TEXT
The cyanobacterium Anabaena needs a variety of metals for its cellular biochemistry. The general problem is to supply all the different metals to the right enzymes, thereby avoiding toxic side reactions and interference between these metals. Anabaena has to deal with surplus metals but also with metal starvation, and this problem has to be solved for many environments, each with a different availability of metal cations.
In this issue, Napolitano et al. (34) report a study in which they unraveled the zinc starvation response in Anabaena. Zinc is an important essential trace element in all organisms. More than 100 zinc-dependent or -binding proteins are known in Escherichia coli (39), e.g., a fructose-bisphosphate aldolase, DNA primase, carbonic anhydrase, alkaline phosphatase, and RNA polymerase. In mammals, including humans, zinc is required for proper brain function and for insulin and semen release and acts as cellular signal (30).
The story told by Napolitano et al. (34) is wonderful to read. They identified the main transcriptional regulator to cope with zinc deficiency in Anabaena, Zur, and dozens of genes under Zur control. Most interesting among those were putative zinc-binding metallochaperones, other regulatory proteins, and, surprisingly, TonB-dependent outer membrane proteins, which may be involved in active transport of zinc or zinc chelates across the outer membrane. Anabaena might even synthesize and excrete a zinc chelator (a “zincophore”?), reminiscent of siderophores for iron or chalkophores for copper acquisition. To place this work (34) in a proper context, it is important to reiterate what is known about Anabaena, how this bacterium grows, why it needs metal cations, how zinc homeostasis in the context of general metal homeostasis might function, and what is new in the report by Napolitano et al. (34) (Fig. 1).
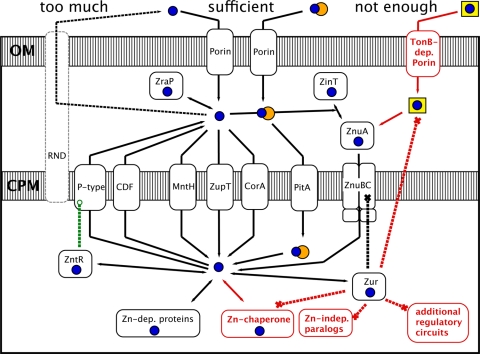
Fig 1.
What we knew about zinc homeostasis and what is new. What was already known concerning bacterial zinc homeostasis is shown in black; the additional insights by Napolitano et al. (34) are in red. With sufficient zinc supply, zinc (blue dots) or zinc phosphate (adjacent blue and orange dots) diffuses via porins through the outer membrane (OM) into the periplasm between the OM and the cytoplasmic membrane (CPM), and is further transported across the CPM to the cytoplasm by fast and unspecific uptake systems such as ZupT, MntH, CorA, and PitA. Here, zinc is supplied to zinc-dependent proteins. Surplus zinc is moved back into the periplasm by CDF proteins or P-type ATPases that may be under the control of a ZntR-type regulator (green dashed line). In some bacteria, an RND-driven efflux system may export periplasmic zinc across the OM back to the outside (white box with dashed outline), or Zn2+ is bound by a periplasmic zinc chaperone such as ZraP. Under conditions of low zinc, Zur-type proteins upregulate expression of ZnuBC-like uptake systems that interact with ZnuA- and ZinT-type periplasmic metal binding proteins. In addition to this picture, Napolitano et al. (34) have found in Anabaena sp. strain PCC 7120 evidence for paralogs of zinc-dependent proteins, cytoplasmic zinc chaperones, additional regulatory circuits, and possibly zinc acquisition by a “zincophore” (or another kind of zinc chelate [yellow squares]) and its uptake by TonB-dependent outer membrane proteins, all under Zur control.
Anabaena sp. PCC 7120 is a cyanobacterium. Without these organisms, life on this planet would be different. They are a major phylum of the superkingdom Bacteria, and their ecological niche is a physiological one, namely, oxygenic photosynthesis. Cyanobacteria are the only organisms able to perform this reaction, either as free-living organisms or as plastid endosymbionts (or slaves?) of eukaryotic cells. The product of oxygenic photosynthesis is molecular oxygen. With a half-cell potential (Eo′) of +816 mV (61), transfer of electrons from NADH (Eo′ = −320 mV) releases about −238 kJ/mol under standard conditions (molar concentrations; pH = 7), enough to conserve 3 ATP per electron pair transferred. No other respiratory electron acceptor allows such a high energy conservation by electron transfer-dependent phosphorylation. Production of molecular oxygen by cyanobacteria led to two major oxygenation events about 2.4 billion and 700 million years ago, which may have sparked evolution of eukaryotes and multicellular organisms, respectively (22, 25, 45). Thus, cyanobacteria have efficiently changed the biogeochemistry of our planet.
