Abstract
We previously reassigned the amber UAG stop triplet as a sense codon in Escherichia coli by expressing a UAG-decoding tRNA and knocking out the prfA gene, encoding release factor 1. UAG triplets were left at the ends of about 300 genes in the genome. In the present study, we showed that the detrimental effect of UAG reassignment could be alleviated by increasing the efficiency of UAG translation instead of reducing the number of UAGs in the genome. We isolated an amber suppressor tRNAGln variant displaying enhanced suppression activity, and we introduced it into the prfA knockout strain, RFzero-q, in place of the original suppressor tRNAGln. The resulting strain, RFzero-q3, translated UAG to glutamine almost as efficiently as the glutamine codons, and it proliferated faster than the parent RFzero-q strain. We identified two major factors in this growth enhancement. First, the sucB gene, which is involved in energy regeneration and has two successive UAG triplets at the end, was expressed at a higher level in RFzero-q3 than RFzero-q. Second, the ribosome stalling that occurred at UAG in RFzero-q was resolved in RFzero-q3. The results revealed the importance of “backup” stop triplets, UAA or UGA downstream of UAG, to avoid the deleterious impact of UAG reassignment on the proteome.
INTRODUCTION
Most organisms, from Escherichia coli to humans, use the “universal” genetic code, which was presumably established in the ancestor of all organisms. The code has not changed over billions of years, except for rare deviations established in mitochondria and the nuclear genomes of certain organisms (23, 31). The UAA and UAG triplets, which mean “translation stop” in the universal code, specify glutamine in the nuclear code of ciliates, UGA specifies tryptophan in the eubacterium Mycoplasma (34), and the “leucine” CUG codon primarily specifies serine in certain yeasts (15, 28, 32). The development of these deviations is a slow evolutionary process, established over tens or hundreds of thousands of years (16). There are two theories explaining the emergence of such noncanonical codes. The “Osawa and Jukes” theory assumes that the codon to be redefined first disappears from the genome and then reappears with a new meaning (22). The other theory assumes the status of “ambiguous” decoding, in which the codon to be redefined gains a second meaning while it retains the original meaning at the same time (29). The genome of the organism then gradually adapts to the new meaning, and the original meaning is eventually lost safely. Both scenarios hypothesize the accumulation of a large number of mutations in the genome prior to the codon reassignment event.
We previously achieved the reassignment of the amber UAG stop triplet as a sense codon in E. coli by expressing a UAG-decoding tRNA and knocking out the prfA gene, which encodes release factor 1 (RF-1) (18). RF-1 is the essential cellular component recognizing UAG to terminate translation, and its elimination from the cell should be lethal (26, 30). To avoid this lethality, we engineered UAA ends for the seven essential open reading frames (ORFs) ending with UAG, and we introduced them into E. coli, leaving the remaining 300 UAG stop triplets intact in the genome. The expression of a UAG-decoding tRNA was also necessary to avoid lethality. The new meaning of UAG corresponds to the amino acid specificity of this tRNA, and the triplet has thus been reassigned to glutamine, tyrosine, and various nonnatural amino acids (18, 19). The E. coli strain lacking prfA, called RFzero, provides a laboratory model of genetic-code evolution.
The most obvious phenotype of RFzero is that it does not grow as fast as the parent strain. There were two conceivable effects of the UAG reassignment at the molecular level. First, the characteristics of the UAG-decoding tRNA and its cognate aminoacyl-tRNA synthetase (aaRS) are not optimized to translate UAG. Therefore, the efficiency of reassigned UAG codon translation should be lower than those for translation of other sense codons. Second, the translation of UAG-ending ORFs should proceed downstream of UAG, and their products are expected to have extra C-terminal peptides. In the present study, we showed that increasing the efficiency of UAG translation enhances the growth of RFzero. The facilitated expression of the extended gene products conferred favorable effects on the growth. Their expression assumes the presence of a second stop triplet, UAA or UGA, downstream of UAG. The results implied that the E. coli genome has prepared itself for the UAG redefinition, with most of the UAG-ending ORFs followed by such “backup” stop triplets.
MATERIALS AND METHODS
Strains and plasmids.
HST08 and DH10B were purchased from TaKaRa Bio Inc. (Otsu, Japan) and Invitrogen (Carlsbad, CA), respectively. The RFzero strains translating UAG to nonnatural amino acids were described previously (19). Luria-Bertani (LB) medium was prepared using LB broth (Miller) purchased from Nacalai Tesque (Kyoto, Japan). The premixed M9 minimal medium salts were purchased from MP Biomedical Japan (Tokyo, Japan). The minimal medium used in this study contained M9 salts, magnesium sulfate (1 mM), and glycerol (0.5%, wt/vol). The optical densities of the cell cultures were measured by an Ultrospec spectrophotometer (GE Healthcare, Chalfont St. Giles, United Kingdom). The amber mutant chloramphenicol acetyltransferase genes cat(3amb) and cat(10amb) were previously cloned in pACYC184-kan, a kanamycin-resistant derivative of pACYC184 (18). The three glutamine codons remaining in cat(10amb) were replaced with UAG to create cat(13amb), and the resulting plasmid was designated pCAT(13amb). The sucB(UAA) and sucB(CAG) genes were cloned downstream of the bla gene in pBR322 for transcription from the bla promoter, and the resulting plasmids were named pBRsucB(UAA) and pBRsucB(CAG), respectively. The sdhCDAB-sucABCD operon was cloned in the pAp105 plasmid, a derivative of pAp102 (27) with the kanamycin resistance gene in place of bla, to create pApsucB. The sucB coding sequence in the plasmid was replaced with sucB, sucB(UAA), and sucB(CAG), each N-terminally tagged with a FLAG peptide (DYKDDDDK), to create pApsucB, pApsucB(UAA), and pApsucB(CAG), respectively. The yaeJ gene was cloned between the lpp promoter and terminator inserted in pAp105 and pApsucB to create pApyaeJ and pApsucB-yaeJ, respectively. The double UAG at the end of sucB on pApsucB-yaeJ was replaced with UAA and a double CAG to create pApsucB(UAA)-yaeJ and pApsucB(CAG)-yaeJ, respectively. The “fixed” prfB and prfB* genes were cloned in pAp105, together with the upstream sequence starting from just after the recJ ORF, to create pApnPrfBf and pApnPrfB*, respectively. These genes with the upstream sequence were also cloned downstream of the kanamycin resistance gene of pAp105 to create pApkPrfBf and pApkPrfB*, respectively. The plasmid pACYC-TRX-GSTam, carrying the gene encoding a fusion protein of thioredoxin and glutathione S-transferase (GST) in this order, with a UAG triplet at the beginning of GST, was previously described (18).
