iraM, the genes coding for two antiadaptor proteins that stabilize RpoS when overexpressed. The regulation by H-NS of iraM is independent from the previously demonstrated regulation by the PhoP/PhoQ two-component system. Moreover, differences in the behavior of several hns alleles are explained by a role for StpA, an H-NS-like protein, in the regulation of RpoS stability. This finding parallels recent observations for a role of StpA in regulation of RpoS stability in Salmonella.
INTRODUCTION
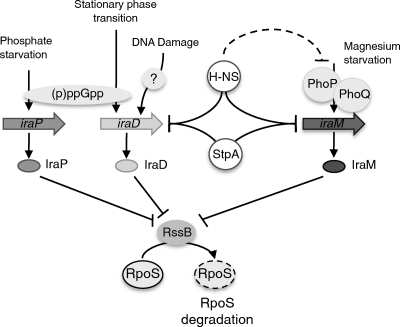
RpoS is the primary sigma factor allowing adaptation of Escherichia coli to stationary phase and many stress conditions. It is highly regulated at the level of transcription and translation. It is also degraded when not needed (for a review, see reference 2). RpoS is degraded by the ATP-dependent protease ClpXP, but to be recognized as a substrate, RpoS first must interact with RssB, an adaptor protein (16, 23, 26, 37). Recently, three new proteins involved in inhibiting this degradation pathway have been discovered (3, 5). They have been called antiadaptor proteins because they allow RpoS stabilization by interacting with RssB and inhibiting its binding to RpoS. These three proteins are induced under different stress conditions: IraP is induced under phosphate starvation, IraM is induced under magnesium starvation, and IraD is induced by DNA damage or during the transition from exponential to stationary phase (3, 5, 20, 21). Expression of iraP and iraD is activated by the nucleotide (p)ppGpp, whereas iraM expression is positively regulated by the PhoP/PhoQ two-component system (3, 4, 21).
Other proteins that influence RpoS degradation have been described (3, 32, 36; A. Battesti, unpublished data). One of them is H-NS, a global transcriptional repressor. In order to regulate gene expression, this histone-like protein binds preferentially to AT-rich regions of DNA. Recently, a high-affinity H-NS binding motif has been defined (18). Although H-NS regulates a large number of genes by itself, it has also been shown that H-NS can interact with StpA, a homologous protein, to regulate the expression of certain genes (29, 35).
The inhibition of RpoS degradation by H-NS was demonstrated by Yamashino and coworkers (32) and further explored by Zhou and Gottesman (36). In a strain from which hns was deleted, RpoS transcription was shown to be slightly increased and its degradation strongly inhibited. Indeed, RpoS was stable in the hns mutant strain, whereas other ClpXP substrates were still degraded (36). RpoS stabilization was the consequence of the loss of RssB activity; higher levels of RssB overcame this effect of an hns mutant. In the study described in this paper, we investigated further the role of H-NS on RpoS degradation. We hypothesized that H-NS could regulate RpoS stability by negatively regulating the expression of one or more of the antiadaptor proteins. We found that H-NS inhibited the expression of two antiadaptor proteins, IraM and IraD, and that the H-NS homolog StpA was involved in the regulation of RpoS degradation.
MATERIALS AND METHODS
Media and growth conditions.
Cells were grown in LB (Lennox broth) or M9 minimal medium containing 2 mM MgSO4, 100 μM CaCl2, and 10 μM FeSO4. Plasmids were maintained with ampicillin (100 μg/ml). Liquid cultures were grown under aerobic conditions at 37°C unless otherwise stated.
For the Mg2+/Ca2+ starvation experiment, M9 medium was made without MgSO4 and CaCl2. Cells were grown overnight in M9 medium containing 2 mM MgSO4 and 100 μM CaCl2, diluted into fresh M9 medium at an optical density at 600 nm (OD600) of ≈0.01, and grown to mid-logarithmic phase (OD600 ≈ 0.3) at 37°C. Cultures were then split in two. Half of the cells were washed twice by filtration with prewarmed starvation medium and resuspended in M9 starvation medium. The other half was washed twice by filtration with prewarmed M9 medium and resuspended in M9 medium. Incubation at 37°C was continued, and samples were harvested before filtration (0 min) and at 30 min, 60 min, and 120 min after filtration.
Strains and plasmids.
All the strains used are derivatives of MG1655 made by P1 transduction, selecting for the appropriate antibiotic resistance marker, as indicated in Table S1 in the supplemental material. Primers are listed in Table S2 in the supplemental material. To construct the stpA-SPA-kan strain (where SPA is the sequential peptide affinity tag) or mutants with deletion mutations of iraM, hns, and phoP, the bacteriophage λ red recombination system was used as described previously (33). Briefly, PCR fragments were obtained by amplifying the SPA-kan cassette of strain DY330 ACP-SPA (where ACP is acyl carrier protein) (6) (primers BA385 and BA386), the zeocin resistance cassette of strain NM1201 (primers BA261/BA262), and the kanamycin or the chloramphenicol resistance cassettes of the TKC strain (primers BA040/BA041 and BA330/BA331, respectively) (27). The PCR fragments were then recombined into the chromosome of strain NM1100 (3). The transformed cells were selected on LB plates containing 25 μg/ml zeocin, 25 μg/ml kanamycin, or 10 μg/ml chloramphenicol at 37°C. Recombinant products were verified by PCR, and mutations were transferred into MG1655 by P1 transduction. The gene conferring resistance to kanamycin was then removed from the stpA-SPA-kan strain by using the pcp20 plasmid (7) to allow the subsequent introduction of the hns-111::kan or Δhns-414 allele by P1 transduction.
Plasmid pBA340 contains the upstream region of hns (605 nucleotides [nt] upstream of the ATG start codon) and the first 111 nt of hns. Plasmid pBA339 contains the upstream region of hns (605 nt upstream of the ATG start codon), the first 111 nt of hns, and 99 nt of the kanamycin resistance cassette. PCR fragments were amplified with primers BA343/361 for pBA339 and BA343/BA362 for pBA340, using genomic DNA from hns-111::kan as the template. PCR fragments were then digested with XhoI and HindIII and cloned into pACYC177 cut by the same enzymes. Constructs were confirmed by sequencing.