Anabaena needs transition metals to perform oxygenic photosynthesis and for nitrogen fixation. Oxygenic photosynthesis occurs in thylakoids, a specialized internal membrane system that contains photosystems I and II. Both contain chlorophyll molecules, which harbor a Mg2+ at their center. A Mn-containing water-splitting complex attached to photosystem II replenishes electrons that are transferred after light absorption from photosystem II to a plastoquinone. The water-splitting complex contains at its core a Mn4CaO5 cluster with two chloride anions in its vicinity (59), oxidizes water to molecular oxygen, and donates the resulting electrons one by one to the center of photosystem II. In the subsequent steps, electrons are transferred from the plastoquinone to an iron-containing cytochrome b6f complex, via the copper-dependent soluble protein plastocyanin to the iron-containing photosystem I, and from here, after being pushed by another photon, to NADP+. This process also conserves energy via the proton motive force and the F1Fo ATPase. Finally, NADPH is used as a redox donor to assimilate CO2 via the Calvin cycle.
Oxygenic photosynthesis, therefore, needs the transition metals manganese, iron, and copper, the earth alkali metals magnesium and calcium, and the halogen chlorine. The nitrogen-fixing nitrogenase complex contains iron and molybdenum. Anabaena sp. strain PCC 7120 may also be able to synthesize an alternative vanadium-iron-dependent nitrogenase (NifH2, all1455) and a vanadium-dependent chloroperoxidase (alr0672). Cobalt is part of the component cobalamin, and nickel is part of urease and of hydrogenase, an enzyme that reassimilates molecular hydrogen produced by the nitrogenases among other physiological functions. If we move along the periodic table of the elements in the first transition period from left to right, Anabaena has use for V, Mo of the second transition period (chromium of the first period is as chromate a sulfate antagonist and very toxic), Mn, Fe, Co, Ni, Cu, and finally Zn.
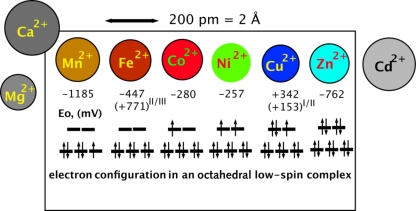
Thus, Anabaena has an impressive need for transition and other metals and, like other organisms, must solve the problem of getting the correct metal to the right protein. In general, about 40% of all enzymes need metals as cofactors, ranking from Mg (16%) > Zn (9%) > Fe (8%) > Mn (6%) > Ca (2%) > Co and Cu (1%) down to K, Na, Ni, V, Mo, W, and in one case Cd (60). The problem of metal allocation is especially difficult for transition metal cations because, at first glance, those of the first transition group (Mn, Fe, Co, Ni, Cu, and Zn) have similar ionic radii (Fig. 2). In the cell, these metal cations interfere with each other along the Irving-Williams series or “pecking order” (21), which has copper on top and manganese, essential for production of molecular oxygen, at the bottom as far as transition metal cations are concerned. Moreover, transition metal cations may cause oxidative stress by binding to thiol groups, as has been shown for Cd2+ (19). Zinc, cadmium, and cobalt may interfere with the metabolism of iron (18, 19, 48, 56), and nickel with that of zinc (29). Fe and Cu can cause Fenton and Fenton-like reactions (14), particularly in oxygen-producing Anabaena cells.
Fig 2.
Comparison of the essential transition metal cations. The essential divalent transition metal cations have all similar radii and can be distinguished only if variations in the complex-forming and redox abilities are considered. Despite this fact, they perform different functions and have to be provided to different proteins, e.g., manganese to the water-splitting complex of photosystem II, copper to plastocyanin, iron to cytochromes, nonheme iron sites and iron-sulfur centers, cobalt to cobalamin, nickel to hydrogenase, and zinc to many enzymes, such as RNA polymerase.