Construction of the library of supE tRNA variants.
The sequence containing the tyrT promoter, TTCTCAACGTAACACTTTACAGCGGCGCGTCATTTGATATGATGCGCCCCGCTTCCCGATAAGGGAGCAGGCCAGTAAAAAGGAT, and the sequence containing the rrnC terminator, AAATTTTTGATCCTTAGCGAAAGCTAAGGATTTTTTTTATCGCGA, were connected to each other by a BstXI recognition sequence (CCATCAGATTGG) and then inserted between the SphI and SalI sites of the pACYC184-kan vector carrying cat(3amb) to create the plasmid pKS3cat(3amb). A double-stranded DNA molecule encoding the sequence of randomly mutagenized supE tRNA was constructed by annealing three oligomers with the following sequences: CGCATTTGGGGTATCGCCAAGCGGTAAGGCACCGNNNNCTANNNNCGGCATTCCGAGGTTCGAATCCTCGTACCCCAGCCATTTATCACAGA (OLG1), TGATAAATGGCTGGGGTACGAGGATTCGAACCTCGGAATGCCG (OLG2), and CGGTGCCTTACCGCTTGGCGATACCCCAAATGCGTCTG (OLG3). In these sequences, N represents any of the four bases, and the italicized letters represent the nucleotides outside the tRNA coding sequence. Before annealing, the OLG1 and OLG2 oligomers (200 pmol each) were separately phosphorylated at the 5′ end in a 20-μl reaction mixture containing 50 mM Tris-HCl (pH 8.0), 10 mM MgCl2, 10 mM 2-mercaptoethanol, 4.7 mM ATP, and T4 polynucleotide kinase (10 units), at 37°C for 30 min. These oligomers were mixed together, and OLG3 (200 pmol) was then added to the mixture. The annealing was performed at 94°C for 0.5 min, 80°C for 1 min, 74°C for 1 min, 65°C for 0.5 min, and 55°C for 1 min in this order, using a Veriti thermal cycler (Applied Biosystems Japan, Tokyo, Japan), and thus a double-stranded DNA molecule with a gap in the “template” strand was formed. The gap corresponded to the anticodon moiety and eight neighboring randomized nucleotides on the nontemplate strand, and it should be filled by the cellular DNA polymerase after transformation. The annealed oligomers were purified by centrifugation through a MicroSpin S200-HR column (GE Healthcare) to remove the ATP and were ligated with the BstXI-digested pKS3cat(3amb) plasmid to clone the variant gene downstream of the tyrT promoter. DH10B, with no suppressor tRNA, was transformed with the ligation products. A library consisting of 2 × 107 clones was thus generated on an LB plate containing kanamycin (30 μg/ml). A total of 23 clones were subjected to sequence analysis, and 15 of them (65%) contained complete tRNA sequences, whereas the others either lacked the entire tRNA sequence or had nucleotides missing in the randomized region. An analysis of the frequencies of the occurrence of the four bases at each position revealed that they were similarly represented in the randomized region.
Chromosome engineering.
Homologous recombination, facilitated by the bacteriophage λ recombination enzymes (20, 21), was employed to modify the E. coli chromosome. The replacement of the glnX gene with cat in HST08 and the disruption of prfA with the Zeocin-resistance gene were performed as described previously (18). We similarly substituted supE3 and supE7 for the knocked-in cat at the supE locus and cat for sucB. The enrichment of chloramphenicol-sensitive cells was performed as described previously (10). Chromosomal DNA was prepared using GenTLE (TaKaRa Bio) for sequence analysis.
Production of GST.
The mutant gst(6amb) gene was expressed from the plasmid pTacGST(6Am) (18). All of these UAG codons were replaced by CAG to create gst(6gln), which was expressed from pTacGST(6gln). The expression of gst(6amb) and gst(6gln) was induced by the addition of isopropyl-β-d-thiogalactopyranoside (Naclai Tesque) to a final concentration of 1 mM. Equal volumes of the cell cultures were obtained from RFzero-q, -q3, and -q7, 4 h after the induction. The GST activity was assayed using a GST detection module (GE Healthcare). The antibody against GST was included in this module.
Analysis of sucB expression.
The cells transformed with pApsucB and pApsucB-yaeJ and their derivatives were grown at 37°C in 100 ml of LB medium containing 15 mg/liter kanamycin and were harvested from a 50-ml aliquot of the culture after overnight incubation. The wet weights of the harvested cells were measured. The same amounts of the cells were lysed and analyzed by SDS-polyacrylamide gel electrophoresis, followed by Western blotting with the antibody against the FLAG peptide.
Isolation of fast-growing RFzero.
RFzero-py is a BW25113-based RFzero strain translating UAG to lysine derivatives, including Nε-allyloxycarbonyl-l-lysine, as previously described (19). This strain was grown in LB supplemented with the α-hydroxy acid analog of Nε-(tert-butoxycarbonyl)-l-lysine, commercially synthesized by Shinsei Chemical Co. Ltd. (Osaka, Japan), at a concentration of 10 mg/liter at 37°C. The expression plasmid for the mutant GST gene with the amber codon at position 25 was previously described (10). Mass spectrometry was performed using a Voyager DE-STR spectrometer (Applied Biosystems, Inc.).
RESULTS
Isolation of supE tRNA variants displaying enhanced amber suppression activities.
We previously created a prfA knockout strain that translates UAG as glutamine, based on HST08, a K-12 strain expressing the amber suppressor tRNAGln (18). The created strain was named RFzero-q (Table 1). The amber suppressor tRNAGln or supE tRNA is encoded by the glnX mutant gene with the supE44 mutation in RFzero-q. This mutation is a G-to-A transition at the third position of the anticodon of tRNA2Gln (9), which changes the codon specificity of the tRNA from the CAG glutamine codon to the amber UAG stop triplet. RFzero-q can express a mutant chloramphenicol acetyltransferase (CAT) gene, cat(10amb), bearing UAG codons in place of 10 glutamine codons (18). The level of chloramphenicol (Cm) resistance conferred by cat(10amb) is lower than that conferred by the wild-type cat gene. This observation indicated that the supE tRNA still translates UAG less efficiently in the absence of RF-1, compared to tRNAGln translation of the glutamine codons. Translation by tRNA mainly involves two distinct activities: aminoacylation and codon recognition. A base change at the third anticodon position of tRNAGln reportedly causes a moderate reduction in the aminoacylation activity (8, 13). Instead of engineering glutaminyl-tRNA synthetase (GlnRS) to restore the activity, we attempted to enhance the UAG-reading efficiency of supE tRNA.