Strains AB041, AB042, and AB050 are gifts from A. Bougdour and were constructed as described previously (3). The transcriptional fusions contain a fragment from −462 to +255 bp relative to the ATG start codon for iraP, from −700 to +321 bp relative to the ATG start codon for iraM, and from −672 to +386 bp relative to the ATG start codon for iraD.
Assay for protein degradation in vivo.
Cells were grown overnight in LB medium, diluted into fresh LB medium at an OD600 of ≈0.01, and grown to mid-logarithmic phase (OD600 ≈ 0.3) at 37°C. Chloramphenicol was added (200 μg/ml). Samples (1 ml) were harvested at the indicated time points and treated as described below.
Protein electrophoresis and Western blotting.
For RpoS and StpA-SPA stability experiments, samples were precipitated with a final concentration of 5% ice-cold tricarboxylic acid (TCA). Precipitated pellets were washed with 500 μl of 80% cold acetone and resuspended in a volume of SDS sample buffer normalized to the OD600.
For H-NS expression experiments, 200 μl of overnight cultures (32°C) was pelleted. Pellets were resuspended in a volume of SDS sample buffer normalized to the OD600.
Samples were analyzed using Nu-PAGE 12% bis-Tris gels (Invitrogen, CA), transferred to a nitrocellulose membrane, and probed with a 1:5,000 dilution of anti-RpoS antiserum, a 1:1,000 dilution of anti-H-NS antiserum, or a 1:1,000 dilution of anti-Flag antiserum (Sigma-Aldrich). The blots were developed with the Lumi-Phos Western blotting chemiluminescent substrate (Thermo Scientific) using a luminescence image analyzer (LAS-4000 Mini; Fujifilm). Quantification was performed by using Multi-Gauge software (Fujifilm). Values presented are the mean of at least three independent assays.
β-Galactosidase assays.
Cells were grown in LB medium. For the starvation experiment, cells were grown in M9 medium at 37°C and treated as described above. β-Galactosidase activity was determined using the standard assay described by Miller (22). Values presented are the mean of at least three independent assays.
RESULTS
As noted above, stabilization of RpoS in hns mutants has been reported by a number of groups. In initial experiments, we found some unexpected differences in results with different hns alleles. As described below, an hns-kan insertion mutation, now referred to as hns-111::kan, led to RpoS stabilization, while hns-205::tet did not. More surprisingly, a full deletion of hns, Δhns, had only a modest effect on RpoS turnover. We first investigate the basis for the stabilization of RpoS in the hns-111::kan strain and then compare these results to those in strains carrying the other hns mutations.
iraD and iraM deletions restore RpoS proteolysis in an hns::kan strain.
In the first set of experiments, we used the hns-111::kan allele, which is not a null hns allele (see below). This strain contains an insertion after amino acid 37; translation continues into the insertion, adding a 33-amino-acid tail to the truncated H-NS protein (35). This hns allele led to RpoS stabilization (Fig. 1A).
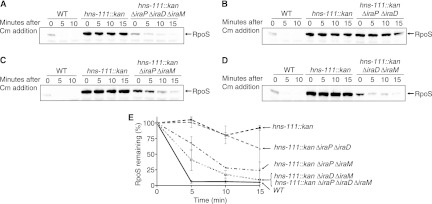
Fig 1.
Effect of antiadaptors on RpoS degradation in hns-111::kan strains. Strains were grown in LB at 37°C. At an OD600 of ≈0.3, protein synthesis was inhibited by chloramphenicol (Cm) addition. Samples were then removed at the indicated time points after chloramphenicol addition and RpoS levels were analyzed by Western blotting using anti-RpoS antiserum. Strains used are WT (MG1655), hns-111::kan (AB053), and the following isogenic derivatives: hns-111::kan ΔiraP ΔiraD ΔiraM (DP004) (A), hns-111::kan ΔiraP ΔiraD (DP002) (B), hns-111::kan ΔiraP ΔiraM (BA171) (C), and hns-111::kan ΔiraD ΔiraM (BA173) (D). (E) Quantification of RpoS level in different genetic backgrounds. The intensity measured at time zero for each strain in each experiment was set at 100%. The mean from 3 replicates is presented, and the error bars indicate the standard error (SEM).
In order to determine if H-NS influenced RpoS degradation by regulating the expression of antiadaptor proteins, we constructed an hns-111::kan strain that is wild type (AB053) or has a deletion of the genes encoding the three known antiadaptors, iraP, iraD, and iraM (strain DP004). If the stabilization of RpoS in an hns-111::kan strain is solely the consequence of the expression of one or more of these antiadaptors, deleting the antiadaptor should restore the degradation of RpoS.
We compared RpoS stability in a wild-type (WT) parent, an hns-111::kan strain, and an hns-111::kan ΔiraP ΔiraD ΔiraM strain by inhibiting new protein synthesis with chloramphenicol and following the fate of RpoS by Western blotting. In exponential phase at 37°C, RpoS is rapidly degraded; RpoS was detectable only at time zero in the WT strain (Fig. 1A and E). As expected, in the hns-111::kan strain, the level of RpoS was still detectable after a 15-min chase (Fig. 1A and E), consistent with no or very slow RpoS degradation in this strain. In contrast, in the hns-111::kan ΔiraP ΔiraD ΔiraM strain, the RpoS level was initially lower and decreased much more rapidly than in the hns-111::kan strain (Fig. 1A and E). However, the level of RpoS detected was still higher than that in the WT strain, and the rate of RpoS degradation was slower than that in the hns+ strain, with some RpoS detectable at 15 min (Fig. 1A and E). These results strongly suggest that the stabilization of RpoS in an hns-111::kan background is primarily, but not solely, due to overexpression of one or more of the three known antiadaptors.