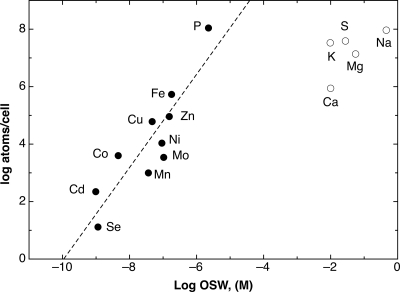
So, the toxicity of transition metals is a real problem, but so is starvation for these metals. This situation demands sophisticated transition metal homeostasis systems, but particularly in Anabaena, which has three cellular compartments to deal with: the periplasm, the cytoplasm, and the lumen of the thylakoids. Understanding the solution to this problem might come from a simple observation. Cyanobacteria and other bacteria living in seawater, which can serve as a kind of global standard or model environment, are confronted with a “mélange” of transition metal cations that approximates that inside the bacterial cell (Fig. 3). If we regard a bacterial cell as a simple “tea bag,” it would be sufficient to simply accumulate these metals by a high-rate and nonselective import process with an energy of about 90 mV, about half of that of the proton motive force. This would lead to a cellular metal content that is “just right” and avoid competition along the Irving-Williams series due to the low concentrations of nickel and cobalt. Achievement of metal homeostasis by highly redundant metal uptake systems, which have only minimal cation selectivity, has been shown for the bacterium Cupriavidus metallidurans (27). The genes for the respective uptake systems are also present in the Anabaena genome: all4110 (CorA), alr3096 (PitA), all0473 (ZupT), and all7601 (MntH). Thus, Anabaena, which actually dwells in fresh water and microbial mats, might nevertheless use the “take what you can get” approach to homeostasis of transition metals, including zinc (Fig. 1).
Fig 3.
Plot of the elements per cell against their occurrence in seawater (OSW). A double logarithmical plot of the number of atoms in the cell of the bacterium C. metallidurans as determined by ICP-MS (24) against OSW as a model of a standard environment (61). Please note that the transition metals plus phosphate and selenium (black dots) reside on a line. Assuming the cell is a “tea bag” without internal metal-chelating capacity with a cellular volume of 0.57 fl (12), this line would represent accumulation of the shown metal cations with an energy of 93 ± 20 mV across the cytoplasmic membrane. The five major bioelements shown as open symbols in the upper-right corner are outside of this line and therefore much easier to acquire than the elements on the line.
This approach to transition metal homeostasis in bacteria is refined by (i) flux control, which seems to be used by many metal transporters in addition to expression control of the respective genes to prevent overuptake of substrates by metal importers and overefflux of essential metal by metal efflux systems (38); (ii) metal chaperones; (iii) efflux of surplus metals; and (iv) modes of dealing with metal starvation. Napolitano et al. (34) have added pieces to the homeostasis puzzle: a possible zinc chaperone (to number ii); cross regulation that might influence metal efflux (to number iii); and maybe a new way to deal with zinc starvation (to number iv). Let us follow their line of thoughts now before we merge their insights into the current picture of zinc homeostasis.
Their story starts with an idea and a gene region with unknown function. One response to starvation might be reallocation and redistribution. In such a case, zinc would be redistributed from less important enzymes (or those that show redundancy) to the essential ones, such as RNA polymerase. Examples for such a process are known for copper in the alga Chlamydomonas (6) and for iron in E. coli (31). There is also evidence for a shift between zinc-binding and nonbinding ribosomal proteins in Streptomyces (43, 44, 54). In Anabaena, Napolitano et al. (34) found a gene region (all4727 to all4721) that might encode paralogs of other proteins. Under several conditions tested, it was the presence of the zinc chelator TPEN [N,N,N′,N-tetrakis(2-pyridylmethyl)ethylenediamine] that increased the transcription of some of these genes (all4726 and all4727, encoding a two-component regulatory system) slightly but that of others (all4721 to all4725) dramatically. These five genes are in an operon in the direction from all4725 to all4721, as shown by reverse transcription-PCR (RT-PCR), and encode a putative delta-aminolevulinic acid dehydratase (all4725), a threonyl-tRNA synthetase (all4723), a GTP cyclohydrolase (all4721), and two putative proteins with unknown functions. Several promoters were identified as being responsible for generation of the respective transcripts, with the most important being 19 bp upstream of the start codon of the all4725 open reading frame. The genes all4726 and all4727, encoding the two-component regulatory system, were also transcribed as an operon in a dicistronic mRNA from a promoter 68 bp upstream of all4727 (34).