Table 1.
Genotypes and sources of the strains used in the present study
| Strain | Genotype | Source or reference |
|---|---|---|
| HST08 | F−endA1supE44thi-1recA1relA1gyrA96phoA ΔmcrA ϕ80dlacZΔM15 (lacZYA-argF)U169 Δ(mrr-hsdRMS-mcrBC) λ− | TaKaRa Bio Inc. |
| HST08-3 | HST08, supE3 substituting for supE44 | This study |
| HST08-7 | HST08, supE7 substituting for supE44 | This study |
| RFzero-q | HST08, BAC7gent, prfA::zeo | 18 |
| RFzero-q3 | HST08-3, BAC7gent, prfA::zeo | This study |
| RFzero-q7 | HST08-7, BAC7gent, prfA::zeo | This study |
| HST08[ΔsucB] | HST08, sucB::cat | This study |
| RFzero-q[ΔsucB] | RFzero-q, sucB::cat | This study |
| RFzero-q3[ΔsucB] | RFzero-q3, sucB::cat | This study |
| RFzero-iy | BW25113, BAC8, prfA::zeo, piodoTyrRS-MJR1-gent | 19 |
| RFzero-py | RFzero-iy, pPylF (a plasmid expressing a PylRS variant and tRNAPyl) | 19 |
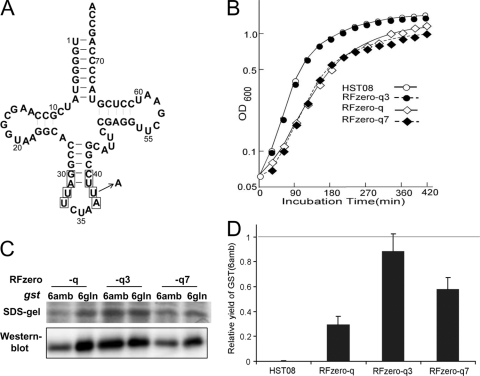
We constructed a library of supE tRNA variants by randomizing the nucleotides at positions 30 to 40, except for the anticodon moiety (positions 34, 35, and 36) (Fig. 1A). DH10B cells were transformed with this library, together with a mutant cat gene with three in-frame UAG codons [cat(3amb)]. We obtained 18 clones growing on an LB plate containing Cm at a concentration of 150 μg/ml, while the parent supE tRNA confers resistance to 150 to 200 μg/ml Cm. The Cm resistance levels of these clones were then determined by inoculating them on LB plates containing Cm at 150 to 500 μg/ml. All of them displayed higher levels of Cm resistance than the cells expressing the supE tRNA (Table 2), suggesting that the isolated supE tRNA variants translate UAG more efficiently than the parent. A sequence analysis revealed that these variants contained 13 different tRNA sequences, but all of them had U33, A37, and nucleotides 30 and 40 base paired with each other; therefore, these three characteristics are probably essential for efficient UAG decoding. Variants 2, 3, 4, 5, 7, 9, and 11 conferred the highest levels of Cm resistance, and variant 3 was identical to the parent supE tRNA, except for a U-to-A substitution at position 38 (Fig. 1A). The U38A substitution was present in six of the highest-ranking variants, except for variant 2, and probably contributed to efficient UAG decoding. On the other hand, variants 4 and 7 differed the most from the parent tRNA, with six base substitutions at the eight randomized positions. We decided to use variants 3 and 7, with the smallest and largest numbers of mutations, respectively, relative to the parent tRNA, to achieve efficient UAG translation in RFzero. These variants were designated supE3 and supE7.
Fig 1.
Growth enhancement by increasing UAG decoding efficiency in RFzero. (A) Secondary structure of the unmodified supE tRNA. The nucleotides in the boxes were randomized. The indicated U-to-A change at position 38 occurs in the supE3 tRNA. The nucleotides are numbered according to the standard numbering system for tRNA. (B) Growth profiles of HST08 and RFzero-q, -q3, and -q7 in LB medium with no antibiotic at 37°C. HST08 did not harbor BAC7gent. (C) Western blot analysis of the gst(6amb) and gst(6gln) products in the RFzero-q strains. The products were subjected to SDS-polyacrylamide gel electrophoresis and then stained or detected using the antibody against GST. (D) Yields of the gst(6amb) product in the HST08 and RFzero strains, relative to those of the gst(6gln) products in the same strains.
Table 2.
Cm resistance conferred by the supE tRNA variants on DH10B
| tRNAc | Base sequenceb | Resistance at Cm concna |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 150 | 200 | 250 | 300 | 350 | 400 | 450 | 500 | ||
| supE | GAUU—AUUC | + | ± | − | − | − | − | − | − |
| 1 | GUCU—AAUC | + | + | + | + | + | + | ± | − |
| 2, (6) | GCUU—ACUC | + | + | + | + | + | + | + | − |
| 3 | GAUU—AAUC | + | + | + | + | + | + | + | − |
| 4 | UUCU—AAAG | + | + | + | + | + | + | + | − |
| 5 | GGCU—AACC | + | + | + | + | + | + | + | − |
| 7, (8) | CUCU—AAAG | + | + | + | + | + | + | + | − |
| 9, (13, 17) | UACU—AAUA | + | + | + | + | + | + | + | − |
| 10, (12) | GACU—AACC | + | + | + | + | + | + | − | − |
| 11 | GCCU—AAUU | + | + | + | + | + | + | + | − |
| 14 | ACCU—AACU | + | + | + | + | + | − | − | − |
| 15 | ACCU—AAAU | + | + | + | + | ± | − | − | − |
| 16 | CGCU—AACG | + | + | + | + | + | + | ± | − |
| 18 | GCCU—AACC | + | + | + | + | + | + | ± | − |
Cm concentration (mg/liter) in the growth medium. +, resistance; ±, weak resistance; −, susceptibility.
The base sequence from positions 29 to 40, with the invariant anticodon sequence omitted. Bold letters represent the nucleotides that differ from those of the supE tRNA at the corresponding positions.
The number(s) in parentheses shows the second (and third) clone(s) with the same sequence.
Introduction of supE3 into RFzero-q enhances the bacterial growth.