To identify which of the antiadaptor proteins was regulated by H-NS, we constructed an isogenic set of hns-111::kan derivatives, each with one or two genes coding for antiadaptor proteins deleted. Each of the strains was assayed for RpoS stability. Single deletions of each of the antiadaptors in the hns-111::kan background had either no effect on stabilization (ΔiraP) or modest effects (ΔiraM or ΔiraD) (data not shown), suggesting that H-NS was likely to be regulating multiple antiadaptor proteins. The stability of RpoS in a strain with iraP and iraD deletions was comparable to that in an hns-111::kan strain (Fig. 1B and E). In the strain with iraP and iraM deletions, RpoS was significantly less stable than it was in the hns-111::kan strain (Fig. 1C and E). Since we observed no change in RpoS stability in the strain with iraP and iraD deletions, we attributed this decreased stability to the iraM deletion. The RpoS level in the hns-111::kan ΔiraP ΔiraM strain was still higher than the level observed in the hns-111::kan ΔiraP ΔiraD ΔiraM strain (compare the 0-min and 5-min time points in Fig. 1A and C), suggesting that there was still some RpoS stabilization in the strain with the iraP and iraM deletions. Finally, in a strain with both iraM and iraD deleted, RpoS degradation was rapid and comparable to that seen in the hns-111::kan ΔiraP ΔiraD ΔiraM strain (compare Fig. 1D and A; Fig. 1E).
Thus, these results suggest that H-NS inhibits the expression of iraM and iraD; when H-NS is mutant, an increased expression of either or both of these antiadaptors leads to RpoS stabilization. These two antiadaptors account for most but not all of the effect on RpoS, with IraM being sufficient to stabilize (Fig. 1B), while IraD alone has a modest effect on stability (Fig. 1C). Because deletion of all three antiadaptors (Fig. 1A) or both IraD and IraM (Fig. 1D) leaves some residual RpoS stabilization, there may be yet another unidentified antiadaptor or other pathway for RpoS stabilization that is repressed by H-NS.
iraM and iraD expression are regulated by H-NS.
Based upon our results in Fig. 1, we predicted that the transcription of iraM and iraD, but not iraP, should be negatively regulated by H-NS. We confirmed this by constructing transcriptional lacZ fusions to iraM, iraD, and iraP and measuring the expression of β-galactosidase from these fusions in the absence or presence of H-NS. As shown in Fig. 2, inactivation of hns in hns-111::kan strains led to a strong increase in both iraM and iraD expression. However, iraP expression was almost unchanged (note that the basal level in a wild-type strain was also much higher for this fusion) (Fig. 2). These results strongly suggest that H-NS inhibits the transcription of iraM and iraD expression but not that of iraP expression.
Fig 2.
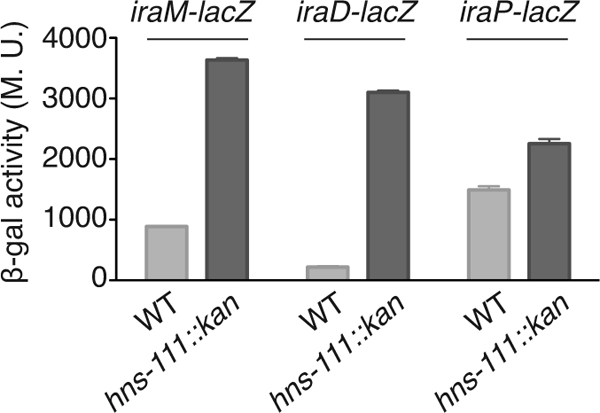
H-NS repression of iraM and iraD transcription. Strains containing transcriptional fusions iraM-lacZ (WT, AB042; hns-111::kan, YMT79), iraD-lacZ (WT, AB050; hns-111::kan, YMT82), or iraP-lacZ (WT, AB041; hns-111::kan, YMT80) were grown at 32°C overnight in LB. Samples were taken from each culture, and β-galactosidase activity was measured as described by Miller (22). The mean from 3 replicates is presented, and the standard error (SEM) is indicated by the error bars. M.U., Miller units.
Effect of H-NS on iraM regulation by PhoP/PhoQ.
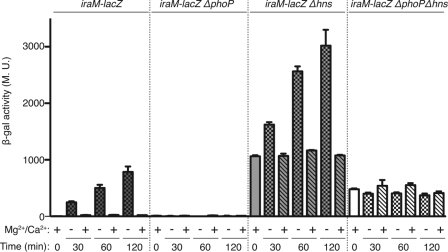
As mentioned earlier, IraM is produced during magnesium starvation. Its expression is under the positive control of the PhoP/PhoQ two-component system (3). If H-NS acts directly as a negative regulator of the iraM promoter, PhoP might act by displacing H-NS, as is found in some other H-NS-silenced promoters (reviewed in reference 9). If so, PhoP should become dispensable for iraM expression in the absence of H-NS and IraM levels would be the same in a Δhns strain and in a Δhns ΔphoP strain. Therefore, we compared iraM expression in WT, ΔphoP, Δhns, and Δhns ΔphoP strains, after magnesium starvation (Fig. 3). Note that instead of the hns insertion (hns-111::kan) used in the experiments described above, we used the Δhns-414 allele, which has a full deletion of hns (see Materials and Methods); however, results were similar with the hns-111::kan insertion mutant (data not shown). This mutation is referred to here as Δhns. In a WT strain, the expression of iraM increased over time during magnesium starvation, whereas almost no activity was measured under normal growth conditions (Fig. 3, leftmost set of data). As previously shown (3), in a strain with phoP deleted, iraM expression was very low in the presence or absence of magnesium (Fig. 3, second set of samples). The deletion of hns led to a dramatic increase in the basal level of iraM expression, but the regulation by PhoP was still detectable; expression increased under starvation conditions (Fig. 3, third set of samples). This result suggests that iraM regulation by PhoP and H-NS is at least partially independent. However, in a Δhns ΔphoP double mutant, the basal level of iraM expression was lower than that in a Δhns single mutant but significantly higher than that in the wild-type strain and, as expected, not responsive to Mg2+ starvation (Fig. 3, fourth set of samples). One interpretation of these results is that H-NS acts at two levels to affect iraM. It directly affects the iraM promoter, since there was a PhoP-independent increase in the basal level. In addition, it negatively regulates PhoP synthesis or activity, resulting in higher PhoP-dependent expression of the fusion in Δhns samples without Mg2+ starvation. These experiments are consistent with observations by Kong et al. (17), who demonstrated a role for H-NS in regulation of PhoP-dependent genes in Salmonella. They found some promoters that were fully PhoP dependent and other promoters in which PhoP acted solely as an antirepressor for H-NS. Our results show that neither H-NS nor PhoP is fully epistatic for regulation of iraM.