Response to iron deficiency in E. coli and other bacteria is regulated by the Fur protein (5, 16, 55). Fur binds to its operators probably with iron as the corepressor and is the archetype of the Fur protein family of regulators that comprises, for instance, Nur for nickel, Mur for manganese, PerR for oxidative stress (20), and Zur for zinc (15, 46). Anabaena contains the genes for three members of this family, and FurB (all2473) was identified as Zur. Only Zur was able to bind to the operator region upstream of all4725, not FurA (all1691) or FurC (alr0957). In a Δzur mutant, the all4725-all4721 pentacistronic operon was highly expressed in the absence of TPEN, and the mutant strain was sensitive to zinc but not to other metal cations. Finally, Zur bound to the operator region only in the presence of zinc (34).
To get a wider view of zinc starvation response in Anabaena, the authors first determined the consensus sequence of the Fur box by measuring the affinity of Zur for mutant derivatives of the Zur box upstream of all4725. Equipped with this information, they searched for putative Zur boxes in the genome of Anabaena, found 33 candidates, and tested them for increased expression in the Δzur mutant compared to the wild type (34). This led them, as mentioned above, to indications of a zinc chaperone, to evidence for cross regulation, and to a new answer to the problem of zinc starvation.
Metal chaperones (number ii above) are small proteins in the cytoplasm or periplasm which bind transition metal cations to decrease toxic reactions and interference with other metals and to provide the cations as cofactors to their specific enzymes (41). Copper chaperones sequester copper in the cytoplasm of many organisms (9, 41) because copper is at the top of the Irving-Williams series, plus a strong thiol-binding metal and a dangerous Fenton reagent. Metal chaperones are also used to control availability of nickel and cobalt (17), although the amount of these metals in the cell is kept low to avoid interference with iron. Anabaena has specific nickel chaperones for urease (UreE, alr0733) and hydrogenase (HypA/HupA, alr0699) biosynthesis (7, 11, 32). There are also several systems that buffer the intracellular iron pool in bacteria (1).
The manganese-binding chaperone MncA is used by the cyanobacterium Synechocystis strain PCC 6803 to solve the problem of sorting manganese to the correct site. MncA folds in the cytoplasm, thereby binding Mn2+. The available Zn2+ and Cu+ concentrations are 10,000-fold lower here, so that these metals cannot compete with Mn2+. The loaded MncA is subsequently transported by the twin-arginine transport pathway (that transports folded proteins) into the periplasm. Here, MncA remains kinetically stable, so that copper and other metals cannot displace Mn2+. On the other hand, the periplasmic copper chaperone CucA is exported unfolded by the Sec pathway into the periplasm and readily binds copper cations in this compartment (58). Anabaena also contains a candidate gene (all7319) for a MncA or CucA ortholog.
There are two periplasmic zinc chaperones in E. coli: ZraP, which is strongly upregulated in the presence of zinc (10) by a two-component regulatory system (28), and ZinT, which binds periplasmic zinc and may provide it for further uptake by ZnuABC (47). So ZraP might buffer the periplasmic zinc pool at high and ZinT at low concentrations. Both proteins have no homologs in Anabaena. A cytoplasmic zinc chaperone was missing, however, until Napolitano et al. (34) found the first candidates in Anabaena.