RFzero-q was previously created by knocking out prfA in the HST08 strain transformed with the BAC7gent plasmid, which carried the seven essential UAG-ending ORFs engineered to end with UAA (18). Before creating RFzero strains expressing the isolated supE tRNA variants, we engineered HST08 by replacing the supE coding sequence in the chromosome with the supE3 and supE7 sequences to create HST08-3 and HST08-7, respectively (Table 1). The replacements were confirmed by sequencing the glnX loci of the created strains.
We examined the Cm resistance levels of HST08-3 and HST08-7 harboring cat(3amb). HST08-7 exhibited a resistance level (10 to 17 μg/ml Cm) similar to that of the parent HST08 strain, while the resistance level (50 μg/ml Cm) of HST08-3 was significantly higher. This observation suggested that the supE3 tRNA translates UAG more efficiently than the supE7 tRNA. The prfA gene was then knocked out in the HST08-3 and HST08-7 strains, each transformed with BAC7gent, to create RFzero-q3 and RFzero-q7, respectively. The knockout was confirmed by sequencing the prfA loci of the chromosomes of the created strains.
We compared the growth rates of HST08 and the RFzero-q, -q3, and -q7 strains in LB medium at 37°C. RFzero-q grew slowly, compared with the parent HST08, while RFzero-q7 displayed a minimally improved growth profile (Fig. 1B). On the other hand, the growth profile of RFzero-q3 was indistinguishable from that of HST08, revealing the favorable effect of the supE3 mutation on cell proliferation.
RFzero-q3 translates UAG almost as efficiently as the glutamine codons.
To examine if the UAG-decoding efficiency was actually improved in RFzero-q3 and -q7, we transformed these strains with the mutant cat gene with all 13 of the glutamine codons replaced by UAG or cat(13amb). The wild-type cat conferred resistance up to 400 μg/ml Cm on HST08 and RFzero-q (Table 3). RFzero-q and -q7 expressed the cat(13amb) mutant and exhibited Cm resistance up to 200 μg/ml. On the other hand, RFzero-q3 with cat(13amb) displayed Cm resistance almost equal to that conferred by the wild-type cat. This observation suggested that the efficiency of UAG translation in RFzero-q3 was almost equal to that for glutamine codon translation.
Table 3.
Growth of RFzero-q strains transformed with the cat genes on Cm-containing platesa
| Strain | cat gene/plasmid | Growth at CM concnb |
||||
|---|---|---|---|---|---|---|
| 100 | 200 | 300 | 400 | 500 | ||
| HST08 | WT/pACYC184-kan | + | + | + | + | ± |
| RFzero-q | WT/pACYC184-kan | + | + | + | + | ± |
| RFzero-q | cat(13amb)/pCAT(13amb) | + | + | ± | − | − |
| RFzero-q3 | cat(13amb)/pCAT(13amb) | + | + | + | + | − |
| RFzero-q7 | cat(13amb)/pCAT(13amb) | + | + | ± | − | − |
HST08 and its derivatives generally exhibit lower levels of Cm resistance than DH10B, when transformed with cat.
Cm concentration (μg/ml) in the LB medium.+, resistance; ±, weak resistance; −, susceptibility.
Next, we compared the translation efficiencies with UAG and the CAG glutamine codon in a more quantitative manner, in terms of the yields of a reporter protein in the RFzero-q strains. We constructed a mutant glutathione S-transferase (GST) gene, gst(6amb), bearing six in-frame UAG triplets in an additional sequence at the N terminus. For comparison, we constructed another gst gene, gst(6gln), which had CAG in place of the six UAG triplets. The expression of gst(6amb) and gst(6gln) was assessed by SDS-polyacrylamide gel electrophoresis and Western blot analysis. In RFzero-q, a significantly smaller amount of the full-length GST was expressed from gst(6amb) than from gst(6gln) (Fig. 1C). On the other hand, the expression levels of gst(6amb) and gst(6gln) were similar in RFzero-q3, and the expression level of gst(6amb) was slightly lower than that of gst(6gln) in RFzero-q7. The yields of GST from RFzero-q3 were larger than those from RFzero-q and -q7, because RFzero-q3 grew the fastest, with the largest cell yield per culture volume.
The yield of the gst(6amb) product was quantified, relative to that of gst(6gln) obtained from the same strain, by measuring the GST activity in the cell extracts (Fig. 1D). The gst(6amb) gene was hardly expressed in the parent HST08 strain, bearing the intact prfA. The prfA knockout drastically increased the yield of the gst(6amb) product in RFzero-q, which gave a relative yield of 29%. This yield was doubled in RFzero-q7 (59%) and tripled in RFzero-q3 (89%), and the yields of the gst(6amb) and gst(6gln) products were almost identical in RFzero-q3. This result supported our claim that RFzero-q3 translates UAG almost as efficiently as the glutamine codons.
The introduction of the sucB gene ending with UAA enhances the growth of RFzero-q.
We attempted to identify the factors involved in the restored growth of RFzero-q3. Efficient UAG decoding should promote translation into the downstream region of UAG. The expression of the gene products with C-terminal extensions should have a favorable effect, because efficient UAG decoding enhances the growth of RFzero-q3. The sucB gene encoding dihydrolipoyltranssuccinase is one of the candidate genes with facilitated expression that contributed to cell growth (19). Although sucB is neither essential nor among the seven genes engineered to end with UAA in place of UAG, it is involved in energy regeneration, and its ORF ends with two successive UAG triplets. Therefore, the sucB expression may be affected by the efficiency in UAG translation. We previously reported that the introduction of sucB(UAA), a sucB gene with UAA in place of the double UAG, improved the growth of RFzero strains translating UAG to nonnatural amino acids (19). The sucB(UAA) gene was expected to produce the SucB protein with the original length in RFzero. Figure 2A shows that the introduction of sucB(UAA) also improved the growth of RFzero-q, although the strain still grew more slowly than RFzero-q3. This observation suggested that the sucB activity is lacking in RFzero-q and is restored to some level in RFzero-q3. There are two other genes ending with successive UAG triplets: nanC and ybjK. The introduction of nanC(UAA) did not improve the growth of RFzero (19), while ybjK, with an unidentified function, is dispensable for cell growth in rich media (Genobase, http://ecoli.aist-nara.ac.jp/GB8/).