Fig 3.
Effect of Mg2+/Ca2+ starvation on iraM expression in different genetic contexts. Wild-type (AB042), ΔphoP (BA218), Δhns (YMT94), and Δhns ΔphoP (BA222) strains containing an iraM-lacZ transcriptional fusion were grown at 37°C in M9 medium. At an OD600 of ≈0.3, cultures were split in two, filtered, washed, and cultured in the presence (+) or absence (−) of Mg2+/Ca2+. Samples were collected at the indicated time points, and β-galactosidase activity was measured as described by Miller (22). The mean from 3 replicates is presented; the standard error (SEM) is indicated by the error bars. M.U., Miller units.
Different hns alleles have different effects on RpoS stability.
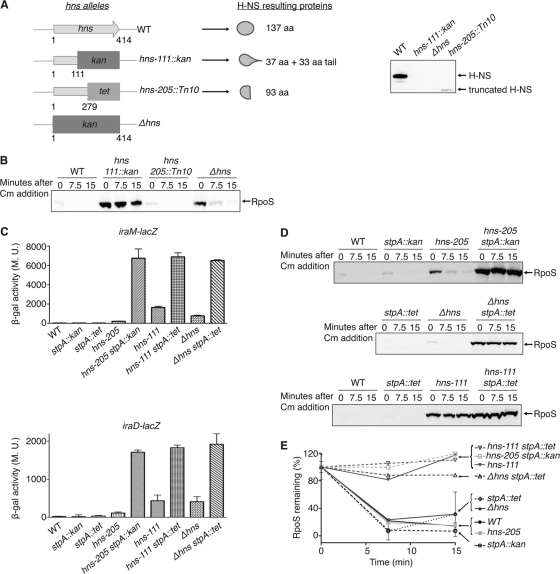
The experiments whose results are shown in Fig. 1 and 2 were performed with an hns-111::kan strain. However, during the course of this study, we used several strains containing different hns insertions and a deletion (Δhns) that we created (Fig. 4A) and observed that they did not have the same effect on RpoS stability (Fig. 4B). In the hns-111::kan strain, a hybrid H-NS protein containing the first 37 amino acids of H-NS and 33 amino acids encoded by the kanamycin resistance cassette is produced (30, 35). The tetracycline insertion in the hns-205::Tn10 strain is after the 93rd codon, leading to the production of a 93-amino-acid truncated H-NS protein (8, 12). In the Δhns strain, the hns gene was fully deleted (see Materials and Methods).
Fig 4.
Effect of different hns alleles and StpA on RpoS stability. (A) Schematic representation of the different hns alleles used in this study and expression of truncated H-NS proteins. The number under the hns arrows corresponds to the hns nucleotide number. aa, amino acids; kan, kanamycin; tet, tetracycline. The wild-type (MG1655), hns-111::kan (AB053), Δhns(BA054), or hns-205::Tn10 (AB052) strains were grown at 32°C overnight in LB. Samples were collected and analyzed by Western blotting using an anti-H-NS antiserum. (B) Effect of the different hns alleles on RpoS stability. Strains were grown at 37°C in LB to an OD600 of ≈0.3, and protein synthesis was inhibited by chloramphenicol (Cm) addition. Samples were then removed at the indicated time points and RpoS levels were analyzed by Western blotting using an anti-RpoS antiserum. The following strains were used: WT (MG1655), hns-111::kan (AB053), hns-205::Tn10 (AB052), and Δhns (BA054). (C) Effect of the different hns alleles on iraM and iraD expression. Strains containing transcriptional fusions iraM-lacZ (WT [AB042], stpA::kan [YMT106], stpA::tet [BA204], hns-205::Tn10 [YMT85], hns-205::Tn10 stpA::kan [YMT108], hns-111::kan [YMT79], hns-111::kan stpA::tet [BA206], Δhns [YMT94], and Δhns stpA::tet [BA214]) or iraD-lacZ (WT [AB050], stpA::kan [YMT105], stpA::tet [BA202], hns-205::Tn10 [YMT84], hns-205::Tn10 stpA::kan [YMT107], hns-111::kan [YMT82], hns-111::kan stpA::tet [BA208], Δhns [YMT95], and Δhns stpA::tet [BA216]) were grown at 37°C in LB. At an OD600 of ≈0.3, samples were taken from each culture and β-galactosidase activity was measured as described by Miller (22). The mean from 3 replicates is presented, and the standard error (SEM) is indicated by the error bars. M.U., Miller units. (D) Strains were grown at 37°C in LB to an OD600 of ≈0.3, and protein synthesis was inhibited by chloramphenicol addition. Samples were then removed at the indicated time points after chloramphenicol addition and RpoS levels were analyzed by Western blotting using an anti-RpoS antiserum. Strains used are WT (MG1655), stpA::kan (BA228), hns-205::Tn10 (AB052), hns-205::Tn10 stpA::kan (BA210), stpA::tet (BA232), Δhns (BA054), Δhns stpA::tet (BA212), hns-111::kan (AB053), and hns-111::kan stpA::tet (BA230). (E) Quantification of RpoS level in the different genetic backgrounds. The intensity measured at time zero for each strain in each experiment was set at 100%. In panels C and E, the mean from 3 replicates is presented and the standard error (SEM) is indicated by the error bars. M.U., Miller units.
Although the hns-111::kan allele fully stabilized RpoS (Fig. 1 and 4B), the hns-205::Tn10 allele as well as the hns deletion showed only a mild effect on RpoS stability (Fig. 4B). RpoS was visible in a WT strain only at 0 min; however, in the strains containing the hns-205::Tn10 and Δhns alleles, the level of RpoS was higher at 0 min (averages, 5-fold for hns-205::Tn10 and 9-fold for Δhns) and we were able to detect some remaining RpoS at the 7.5- and 15-min time points (Fig. 4B).