Surplus cations, especially Cu+, are removed by efflux pumps (number iii above), which are synthesized as a response to an excess of a metal in the total metal cation mélange (Fig. 1). PIB-type ATPases (TC 3.A.3) (40, 49, 50) and CDF proteins (TC 2.A.3) export surplus metals across the cytoplasmic membrane, and huge RND-driven (TC 2.A.6) transenvelope efflux systems in Gram-negative bacteria move metals from the periplasm further on to the outside (36). Anabaena contains homologs of the CDF proteins CzcD and DmeF (all7610 and all2845) that export Zn2+, Cd2+, Co2+ and a homolog of the Fe2+-exporting FieF protein (all2900) (13, 33, 35). There are two P-type ATPases of the Zn/Cd/Pb-exporting subgroup PIB2 (alr7622 and all3161) and four (alr7635, all7592, alr1627, and all3782) putative PIB1-type copper exporters (2). Depending on sequence similarities and signature sequences (37) of the putative RND proteins, there are two putative metal transporters (all7631 and all7618) of the HME5 subgroup of RND proteins that might be nickel exporters, an AcrB-like (all3143) and an MdtB-like (alr4267) protein, and two very unusual putative RND proteins (alr5294 and alr1656). So there is sufficient efflux competence in Anabaena to deal with surplus metal cations and avoid their toxicity.
If, in contrast, a metal cation is limited in the cell (number iv above), bacteria synthesize high-affinity import systems, usually belonging to the ABC protein family (TC 3.A.1). In some bacteria such as Escherichia coli, there are “matched pairs” consisting of an import and export system, which are both inversely controlled by their substrate (42) and thus keep the cellular zinc concentration in a tight range. For zinc in E. coli, these are the ZnuABC (46) uptake and the ZntA (51) efflux systems. Candidate genes for ZntA (alr7622) and Zur-controlled ABC uptake systems also exist in Anabaena (34), so such a matched pair might also operate in this cyanobacterium.
The two-component regulatory system (all4727 and all4726) that is cotranscribed with the pentacistronic zinc starvation operon described by Napolitano et al. (34) is related to the BaeSR two-component system of E. coli. BaeSR seems to control expression of efflux pumps in response to cell-damaging agents (26). Moreover, in a Zur-dependent operon for a ZnuABC-type zinc uptake system, there is also a putative regulator of the ArsR/SmtB protein family. These regulators also control expression of efflux pumps and metal detoxification systems (57). So, Anabaena seems not only to redistribute its zinc resources but also tries to acquire additional zinc. At the same time, production of regulators for efflux systems might enhance the ability to increase the efflux power by enabling a matched-pair situation to deal with any possible threat that might result from this effort.
Expression of an ABC uptake system, which usually operates with the help of a periplasmic metal-binding protein, might not be adequate to acquire sufficient metals if these metals are bound in the environment by organic or inorganic compounds or are unavailable due to formation of insoluble hydroxide complexes, as in the case of Fe(III). Bacteria and other organisms deal with this problem by producing substances that sequester the respective metal, making it available for uptake. Siderophores that complex Fe(III) have long been known to be important for iron supply under oxic conditions (4, 5). Methylosinus trichosporium grows on methane with the help of an efficient, copper-dependent methane monooxygenase. This bacterium acquires copper with methanobactin, a copper-binding compound called a “chalkophore” (8, 23). Moving from copper to cobalt, exchange of cobalamin and derivatives thereof may be the “trading form” for cobalt in various bacteria (39).
Complicated and large molecules such as siderophores and cobalamin, however, cannot be transported efficiently across the outer membrane of Gram-negative bacteria by facilitated diffusion through porins. Such a process needs active transport across this membrane. The energy needed here stems from the proton motive force, which is transformed into conformational energy by the ExbB and ExbD proteins of the inner cytoplasmic membrane and is transmitted across the periplasm by the TonB protein (4, 5). Therefore, TonB-dependent outer membrane proteins are a group of proteins that catalyze active transport of substances across this barrier.
In Anabaena, two TonB-dependent receptor genes are under Zur control (alr3242 and alr4028-4029), both in the vicinity of genes for periplasmic metal-binding proteins that cooperate with ABC import systems. This situation is reminiscent of the import of siderophores (34). Does Anabaena use siderophores for uptake of zinc under starvation conditions? Or is there an altogether different and new compound group that may be named a “zincophore”? Similar to siderophores, the genes under the control of the two-component regulatory system encoded by all4727 and all4726 might also be required to produce the export pathway for a zinc-chelating substance (3). Or does the TonB-dependent system merely import some form of zinc chelate reminiscent of the import of nickel chelates (52, 53)? As always, a good publication answers one question but ends up asking a couple of new ones!
ACKNOWLEDGMENTS
Work in my lab on zinc uptake in C. metallidurans is funded by the Deutsche Forschungsgemeinschaft (grant Ni262/10 and for the ICP-MS INST 271/266-1).