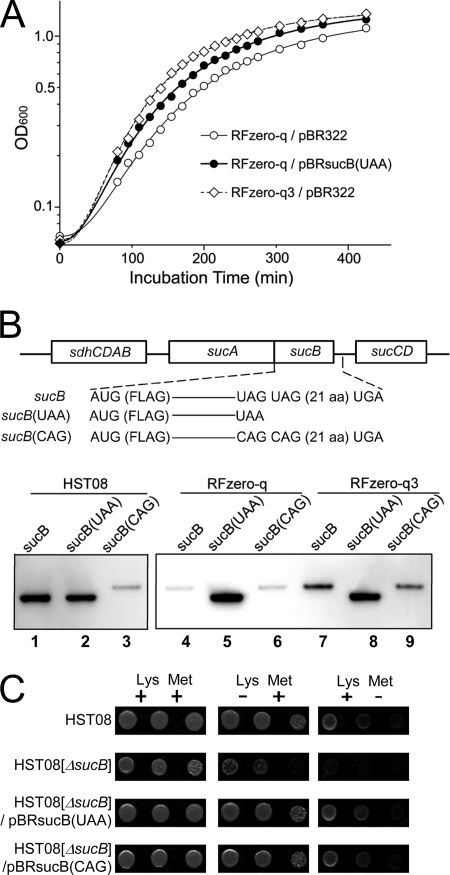
Fig 2.
Facilitated sucB expression contributes to the improved growth of RFzero-q3. (A) Growth profiles in LB medium with ampicillin at 37°C of RFzero-q harboring pBRsucB(UAA) (open circles) and RFzero-q (filled circles) and RFzero-q3 (diamonds) each harboring pBR322, an empty vector. (B) Illustration of sucB and its variants, each N-terminally FLAG tagged, in the sdhCDAB-sucABCD operon, and the Western blot analysis of the sucB products from identical cell amounts of HST08[ΔsucB], RFzero-q[ΔsucB] and -q3[ΔsucB], each harboring the operons with the indicated sucB genes. (C) Growth of the HST08, HST08[ΔsucB], and HST08[ΔsucB] strains transformed with pBRsucB(UAA) and pBRsucB(CAG) on minimal plates supplemented with lysine and methionine or supplemented with either amino acid. Each row shows dilution series of the cell culture inoculated on the plates.
The cellular level of SucB is higher in RFzero-q3 than RFzero-q.
We compared the expression levels of sucB in RFzero-q and RFzero-q3. The sucB ORF has a second stop codon, UGA, 21 triplets downstream of the double UAG. The translation of UAG thus produces a SucB protein with 23 extra amino acids at the C terminus. To compare the sizes of the translation products, we constructed a sucB mutant, sucB(CAG), with two CAG glutamine codons in place of the double UAG and no alteration in the downstream sequence. The sucB gene is transcribed as part of a transcriptional unit consisting of sdhCDAB and sucABCD in this order (5). First, we substituted the cat coding sequence for the sucB ORF in this operon to remove it from the chromosomes of HST08, RFzero-q, and RFzero-q3 and to create HST08[ΔsucB], RFzero-q[ΔsucB], and RFzero-q3[ΔsucB], respectively (Table 1). The whole sdhCDAB-sucABCD operon was subsequently cloned in a low-copy-number plasmid, and then sucB was tagged with a FLAG peptide at the N terminus to create the plasmid pApsucB. The copy number of this plasmid, ColIb-P9, is 1.7 per cell (4). The sucB gene on this plasmid was further mutagenized to sucB(UAA) and sucB(CAG) to create pApsucB(UAA) and pApsucB(CAG), respectively. These plasmids were introduced into the sucB-lacking HST08, RFzero-q, and RFzero-q3 strains.
Western blot analyses revealed that, in HST08, sucB and sucB(UAA) expressed gene products with the same size in similar amounts, whereas sucB(CAG) expressed a longer product at a significantly lower level (Fig. 2B, lanes 1 to 3). In RFzero-q, sucB expressed only the extended product, with the same length as that of the sucB(CAG) product (Fig. 2B, lanes 4 and 6). This observation indicated that translation did not stop at UAG, reaching the second stop triplet. The expression levels of the extended products were significantly lower than that of sucB(UAA). RFzero-q3 also expressed only the extended product from sucB, and its expression level was 2-fold higher than in RFzero-q (Fig. 2B, lane 7). The level of the sucB(CAG) product was slightly increased, compared with its production in RFzero-q (Fig. 2B, lane 9).
The higher sucB level in RFzero-q3, together with the favorable effect of the sucB(UAA) introduction, strongly suggested that the facilitated expression of the extended SucB protein contributes to the growth enhancement of RFzero-q3. We then examined whether this extended protein retains its activity. The sucB knockout in E. coli reportedly causes lysine and methionine auxotrophy (7). The removal of lysine or methionine from the medium actually impaired the growth of HST08[ΔsucB], and the introduction of sucB(UAA) restored the growth (Fig. 2C). The introduction of sucB(CAG) also had the same effect, showing that the extended product retains its activity.
The ribosome stalling in RFzero-q is resolved in RFzero-q3.
We examined whether inefficient UAG translation causes ribosome stalling at UAG in the absence of RF-1. If it occurs, then the stalling was expected to be severe at the double UAG of sucB in RFzero-q and to be alleviated in RFzero-q3. We analyzed the effect of a ribosome recycling factor on the sucB expression in the RFzero strains. The yaeJ gene encodes a peptidyl-tRNA hydrolyzing factor and reportedly can compensate for the deficient ssrA pathway (6), which is the major rescue pathway for stalled ribosomes. Unlike the ssrA pathway, the polypeptides released from the ribosomes by the function of yaeJ are not destined for degradation and thus may be detected. The yaeJ gene under the control of a strong promoter was cloned in pApsucB and introduced into RFzero-q[ΔsucB] and RFzero-q3[ΔsucB]. Two sucB products were thus detected in RFzero-q[ΔsucB], and their lengths were identical to those of the sucB(UAA) and sucB(CAG) products, respectively (Fig. 3A). The detection of the SucB protein with the original length strongly suggested that the ribosomes actually stalled at the UAG at the end of sucB in RFzero-q and that YaeJ probably resolved the stalling and “rescued” the SucB protein by bypassing the ssrA pathway. On the other hand, the SucB protein with the original length was not detected in RFzero-q3 (Fig. 3A), showing that the alleviation of ribosome stalling in this strain was probably due to the efficient translation of UAG. The overexpression of yaeJ in RFzero-q improved the growth profile (Fig. 3B), strongly suggesting that ribosome stalling is another factor contributing to the slow growth of RFzero-q. The growth enhancement was observed only after the late logarithmic phase, and thus yaeJ apparently affects the cells entering the stationary phase. On the other hand, the introduced sucB(UAA) exerted its influence, starting from the mid-log phase (Fig. 2A).
Fig 3.