We next compared the effect of these hns alleles on iraM and iraD expression using transcriptional lacZ fusions. All three alleles increased expression of both fusions compared to wild type, but to different extents (Fig. 4C). In the hns-205::Tn10 strains, the expression of the fusions was reduced compared to that in hns-111::kan strains (Fig. 4C). Thus, the truncated H-NS protein produced in the hns-205::Tn10 strain may still retain some ability to repress the transcription of the antiadaptors. The behavior of the Δhns strain is somewhat harder to explain. This mutation had almost no effect on RpoS stability (Fig. 4B), but the mutant expressed the iraD fusion at a level similar to that seen in the hns-111::kan strain and expressed the iraM fusion at a level lower than that seen in the hns-111::kan strain but well above that seen with the hns-205::Tn10 strain (Fig. 4C). The simplest explanation for these results is that levels of iraM transcription are most critical for stabilization of RpoS in hns mutants; expression of the iraM fusion is highest for hns-111::kan (RpoS fully stable), intermediate for Δhns (RpoS slightly stabilized), and lowest for hns-205::Tn10 (RpoS unstable).
As mentioned earlier, a strain containing the hns-205::Tn10 allele is still able to produce a truncated H-NS protein of 93 amino acids (8). It has been shown previously that this truncated H-NS protein is able to interact with the homologous protein StpA to regulate the bgl promoter and to a lesser extent the proU promoter (10, 11). On the basis of these data, it seemed possible that the inhibition of iraM and iraD expression was not completely abolished in an hns-205::Tn10 strain because the truncated H-NS protein is active in combination with StpA.
We first confirmed that a truncated H-NS protein was produced in our strain (Fig. 4A). We were not able to detect a truncated form of H-NS in the hns-111::kan strain, and, as expected, no H-NS protein was detected in the Δhns strain (Fig. 4A). These results also demonstrate that our antibody does not detect the related StpA protein. The signal obtained for the detection of the 93-amino-acid H-NS form was low (Fig. 4A) and was not improved by deletion of stpA (data not shown), consistent with the observations of Free et al. (10). If the truncated H-NS protein produced in an hns-205::Tn10 strain was still able to repress iraM and iraD expression by interacting with StpA, the deletion of stpA should release this repression. This was tested by introducing a deletion of stpA into the wild-type and hns mutant strains. As has been previously shown for bgl, proU, and other H-NS-repressed genes (10, 11, 30), deletion of stpA in an hns+ strain had no effect on either iraM or iraD expression (Fig. 4C). However, in an hns-205::Tn10 ΔstpA strain, iraM and iraD expression was increased compared to that in a strain containing only the hns-205::Tn10 allele, well above the level seen in the hns-111::kan and Δhns strains (Fig. 4C). Interestingly, a deletion of stpA in the hns-111::kan or Δhns strains also increased the activity of iraM and iraD fusions (Fig. 4C). The results obtained here suggest that StpA plays a role in the regulation of iraM and iraD expression, at least when H-NS is absent or not fully functional. StpA has been described as a backup protein for H-NS (35). In our experiments, we hypothesize that when H-NS is present, StpA has no effect on iraM and iraD expression because the main regulator, H-NS, efficiently silences these genes. In addition, StpA levels may be lower, because H-NS negatively regulates stpA (35). In the absence of H-NS, however, StpA exerts an inhibitory effect on iraM and iraD, although less efficiently than the usual H-NS repression. The deletion of both hns and stpA allows a total derepression of iraM and iraD expression.
In parallel, we looked at the effect of deleting hns, stpA, or both on RpoS stability. As expected, there was no stabilization of RpoS when stpA was inactivated in an hns+ host (Fig. 4D). In the double-deletion strains, the results obtained correlate with what was observed for iraD and iraM expression; all of the double deletions, including hns-205::Tn10 ΔstpA::kan, and Δhns ΔstpA::tet, led to full stabilization of RpoS (Fig. 4D). The deletion of stpA in an hns-111::kan context did not have a strong effect, presumably because RpoS is already stable in the hns-111::kan strain (Fig. 4D, bottom). The quantification of these decay curves suggests a very similar pattern of RpoS degradation for the WT and the hns-205::Tn10 and Δhns derivatives (Fig. 4E).
A possible explanation for why the truncated fragment of H-NS in the hns-111::kan strains leads to stabilization even in an stpA+ host is provided by the work of Wolf et al. (30). They found that the 37-amino-acid-truncated H-NS, linked to a tail of 33 amino acids, is still able to interact with StpA and inhibit stpA transcription. At the same time, this truncated H-NS protein was not able to protect StpA from degradation by the Lon protease (30). Overall, the production of this truncated H-NS protein thus decreases the amount of StpA in the cell and brings it to a level lower than that in a WT cell (in which stpA expression is repressed by H-NS but StpA is stable).
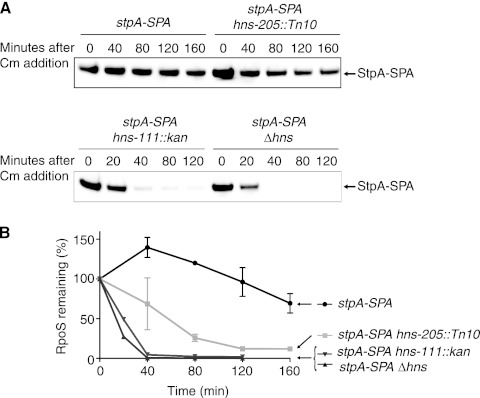
We tested one aspect of this model by inserting a SPA tag at the C terminus of StpA in the chromosome and examining the stability of StpA-SPA. The results of these experiments are shown in Fig. 5. In exponentially growing hns+ cells, StpA-SPA was fully stable. In the hns-205::Tn10 strain, there was slow degradation of StpA-SPA (half-life, 62 min). StpA became significantly more unstable in both the hns-111::kan and the Δhns strains. These results agree well with previous observations on StpA turnover (15, 30). However, we note that the degradation was not sufficient to significantly lower the starting level of StpA in exponential-phase cells (see the 0-min samples in Fig. 5).
Fig 5.