Thanks go to Gary Sawers for his helpful suggestions and for carefully reading the manuscript.
Footnotes
Published ahead of print 2 March 2012
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
ADDENDUM IN PROOF
I learned at a meeting that J. Tommassen has previously published data regarding TonB-dependent receptors that are involved in zinc uptake. I was not aware of this publication (M. Stork et al., PLoS Pathog. 6:e1000969, 2010).
REFERENCES
- 1. Andrews SC, Robinson AK, Rodriguez-Quinones F. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215–237 [DOI] [PubMed] [Google Scholar]
- 2. Argüello JM, Eren E, Gonzalez-Guerrero M. 2007. The structure and function of heavy metal transport P-1B-ATPases. Biometals 20:233–248 [DOI] [PubMed] [Google Scholar]
- 3. Bleuel C, et al. 2005. TolC is involved in enterobactin export across the outer membrane of Escherichia coli. J. Bacteriol. 187:6701–6707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braun V, Braun M. 2002. Active transport of iron and siderophore antibiotics. Curr. Opin. Microbiol. 5:194–201 [DOI] [PubMed] [Google Scholar]
- 5. Braun V, Hantke K. 2007. Acquisition of iron by bacteria, p 189–217 In Nies DH, Silver S. (ed), Molecular microbiology of heavy metals, vol 6 Springer-Verlag, Berlin, Germany [Google Scholar]
- 6. Castruita M, et al. 2011. Systems biology approach in Chlamydomonas reveals connections between copper nutrition and multiple metabolic steps. Plant Cell 23:1273–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan Chung KC, Zamble DB. 2011. Protein interactions and localization of the Escherichia coli accessory protein HypA during nickel Insertion to [NiFe] hydrogenase. J. Biol. Chem. 286:43081–43090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi DW, et al. 2006. Spectral, kinetic, and thermodynamic properties of Cu(I) and Cu(II) binding by methanobactin from Methylosinus trichosporium OB3b. Biochemistry 45:1442–1453 [DOI] [PubMed] [Google Scholar]
- 9. Cobine P, et al. 1999. The Enterococcus hirae copper chaperone CopZ delivers copper(I) to the CopY repressor. FEBS Lett. 445:27–30 [DOI] [PubMed] [Google Scholar]
- 10. Egler M, Große C, Grass G, Nies DH. 2005. Role of ECF sigma factor RpoE in heavy metal resistance of Escherichia coli. J. Bacteriol. 187:2297–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fong YH, et al. 2011. Assembly of preactivation complex for urease maturation in Helicobacter pylori. Crystal structure of the UreF-UreH protein complex. J. Biol. Chem. 286:43241–43249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goris J, et al. 2001. Classification of metal-resistant bacteria from industrial biotopes as Ralstonia campinensis sp. nov., Ralstonia metallidurans sp. nov. and Ralstonia basilensis Steinle et al. emend. Int. J. Syst. Evol. Microbiol. 51:1773–1782 [DOI] [PubMed] [Google Scholar]
- 13. Grass G, et al. 2005. FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch. Microbiol. 183:9–18 [DOI] [PubMed] [Google Scholar]
- 14. Haber F, Weiss J. 1932. Über die Katalyse des Hydroperoxydes. Naturwissenschaften 20:948–950 [Google Scholar]
- 15. Hantke K. 2005. Bacterial zinc uptake and regulators. Curr. Opin. Microbiol. 8:196–202 [DOI] [PubMed] [Google Scholar]
- 16. Hantke K. 1987. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Mol. Gen. Genetics 210:135–139 [DOI] [PubMed] [Google Scholar]
- 17. Hausinger RP, Zamble DB. 2007. Microbial physiology of nickel and cobalt, p 287–320 In Nies DH, Silver S. (ed), Molecular microbiology of heavy metals, vol 6 Springer-Verlag, Berlin, Germany [Google Scholar]
- 18. Helbig K, Bleuel C, Krauss GJ, Nies DH. 2008. Glutathione and transition metal homeostasis in Escherichia coli. J. Bacteriol. 190:5431–5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Helbig K, Grosse C, Nies DH. 2008. Cadmium toxicity in glutathione mutants of Escherichia coli. J. Bacteriol. 190:5439–5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Helmann JD, Soonsange S, Gabriel S. 2007. Metalloregulators: arbiters of metal sufficiency, p 37–71 In Nies DH, Silver S. (ed), Molecular microbiology of heavy metals, vol 6 Springer-Verlag, Berlin, Germany [Google Scholar]