Ribosome stalling is alleviated by yaeJ. (A) Western blot analysis of the expression of FLAG-tagged sucB in the RFzero-q[ΔsucB] and RFzero-q3[ΔsucB]strains, each transformed with pApsucB-yaeJ, pApsucB(UAA)-yaeJ, and pApsucB(CAG)-yaeJ. The amount of the cells harboring sucB(UAA) used for analysis was smaller than those of the cells harboring the other two genes. (B) Growth profiles of the RFzero-q strains transformed with an empty vector (pAp105) (open circles) and pApyaeJ (filled circles) in LB with kanamycin at 37°C.
A spontaneous mutation in a tRNA gene improves the growth of an RFzero strain.
To support our claim that the detrimental effect of UAG reassignment can be reduced by increasing the UAG-decoding efficiency, we characterized a spontaneous mutation occurring within an RFzero strain that improved the cell growth. The original RFzero strain, designated RFzero-py (Table 1), is based on BW25113, a K-12 E. coli strain. The UAG translation in RFzero-py involves the expression of the archaeal pair of pyrrolysine tRNA and a pyrrolysyl-tRNA synthetase (PylRS) variant attaching lysine derivatives to the tRNA (19). The growth of RFzero-py requires the supplementation of one of these derivatives in the medium, and the growth rate varies with the supplements. Therefore, we can create conditions in which RFzero-py is allowed to grow only poorly by supplementing a poor substrate for the PylRS variant. The cell culture attained an optical density at 600 nm (OD600) of 0.3 overnight, and it remained at this density for two more days. The cell density jumped to an OD600 of 1.6 on the fourth day, although no mutagenic chemicals had been added to the medium. We isolated eight clones from the final culture, and we found that all of them could grow as fast as the parent prfA+ strain, reaching an OD600 of nearly 3 overnight. The growth of these strains was no longer dependent on the supplementation with lysine derivatives, suggesting that they either restored the ability to terminate translation at UAG or changed the assignment of UAG to a certain amino acid in the medium.
We employed a representative clone, designated RFzero*, to express GST with an in-frame amber codon. This mutant gst gene and another gene lacking an in-frame UAG were expressed in RFzero* at similar levels (data not shown), suggesting that the strain did not restore the ability to terminate translation at UAG. The product of the amber gst mutant was digested by trypsin and analyzed by mass spectrometry. The observed average mass of the peptide encompassing the UAG position corresponded to the theoretical value (m/z = 1,277.7) of the corresponding peptide with leucine or isoleucine at UAG. These results indicated that UAG is efficiently translated to one of these amino acids in RFzero*.
A sequence analysis revealed that the RF-1 coding sequence was still absent in the prfA locus of RFzero*, and there was no mutation in prfB encoding RF-2. This release factor recognizes UAA and UGA, and a mutation in prfB can change the codon specificity of the factor, allowing it to recognize all three of the stop triplets (12). The lack of a mutation within prfB was consistent with the efficient expression of the amber gst mutant gene, conclusively negating the possibility of RFzero* restoring the activity to terminate translation at UAG. We sequenced the tRNALeu genes from RFzero*, and we found that the second letter of the tRNA5Leu anticodon was changed and its codon specificity was converted from UUG to UAG. This mutation was identical to the previously reported supP mutation, and the supP tRNA is an efficient amber suppressor (33). We concluded that the supP mutation occurred in RFzero-py and took over UAG translation from the archaeal molecules inefficiently translating UAG to a lysine derivative. The emergence of the fast-growing RFzero with supP indicated again that efficient UAG translation has a favorable effect on the growth of the prfA-lacking strain.
A prfB mutant allowing the prfA knockout recognizes UAG.
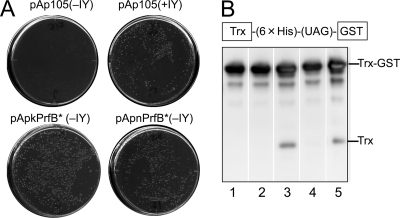
The prfA gene was recently knocked out in an E. coli strain with a reduced number of UAG and UAA triplets in a 15% smaller chromosome (14). Unlike RFzero, this prfA knockout was not dependent on the expression of the UAG-decoding tRNA but required an Ala-to-Glu mutation at position 293 of the “fixed” prfB gene. Since a frameshift mutation and an Ala246Thr replacement naturally occur in prfB, they were removed to “fix” the gene before the prfA knockout. The triple prfB mutant, prfB*, allowed the prfA knockout strain to grow vigorously. Since it failed to complement a temperature-sensitive prfA mutation in another strain (14), prfB* was apparently unable to recognize UAG. To elucidate the basis for the different requirements for the prfA knockout, we characterized prfB* in RFzero strains. First, we performed a complementation test using RFzero-iy, an RFzero strain translating UAG to 3-iodotyrosine (Table 1). The activity of the UAG-decoding tRNA in RFzero-iy strictly depends on the presence of 3-iodotyrosine, and the removal of this amino acid from the medium “inactivates” the tRNA, leading to growth inhibition (Fig. 4A). RFzero-iy was transformed with the plasmids pApnPrfB* and pApkPrfB* each carrying prfB*. The former plasmid, pApnPrfB, was expected to express prfB* from its native upstream sequence, while pApkPrfB* carried the prfB*gene downstream of the kanamycin resistance gene. With either plasmid, the transformed RFzero-iy no longer required 3-iodotyrosine (Fig. 4A). This observation showed that prfB* can complement the lack of the UAG-decoding tRNA.
Fig 4.
UAG-recognizing activity of prfB*. (A) Growth of the RFzero-iy strains transformed with the empty vector pAp105 and the pApkPrfB* and pApnPrfB* plasmids each expressing prfB* on LB plates with kanamycin at 37°C with 3-iodotyrosine (0.1 mg/ml) (+IY) and in its absence (−IY). (B) Western blot analysis of Trx-GST fusion gene expression in RFzero-q3 transformed with pAp105 (lane 1), pApkPrfBf (lane 2), pApkPrfB* (lane 3), pApnPrfBf (lane 4), and pApnPrfB* (lane 5). The sizes corresponding to those of Trx-GST and Trx are marked. The detection of the products was performed using the antibody against the His tag, which was inserted between the C terminus of Trx and the in-frame UAG.