Turnover of StpA-SPA. (A) Strains were grown at 37°C in LB to an OD600 of ≈0.3, and protein synthesis was inhibited by chloramphenicol (Cm) addition. Samples were then removed at the indicated time points after chloramphenicol addition and StpA-SPA levels were analyzed by Western blotting using an anti-Flag antiserum. Strains used are MG1655 stpA-SPA (BA317), stpA-SPA hns-205::Tn10 (BA328), stpA-SPA hns-111::kan (BA330), and stpA-SPA Δhns (BA332). (B) Quantification of StpA-SPA level in the different genetic backgrounds. The intensity measured at time zero for each strain in each experiment was set at 100%. The mean from 2 replicates is presented, and the standard error (SEM) is indicated by the error bars.
The StpA-SPA protein is not fully functional, as judged by RpoS stability in the various hns mutants carrying stpA-SPA in place of stpA+ (see Fig. S1 in the supplemental material). RpoS was stable in the hns-111::kan host, as expected, but was also stable in the Δhns host, a different result from what was seen in an stpA+ host (compare Fig. S1 in the supplemental material to Fig. 4D). However, RpoS was still unstable in the hns-205::Tn10 stpA-SPA strain (Fig. S1 in the supplemental material). These results are discussed further below.
Our results as well as the results of others (30) suggest that the hns-111::kan strain is phenotypically equivalent to a Δhns ΔstpA double mutant strain. This suggests that the truncated form is a gain-of-function allele; the new function is inactivating and/or degrading StpA. This was tested by producing this protein in a Δhns strain. We cloned the sequence coding for the 37 amino acids of H-NS plus the 33-amino-acid tail (pBA339) or the sequence coding only for the 37 first amino acids of H-NS (pBA340) under the control of the H-NS promoter. A Δhns host strain carrying each of these plasmids or the empty vector was examined for RpoS stability. The presence of the empty vector had no effect on RpoS stability; RpoS was visible only at 0 min (Fig. 6). However, when we produced the first 37 amino acids of H-NS plus the tail, the protein expressed in hns-111::kan, we observed a strong stabilization of RpoS, comparable to what was observed in an hns-111::kan strain (Fig. 6). The production of a truncated H-NS protein containing only the first 37 amino acids (without the tail) barely increased RpoS stability; we observed a higher level of RpoS at 0 min, and RpoS was still present after 7.5 min (Fig. 6). This is consistent with what was observed by Wolf et al. (30). In their study, a truncated H-NS protein of 42 amino acids produced in an hns::amp strain did not inhibit stpA transcription as much as the truncated H-NS protein plus tail.
Fig 6.
The production of a truncated H-NS protein containing the first 37 amino acids stabilizes RpoS. Strains containing pBA339 (producing an H-NS truncated protein of 37 amino acids plus 33 amino acids of the tail), pBA340 (producing an H-NS truncated protein of 37 amino acids), or the vector control (pACYC) were grown at 37°C in LB to an OD600 of ≈0.3. Protein synthesis was inhibited by chloramphenicol (Cm) addition. Samples were then removed at the indicated time points after chloramphenicol addition and RpoS levels were analyzed by Western blotting using an anti-RpoS antiserum. Strains used were Δhns (BA054) and hns-111::kan (AB053).
DISCUSSION
H-NS effect on RpoS via antiadaptors.
RpoS stabilization in an hns mutant strain has been reported in several studies, but the basis for this phenotype has never been fully explained (1, 32, 36). In this paper, we show that H-NS inhibits the expression of IraM and IraD, two antiadaptor proteins involved in RpoS stabilization under different stress conditions. Stabilization in an hns mutant is almost completely reversed by deleting the genes coding for these two antiadaptors.
H-NS is likely to have at least one other target important for RpoS stabilization, since a strain with hns, iraD, and iraM deletions still shows some residual RpoS stabilization (Fig. 1D). Possibly, a novel antiadaptor is regulated by H-NS. Interestingly, it has been shown in Salmonella that StpA, a protein homologous to H-NS, regulates RssC, an antiadaptor protein most similar to IraM, but the promoter is not highly conserved. As in E. coli, StpA may regulate multiple antiadaptor proteins in Salmonella, since deletion of rssC does not fully suppress a mutation in stpA (19).
Overall, these results suggest a similar pathway in E. coli and Salmonella to ensure RpoS degradation during exponential growth, with H-NS being the major silencer of antiadaptors in E. coli and StpA being the primary regulator in Salmonella (Fig. 7).
Fig 7.
Model of antiadaptor regulation. Antiadaptor proteins are activated under different stress conditions by various regulators. When expressed, they titrate RssB and allow RpoS stabilization. H-NS inhibits iraM and iraD expression and also affects phoP and phoQ expression. StpA collaborates with H-NS to repress iraM and iraD and may also play an additional role in regulating RpoS degradation.
Interaction of H-NS and StpA for regulation.
Studies of the effects of H-NS have been complicated by the observation that different hns alleles, often all described as null alleles, in fact produce truncated H-NS proteins that display regulatory activity. In the present study, we have used three different hns alleles, each displaying different activities. All three alleles led to increased expression of iraM and iraD, although to various extents (Fig. 4C). Surprisingly, the derepression of the antiadaptor proteins led to RpoS stabilization only in the hns-111::kan strain, although a deletion of hns induced iraM and iraD expression to an extent similar to that seen in the hns-111::kan strain (Fig. 4B and C). The continued degradation of RpoS in the other strains depended on the presence of StpA, partially substituting for H-NS, although inactivation of stpA in an hns+ host had no effect on expression of the antiadaptors or RpoS turnover (Fig. 4C and D).
We suggest that these results can be explained by the effects of the different hns alleles on StpA. H-NS represses stpA transcription but also forms multimers with StpA and protects the StpA protein from degradation by the Lon protease (15, 29, 35). The region of H-NS needed for StpA stabilization lies in amino acids 39 to 60 (14). Whether negative effects of H-NS derivatives on stpA transcription and/or StpA activity or the positive effect on StpA stability is more important may depend on both the hns allele and the target promoter. In strains carrying hns-205::Tn10, producing an H-NS truncated protein containing amino acids 1 to 93, StpA is stable but its expression is repressed, due to the interaction of the H-NS truncated protein with StpA. The stable StpA/H-NS complex is apparently sufficient for partial silencing of the iraM and iraD promoters (Fig. 4C); we suggest that this low level of antiadaptor expression may not be enough to stabilize RpoS (Fig. 4B and D).