- 21. Irving H, Williams RJP. 1948. Order of stability of metal complexes. Nature 162:746–747 [Google Scholar]
- 22. Kerr RA. 2005. The story of O2. Science 308:1730–1732 [DOI] [PubMed] [Google Scholar]
- 23. Kim HJ, et al. 2004. Methanobactin, a copper-acquisition compound from methane-oxidizing bacteria. Science 305:1612–1615 [DOI] [PubMed] [Google Scholar]
- 24. Kirsten A, et al. 2011. Contributions of five secondary metal uptake systems to metal homeostasis of Cupriavidus metallidurans CH34. J. Bacteriol. 193:4652–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kump LR, Barley ME. 2007. Increased subaerial volcanism and the rise of atmospheric oxygen 2.5 billion years ago. Nature 448:1033–1036 [DOI] [PubMed] [Google Scholar]
- 26. Leblanc SK, Oates CW, Raivio TL. 2011. Characterization of the induction and cellular role of the BaeSR two-component envelope stress response of Escherichia coli. J. Bacteriol. 193:3367–3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee SM, et al. 2002. Functional analysis of the Escherichia coli zinc transporter ZitB. FEMS Microbiol. Lett. 215:273–278 [DOI] [PubMed] [Google Scholar]
- 28. Leonhartsberger S, Huber A, Lottspeich F, Böck A. 2001. The hydH/G genes from Escherichia coli code for a zinc and lead responsive two-component regulatory system. J. Mol. Biol. 307:93–105 [DOI] [PubMed] [Google Scholar]
- 29. Macomber L, Elsey SP, Hausinger RP. 2011. Fructose-1,6-bisphosphate aldolase (class II) is the primary site of nickel toxicity in Escherichia coli. Mol. Microbiol. 82:1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maret W. 2011. Metals on the move: zinc ions in cellular regulation and in the coordination dynamics of zinc proteins. Biometals 24:411–418 [DOI] [PubMed] [Google Scholar]
- 31. Masse E, Gottesman S. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:4620–4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mehta N, Olson JW, Maier RJ. 2003. Characterization of Helicobacter pylori nickel metabolism accessory proteins needed for maturation of both urease and hydrogenase. J. Bacteriol. 185:726–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Munkelt D, Grass G, Nies DH. 2004. The chromosomally encoded cation diffusion facilitator proteins DmeF and FieF from Wautersia metallidurans CH34 are transporters of broad metal specificity. J. Bacteriol. 186:8036–8043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Napolitano M, et al. 2012. Characterization of the response to zinc deficiency in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 194:2426–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nies D, Mergeay M, Friedrich B, Schlegel HG. 1987. Cloning of plasmid genes encoding resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus CH34. J. Bacteriol. 169:4865–4868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nies DH. 2007. Bacterial transition metal homeostasis, p 118–142 In Nies DH, Silver S. (ed), Molecular microbiology of heavy metals, vol 6 Springer-Verlag, Berlin, Germany [Google Scholar]
- 37. Nies DH. 2003. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 27:313–339 [DOI] [PubMed] [Google Scholar]
- 38. Nies DH. 2007. How cells control zinc homeostasis. Science 317:1695–1696 [DOI] [PubMed] [Google Scholar]
- 39. Nies DH, Grass G. 2009. Chapter 5. 4. 4. 3. Transition metal homeostasis. In Böck A, Curtiss R, III, Kaper JB, Neidhardt FC, Nyström T, Rudd KE, Squires CL. (ed), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC: http://www.ecosal.org/ [Google Scholar]
- 40. Odermatt A, Suter H, Krapf R, Solioz M. 1992. An ATPase operon involved in copper resistance by Enterococcus hirae. Ann. N. Y. Acad. Sci. 671:484–486 [DOI] [PubMed] [Google Scholar]