Next, we analyzed the expression of a fusion gene of thioredoxin (Trx) and GST in this order, with a UAG triplet located between them. This fusion gene expressed only the full-length fusion protein in RFzero-q3, which translates UAG to glutamine (Fig. 4B, lane 1). The full-length protein was the only product again in the RFzero-q3 strain transformed with the “fixed” prfB gene, which was expressed from pApkPrfBf or pApnPrfBf (Fig. 4B, lanes 2 and 4). Finally, a small amount of a short product, with a size corresponding to that of thioredoxin, was detected in RFzero-q3 transformed with prfB* (Fig. 4B, lanes 3 and 5), strongly suggesting that some rounds of translation terminated at UAG, instead of incorporating glutamine in response to the triplet. This observation, together with the results of the complementation test, shows that RF-2 with the A293E mutation can recognize UAG and terminate translation. However, its activity is probably too weak to complement a prfA(Ts) mutant.
DISCUSSION
As a sense codon, the UAG triplet would not be as efficiently translated as the other sense codons until the characteristics of the decoding tRNA and its cognate aaRS are optimized to UAG. In the present study, we showed that a genetic change to a UAG-reading tRNA achieves the efficient translation of the triplet in the absence of RF-1. The isolated supE3 tRNA, with a U-to-A substitution at position 38, is identical to the previously reported amber suppressor tRNAGln (2). A similar change, a C-to-A substitution at position 38, reportedly improves the UGA-decoding efficiency of the opal suppressor tRNAGlu (24). U at position 38, which is invariant between the two E. coli tRNAGln species, is involved in the recognition of tRNAGln by GlnRS (25), and a U-to-G change at this position moderately decreases the aminoacylation activity of tRNAGln (13). Therefore, the efficient UAG translation by the supE3 tRNA is probably due to an enhanced ability to recognize UAG. When UAG is reassigned to nonnatural amino acids, attaching these novel substrates to tRNA can be a limiting step, and it is probably necessary to engineer aaRSs favoring these amino acids to achieve the efficient translation of UAG.
The analysis of the sucB expression in RFzero revealed the effects of the UAG reassignment at the molecular level. First, only the extended product was expressed from the sucB ORF. Second, the extended product retained its activity, probably contributing to the growth of the bacteria, although its expression was significantly reduced. This reduction in the expression level may be explained by assuming that the addition of the extra C-terminal peptide destabilizes the protein, or that the progression of translation beyond the original stop triplet destabilizes the mRNA. Third, when UAG decoding is not efficient, ribosome stalling occurs at the double UAG at the end of sucB and probably at the ends of other UAG-ending ORFs at lower levels, thus hampering overall translation by preventing the reuse of ribosomes. The increased efficiency of UAG decoding not only facilitates the production of the extended SucB but also probably enhances the overall translation activity by resolving stalled ribosomes. The latter effect may explain the slightly promoted expression of sucB(CAG), which does not specifically involve the translation of UAG.
Among the ∼300 UAG-ending ORFs, sucB is probably not the only gene with an extended product that retains its activity and contributes to bacterial growth. The contribution of this gene is relatively large and severely restricted by the occurrence of the double UAG, and thus it was easier to identify. The observation of the enhanced growth of RFzero-q3 in rich medium does not negate the possibility that some of the extended products could have adverse effects under certain circumstances. For example, the efficient amber suppression at the end of relA, which probably generates an extended product, reportedly impairs the stringent-control mechanism to respond to amino acid starvation (3). The UAG reassignment probably has a mixed effect, and the overall outcome may differ in various environments. A conceivable advantage of RFzero is that there are only two kinds of stop triplets in the strain, thus significantly reducing the frequency of undesirable nonsense mutations compared with that of wild-type strains using the universal code. RFzero might be able to take advantage of such circumstances as the mutation rate is increased and the strain can benefit from the high mutation rate to evolve quickly.
The present results explain the requirement of a UAG-decoding tRNA for knocking out prfA, with the UAG triplets left intact in the chromosome. The UAG-decoding activity is probably required to alleviate ribosome stalling at UAG and to allow the expression of UAG-ending ORFs. The identity of the amino acid to be incorporated at UAG is not important, because the occurrence of a tRNA efficiently incorporating either glutamine or leucine at UAG allows the vigorous growth of RFzero, and UAG has even been reassigned to nonnatural amino acids. On the other hand, the occurrence of a second stop triplet, UAA or UGA, downstream of UAG is the implicit condition needed to knock out prfA: it allows the expression of UAG-ending ORFs in the absence of RF-1. These “backup” stop triplets also have a crucial role when the UAG translation efficiency is increased: they prevent the ribosomes from reaching the end of the mRNA. About half (42%) of the UAG triplets in the E. coli genome have UAA or UGA within 10 triplets after the UAG. This percentage is significantly larger than that expected (27%) if they randomly occur (18). The frequency of the occurrence of the backup stops sharply contrasts with that of the occurrence of a second UAG downstream of UAG; only 8% of the UAG triplets have a second UAG within 10 triplets, whereas the figure expected from random occurrence is 15%. These statistical findings suggest that UAA and UGA might have appeared as backup stops downstream of UAG, while UAG is disappearing at the same time, to avoid the detrimental effect of a situation where UAG largely fails to terminate translation. The nuclear genomes of yeasts and ciliates also reportedly have this backup system (1, 17).
The two hypotheses about the naturally established codon reassignments, as well as two recent attempts to knock out prfA, all assume a major change in the genomic usage of the reassigned codons. The replacement of all of the UAG triplets in the E. coli chromosome is an ongoing project (11), while prfA was knocked out in the E. coli strain with a significantly reduced number of stop triplets (14). However, the E. coli genome might have already undergone a genome-wide change, with the generation of backup stops downstream of UAG. This change underlies our discovery that a small number of mutations can generate vigorously growing E. coli strains with UAG reassigned as a full-fledged sense codon.
ACKNOWLEDGMENTS
We thank A. Ishii and T. Nakayama for clerical assistance.