StpA became unstable in a strain with an hns deletion (Δhns) and in a strain producing the H-NS truncated protein containing 37 amino acids plus a tail (hns-111::kan) (Fig. 5). This is consistent with previous observations (15, 30). However, RpoS is unstable in Δhns strains but not in hns-111::kan strains (Fig. 4). While we do not currently have a full explanation for this difference, the explanation is most likely to reflect different activities and/or amounts of StpA in the two strains. StpA levels would be expected to be higher in the Δhns strain, because the hns-111::kan strain can still partially repress stpA transcription, at least in the presence of active StpA (30). This result also suggests that hns-111::kan is still able to multimerize with StpA, consistent with previous work on regulation of bgl (30).
One explanation of the differential behavior of these two hns alleles for RpoS stability is based on expression of the iraM antiadaptor. We note that the expression of the iraM transcriptional fusion is higher in the hns-111::kan strain (1,600 units) than in the Δhns strain (770 units) (Fig. 4C). Levels of expression of the iraD transcriptional fusion were similar for the two hns alleles (200 units). Thus, it may be that in the Δhns strain, the level of expression of neither IraM nor IraD is sufficiently high to stabilize RpoS, while the increase in IraM expression in the hns-111::kan host is sufficient for stabilization. In fact, we see a larger dependence on IraM than on IraD for stabilization in the hns-111::kan strain (compare Fig. 1C and B). The differences in StpA status and level in the two mutants would presumably explain the differences in transcription.
An alternative explanation is that StpA has an additional role in regulating RpoS stability, apart from transcriptional regulation of iraM and iraD. This additional role could reflect a direct or indirect effect of StpA on translation of one or both of the antiadaptors or possibly an effect on some other target capable of overcoming the antiadaptors. StpA has been shown to bind to RNA (34), and both antiadaptor transcripts have reasonably long untranslated regions, so an effect on translation is certainly possible. In this model, the differences in StpA levels could again be critical, or possibly, the heterodimer between hns-111::kan and StpA blocks this second role of StpA. The gain-of-function activity of hns-111::kan (Fig. 6) suggests that the truncated protein may specifically interact with StpA, inactivating it by multimerizing with it. Further work will be necessary to explore these possibilities, which are not mutually exclusive.
Antiadaptors as horizontally transferred genes.
H-NS is known to repress expression of a large number of apparently unrelated genes by binding to AT-rich regions (reviewed in reference 9). Since the GC content of a gene is characteristic of its organism of origin, it has been proposed that H-NS may silence horizontally acquired genes (19, 24, 28). A number of studies have listed iraM and iraD as horizontally acquired genes and demonstrated association with H-NS (25, 28). In addition, the genome context of the antiadaptor genes is conserved only in E. coli and Shigella species, and the GC contents of iraD (48.9%) and iraM (33.9%) are both somewhat lower than what is expected for an E. coli gene (50.8%). Thus, iraD and iraM are likely transferred from another organism, with inhibition by H-NS contributing to maintaining preexisting regulation in E. coli.
Recently horizontally transferred genes may also have suboptimal codon usage, and purification of both IraM and IraD was aided by overproduction of rare tRNAs (3). A mutation in yhhP was previously identified to be able to partially restore RpoS degradation in an hns mutant (31). This mutant also affected RpoS stability in a WT strain, as well as impaired cell growth, making it unclear whether the role of the yhhP mutations was directly linked to H-NS. YhhP has now been renamed SirA (also called TusA) and identified to be an enzyme involved in modification of uridine at the wobble position of tRNAs for glutamate, glutamine, and lysine (13). This modification is important for accurate translation. It seems possible that the yhhP mutation leads to decreased translation of iraM and iraD; such a decrease would be expected to partially suppress the hns mutant and restore RpoS degradation.
Taken together, these results reinforce the importance of antiadaptors in the regulation of RpoS degradation. As previously shown, each of the three known antiadaptors, when fully induced or overexpressed, is capable of stabilizing RpoS (3, 5). Here we find that lack of H-NS leads to RpoS stabilization also via the antiadaptors. In addition, our results point to the likelihood of yet additional levels of control by StpA of antiadaptor synthesis and/or activity.
Supplementary Material
ACKNOWLEDGMENTS
We thank A. Bougdour for strains and advice throughout this work and comments on the manuscript. We thank K. Ramamurthi, S. Wickner, and members of the Gottesman lab for comments on the manuscript.