- 41. O'Halloran TV, Culotta VC. 2000. Metallochaperones, an intracellular shuttle service for metal ions. J. Biol. Chem. 275:25057–25060 [DOI] [PubMed] [Google Scholar]
- 42. Outten CE, O'Halloran TV. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488–2492 [DOI] [PubMed] [Google Scholar]
- 43. Owen GA, Pascoe B, Kallifidas D, Paget MSB. 2007. Zinc-responsive regulation of alternative ribosomal protein genes in Streptomyces coelicolor involves Zur and σR. J. Bacteriol. 189:4078–4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Panina EM, Mironov AA, Gelfand MS. 2003. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc. Natl. Acad. Sci. U. S. A. 100:9912–9917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parnell J, Boyce AJ, Mark D, Bowden S, Spinks S. 2010. Early oxygenation of the terrestrial environment during the Mesoproterozoic. Nature 468:290–293 [DOI] [PubMed] [Google Scholar]
- 46. Patzer SI, Hantke K. 1998. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 28:1199–1210 [DOI] [PubMed] [Google Scholar]
- 47. Petrarca P, Ammendola S, Pasquali P, Battistoni A. 2010. The Zur-regulated ZinT protein is an auxiliary component of the high-affinity ZnuABC zinc transporter that facilitates metal recruitment during severe zinc shortage. J. Bacteriol. 192:1553–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ranquet C, Ollagnier-de-Choudens S, Loiseau L, Barras F, Fontecave M. 2007. Cobalt stress in Escherichia coli. J. Biol. Chem. 282:30442–30451 [DOI] [PubMed] [Google Scholar]
- 49. Rensing C, Fan B, Sharma R, Mitra B, Rosen BP. 2000. CopA: an Escherichia coli Cu(I)-translocating P-type ATPase. Proc. Natl. Acad. Sci. U. S. A. 97:652–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rensing C, Ghosh M, Rosen BP. 1999. Families of soft-metal-ion-transporting ATPases. J. Bacteriol. 181:5891–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rensing C, Mitra B, Rosen BP. 1997. The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc. Natl. Acad. Sci. U. S. A. 94:14326–14331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schauer K, Gouget B, Carriere M, Labigne A, de Reuse H. 2007. Novel nickel transport mechanism across the bacterial outer membrane energized by the TonB/ExbB/ExbD machinery. Mol. Microbiol. 63:1054–1068 [DOI] [PubMed] [Google Scholar]
- 53. Schauer K, Rodionov DA, de Reuse H. 2008. New substrates for TonB-dependent transport: do we only see the ‘tip of the iceberg’? Trends Biochem. Sci. 33:330–338 [DOI] [PubMed] [Google Scholar]
- 54. Shin JH, Oh SY, Kim SJ, Roe JH. 2007. The zinc-responsive regulator Zur controls a zinc uptake system and some ribosomal proteins in Streptomyces coelicolor A3(2). J. Bacteriol. 189:4070–4077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stojiljkovic I, Bäumler AJ, Hantke K. 1994. Fur regulon in gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a Fur titration assay. J. Mol. Biol. 236:531–545 [DOI] [PubMed] [Google Scholar]
- 56. Thorgersen MP, Downs DM. 2007. Cobalt targets multiple metabolic processes in Salmonella enterica. J. Bacteriol. 189:7774–7781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tottey S, Harvie DR, Robinson NJ. 2007. Understanding how cells allocate metals, p 3–36 In Nies DH, Silver S. (ed), Molecular microbiology of heavy metals, vol 6 Springer-Verlag, Berlin, Germany [Google Scholar]
- 58. Tottey S, et al. 2008. Protein-folding location can regulate manganese binding versus copper- or zinc-binding. Nature 455:1138–1142 [DOI] [PubMed] [Google Scholar]
- 59. Umena Y, Kawakami K, Shen JR, Kamiya N. 2011. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 A. Nature 473:55–60 [DOI] [PubMed] [Google Scholar]
- 60. Waldron KJ, Rutherford JC, Ford D, Robinson NJ. 2009. Metalloproteins and metal sensing. Nature 460:823–830 [DOI] [PubMed] [Google Scholar]
- 61. Weast RC. 1984. CRC handbook of chemistry and physics, 64th ed. CRC Press, Inc., Boca Raton, FL [Google Scholar]