This study was supported by the Targeted Proteins Research Program, a Grant-in-Aid for Young Scientists (B) 21770195 to A.S., and a Grant-in-Aid for Scientific Research (B) 19380195 to K.S., all from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Published ahead of print 16 March 2012
REFERENCES
- 1. Adachi M, Cavalcanti AR. 2009. Tandem stop codons in ciliates that reassign stop codons. J. Mol. Evol. 68:424–431 [DOI] [PubMed] [Google Scholar]
- 2. Bradley D, Park JV, Soll L. 1981. TRNA2Gln Su+2 mutants that increase amber suppression. J. Bacteriol. 145:704–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Breeden L, Yarus M, Cline S. 1980. A cloned suppressor tRNA gene relaxes stringent control. Mol. Gen. Genet. 179:125–133 [DOI] [PubMed] [Google Scholar]
- 4. Clewell DB, Helinski DE. 1970. Existence of the colicinogenic factor-sex factor ColI-b-P9 as a supercoiled circular DNA-protein relaxation complex. Biochem. Biophys. Res. Commun. 41:150–156 [DOI] [PubMed] [Google Scholar]
- 5. Cunningham L, Guest JR. 1998. Transcription and transcript processing in the sdhCDAB-sucABCD operon of Escherichia coli. Microbiology 144:2113–2123 [DOI] [PubMed] [Google Scholar]
- 6. Handa Y, Inaho N, Nameki N. 2011. YaeJ is a novel ribosome-associated protein in Escherichia coli that can hydrolyze peptidyl-tRNA on stalled ribosomes. Nucleic Acids Res. 39:1739–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herbert AA, Guest JR. 1969. Studies with alpha-ketoglutarate dehydrogenase mutants of Escherichia coli. Mol. Gen. Genet. 105:182–190 [DOI] [PubMed] [Google Scholar]
- 8. Ibba M, Hong KW, Sherman JM, Sever S, Söll D. 1996. Interactions between tRNA identity nucleotides and their recognition sites in glutaminyl-tRNA synthetase determine the cognate amino acid affinity of the enzyme. Proc. Natl. Acad. Sci. U. S. A. 93:6953–6958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inokuchi H, Yamao F, Sakano H, Ozeki H. 1979. Identification of transfer RNA suppressors in Escherichia coli. I. Amber suppressor su+2, an anticodon mutant of tRNA2Gln. J. Mol. Biol. 132:649–662 [DOI] [PubMed] [Google Scholar]
- 10. Iraha F, et al. 2010. Functional replacement of the endogenous tyrosyl-tRNA synthetase-tRNATyr pair by the archaeal tyrosine pair in Escherichia coli for genetic code expansion. Nucleic Acids Res. 38:3682–3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Isaacs FJ, et al. 2011. Precise manipulation of chromosomes in vivo enables genome-wide codon replacement. Science 333:348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ito K, Uno M, Nakamura Y. 1998. Single amino acid substitution in prokaryote polypeptide release factor 2 permits it to terminate translation at all three stop codons. Proc. Natl. Acad. Sci. U. S. A. 95:8165–8169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jahn M, Rogers MJ, Söll D. 1991. Anticodon and acceptor stem nucleotides in tRNA(Gln) are major recognition elements for E. coli glutaminyl-tRNA synthetase. Nature 352:258–260 [DOI] [PubMed] [Google Scholar]
- 14. Johnson DB, et al. 2011. RF1 knockout allows ribosomal incorporation of unnatural amino acids at multiple sites. Nat. Chem. Biol. 7:779–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawaguchi Y, Honda H, Taniguchi-Morimura J, Iwasaki S. 1989. The codon CUG is read as serine in an asporogenic yeast Candida cylindracea. Nature 341:164–166 [DOI] [PubMed] [Google Scholar]
- 16. Knight RD, Freeland SJ, Landweber LF. 2001. Rewiring the keyboard: evolvability of the genetic code. Nat. Rev. Genet. 2:49–58 [DOI] [PubMed] [Google Scholar]
- 17. Liang H, Cavalcanti AR, Landweber LF. 2005. Conservation of tandem stop codons in yeasts. Genome Biol. 6:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mukai T, et al. 2010. Codon reassignment in the Escherichia coli genetic code. Nucleic Acids Res. 38:8188–8195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mukai T, et al. 2011. Genetic-code evolution for protein synthesis with non-natural amino acids. Biochem. Biophys. Res. Commun. 411:757–761 [DOI] [PubMed] [Google Scholar]
- 20. Murphy KC. 1998. Use of bacteriophage lambda recombination functions to promote gene replacement in Escherichia coli. J. Bacteriol. 180:2063–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muyrers JP, Zhang Y, Testa G, Stewart AF. 1999. Rapid modification of bacterial artificial chromosomes by ET-recombination. Nucleic Acids Res. 27:1555–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Osawa S, Jukes TH. 1989. Codon reassignment (codon capture) in evolution. J. Mol. Evol. 28:271–278 [DOI] [PubMed] [Google Scholar]
- 23. Osawa S, Jukes TH, Watanabe K, Muto A. 1992. Recent evidence for evolution of the genetic code. Microbiol. Rev. 56:229–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raftery LA, Yarus M. 1987. Systematic alterations in the anticodon arm make tRNA(Glu)-Suoc a more efficient suppressor. EMBO J. 6:1499–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rould MA, Perona JJ, Steitz TA. 1991. Structural basis of anticodon loop recognition by glutaminyl-tRNA synthetase. Nature 352:213–218 [DOI] [PubMed] [Google Scholar]
- 26. Rydén SM, Isaksson LA. 1984. A temperature-sensitive mutant of Escherichia coli that shows enhanced misreading of UAG/A and increased efficiency for some tRNA nonsense suppressors. Mol. Gen. Genet. 193:38–45 [DOI] [PubMed] [Google Scholar]
- 27. Sakamoto K, Ishimaru S, Kobayashi T, Walker JR, Yokoyama S. 2004. The Escherichia coli argU10(Ts) phenotype is caused by a reduction in the cellular level of the argU tRNA for the rare codons AGA and AGG. J. Bacteriol. 186:5899–5905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Santos MA, Tuite MF. 1995. The CUG codon is decoded in vivo as serine and not leucine in Candida albicans. Nucleic Acids Res. 23:1481–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schultz DW, Yarus M. 1994. Transfer RNA mutation and the malleability of the genetic code. J. Mol. Biol. 235:1377–1380 [DOI] [PubMed] [Google Scholar]
- 30. Scolnick E, Tompkins R, Caskey T, Nirenberg M. 1968. Release factors differing in specificity for terminator codons. Proc. Natl. Acad. Sci. U. S. A. 61:768–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Söll D, RajBhandary UL. 2006. The genetic code—thawing the ‘frozen accident’. J. Biosci. 31:459–463 [DOI] [PubMed] [Google Scholar]
- 32. Suzuki T, Ueda T, Watanabe K. 1997. The ‘polysemous’ codon—a codon with multiple amino acid assignment caused by dual specificity of tRNA identity. EMBO J. 16:1122–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thorbjarnardóttir S, et al. 1985. Leucine tRNA family of Escherichia coli: nucleotide sequence of the supP(Am) suppressor gene. J. Bacteriol. 161:219–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamao F, et al. 1985. UGA is read as tryptophan in Mycoplasma capricolum. Proc. Natl. Acad. Sci. U. S. A. 82:2306–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]