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Published ahead of print 9 March 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Barth M, Marschall C, Muffler A, Fischer D, Hengge-Aronis R. 1995. Role for the histone-like protein H-NS in growth phase-dependent and osmotic regulation of σS and many σS-dependent genes in Escherichia coli. J. Bacteriol. 177:3455–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Battesti A, Majdalani N, Gottesman S. 2011. The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 65:189–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bougdour A, Cunning C, Baptiste PJ, Elliott T, Gottesman S. 2008. Multiple pathways for regulation of σS (RpoS) stability in Escherichia coli via the action of multiple anti-adaptors. Mol. Microbiol. 68:298–313 [DOI] [PubMed] [Google Scholar]
- 4. Bougdour A, Gottesman S. 2007. ppGpp regulation of RpoS degradation via an anti-adaptor protein IraP. Proc. Natl. Acad. Sci. U. S. A. 104:12896–12901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bougdour A, Wickner S, Gottesman S. 2006. Modulating RssB activity: IraP, a novel regulator of σS stability in Escherichia coli. Genes Dev. 20:884–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Butland G, et al. 2005. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature 433:531–537 [DOI] [PubMed] [Google Scholar]
- 7. Cherepanov PP, Wackernagel W. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14 [DOI] [PubMed] [Google Scholar]
- 8. Dersch P, Kneip S, Bremer E. 1994. The nucleoid-associated DNA-binding protein H-NS is required for the efficient adaptation of Escherichia coli K-12 to a cold environment. Mol. Gen. Genet. 245:255–259 [DOI] [PubMed] [Google Scholar]
- 9. Fang FC, Rimsky S. 2008. New insights into transcriptional regulation by H-NS. Curr. Opin. Microbiol. 11:113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Free A, Porter ME, Deighan P, Dorman CJ. 2001. Requirement for the molecular adapter function of StpA at the Escherichia coli bgl promoter depends upon the level of truncated H-NS protein. Mol. Microbiol. 42:903–917 [DOI] [PubMed] [Google Scholar]
- 11. Free A, Williams RM, Dorman CJ. 1998. The StpA protein functions as a molecular adapter to mediate repression of the bgl operon by truncated H-NS in Escherichia coli. J. Bacteriol. 180:994–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins CF, et al. 1988. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell 52:569–584 [DOI] [PubMed] [Google Scholar]
- 13. Ikeuchi Y, Shigi N, Kato J, Nishimura A, Suzuki T. 2006. Mechanistic insights into sulfur relay by multiple sulfur mediators involved in thiouridine biosynthesis at tRNA wobble positions. Mol. Cell 21:97–108 [DOI] [PubMed] [Google Scholar]
- 14. Johansson J, Eriksson S, Sonden B, Wai SN, Uhlin BE. 2001. Heteromeric interactions among nucleoid-associated bacterial proteins: localization of StpA-stabilizing regions in H-NS of Escherichia coli. J. Bacteriol. 183:2343–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johansson J, Uhlin BE. 1999. Differential protease-mediated turnover of H-NS and StpA revealed by a mutation altering protein stability and stationary phase survival of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 96:10776–10781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klauck E, Lingnau M, Hengge-Aronis R. 2001. Role of the response regulator RssB in σS recognition and initiation of σS proteolysis in Escherichia coli. Mol. Microbiol. 40:1381–1390 [DOI] [PubMed] [Google Scholar]
- 17. Kong W, Weatherspoon N, Shi Y. 2008. Molecular mechanisms for establishment of signal-dependent regulation in the PhoP/PhoQ system. J. Biol. Chem. 283:16612–16621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lang B, et al. 2007. High-affinity DNA binding sites for H-NS provide a molecular basis for selective silencing within proteobacterial genomes. Nucleic Acids Res. 35:6330–6337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lucchini S, McDermot P, Thompson A, Hinton JCD. 2009. The H-NS-like protein StpA represses the RpoS (σ38) regulon during exponential growth of Salmonella Typhimurium. Mol. Microbiol. 74:1169–1186 [DOI] [PubMed] [Google Scholar]
- 20. Merrikh H, Ferrazzoli AE, Bougdour A, Olivier-Mason A, Lovett ST. 2009. A DNA damage response in Escherichia coli involving the alternative sigma factor RpoS. Proc. Natl. Acad. Sci. U. S. A. 106:611–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Merrikh H, Ferrazzoli AE, Lovett ST. 2009. Growth phase and (p)ppGpp control of IraD, a regulator of rpoS stability, in Escherichia coli. J. Bacteriol. 191:7436–7446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller JH. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 23. Muffler A, Fischer D, Altuvia S, Storz G, Hengge-Aronis R. 1996. The response regulator RssB controls stability of the σS subunit of RNA-polymerase in Escherichia coli. EMBO J. 15:1333–1339 [PMC free article] [PubMed] [Google Scholar]
- 24. Navarre WW, et al. 2006. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 313:236–238 [DOI] [PubMed] [Google Scholar]
- 25. Oshima T, Ishikawa S, Kurokawa K, Aiba H, Ogasawara N. 2006. Escherichia coli histone-like protein H-NS preferentially binds to horizontally acquired DNA in association with RNA polymerase. DNA Res. 13:141–153 [DOI] [PubMed] [Google Scholar]
- 26. Pratt LA, Silhavy TJ. 1996. The response regulator SprE controls the stability of RpoS. Proc. Natl. Acad. Sci. U. S. A. 93:2488–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sharan SK, Thomason LC, Kuznetsov SG, Court DL. 2009. Recombineering: a homologous recombination-based method of genetic engineering. Nat. Protoc. 4:206–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shimada T, Bridler A, Briandet R, Ishihama A. 2011. Novel roles of LeuO in transcription regulation of E. coli genome: antagonistic interplay with the universal silencer H-NS. Mol. Microbiol. 82:378–397 [DOI] [PubMed] [Google Scholar]
- 29. Williams RM, Rimsky S, Buc H. 1996. Probing the structure, function, and interactions of the Escherichia coli H-NS and StpA proteins by using dominant negative derivatives. J. Bacteriol. 178:4335–4343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wolf T, Janzen W, Blum C, Schnetz K. 2006. Differential dependence of StpA on H-NS in autoregulation of stpA and in regulation of bgl. J. Bacteriol. 188:6728–6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamashino T, Isomura M, Ueguchi C, Mizuno T. 1998. The yhhP gene encoding a small ubiquitous protein is fundamental for normal cell growth of Escherichia coli. J. Bacteriol. 180:2257–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamashino T, Ueguchi C, Mizuno T. 1995. Quantitative control of the stationary phase-specific sigma factor, σS, in Escherichia coli: involvement of the nucleoid protein H-NS. EMBO J. 14:594–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu DG, et al. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 97:5978–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang A, Derbyshire V, Salvo JL, Belfort M. 1995. Escherichia coli protein StpA stimulates self-splicing by promoting RNA assembly in vitro. RNA 1:783–793 [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang A, Rimsky S, Reaban ME, Buc H, Belfort M. 1996. Escherichia coli protein analogs StpA and H-HNS: regulatory loops, similar and disparate effects on nucleic acid dynamics. EMBO J. 15:1340–1349 [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou Y, Gottesman S. 2006. Modes of regulation of RpoS by H-NS. J. Bacteriol. 188:7022–7025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou Y, Gottesman S, Hoskins JR, Maurizi MR, Wickner S. 2001. The RssB response regulator directly targets σS for degradation by ClpXP. Genes Dev. 15:627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.