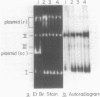
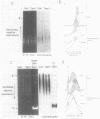
Abstract
The method for detecting the small fraction (1%) of transcriptionally active SV40 minichromosomes, in the presence of the bulk minichromosomes, (14) has been applied to directly analyze the topology of transcribed and non-transcribed minichromosomal DNA. We show here that DNA of both transcribed and non-transcribed minichromosomes have the same number of supercoils which are constrained by nucleosomes. In addition, minichromosomal DNA of both fractions have a low level of unconstrained supercoils (1-2 extra supercoils) which can be relaxed in vitro by topoisomerase I.
Full text
PDF


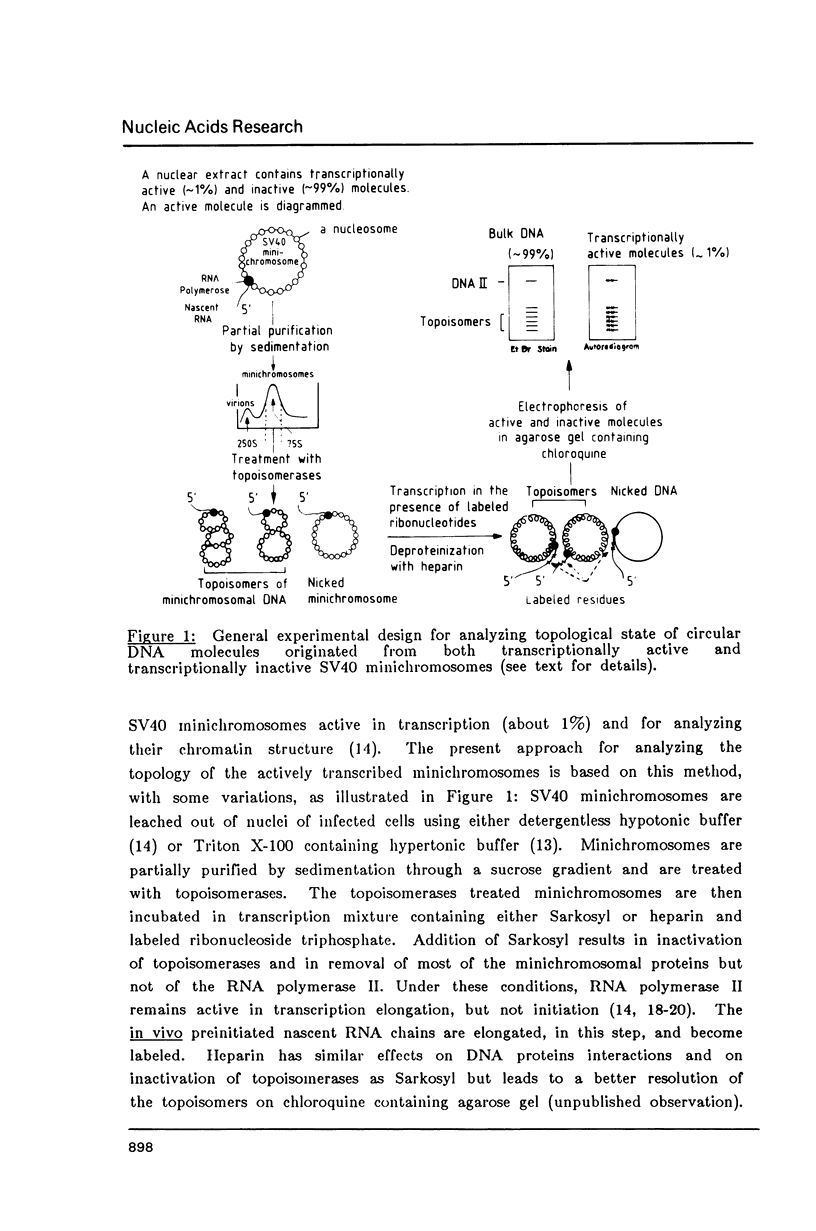

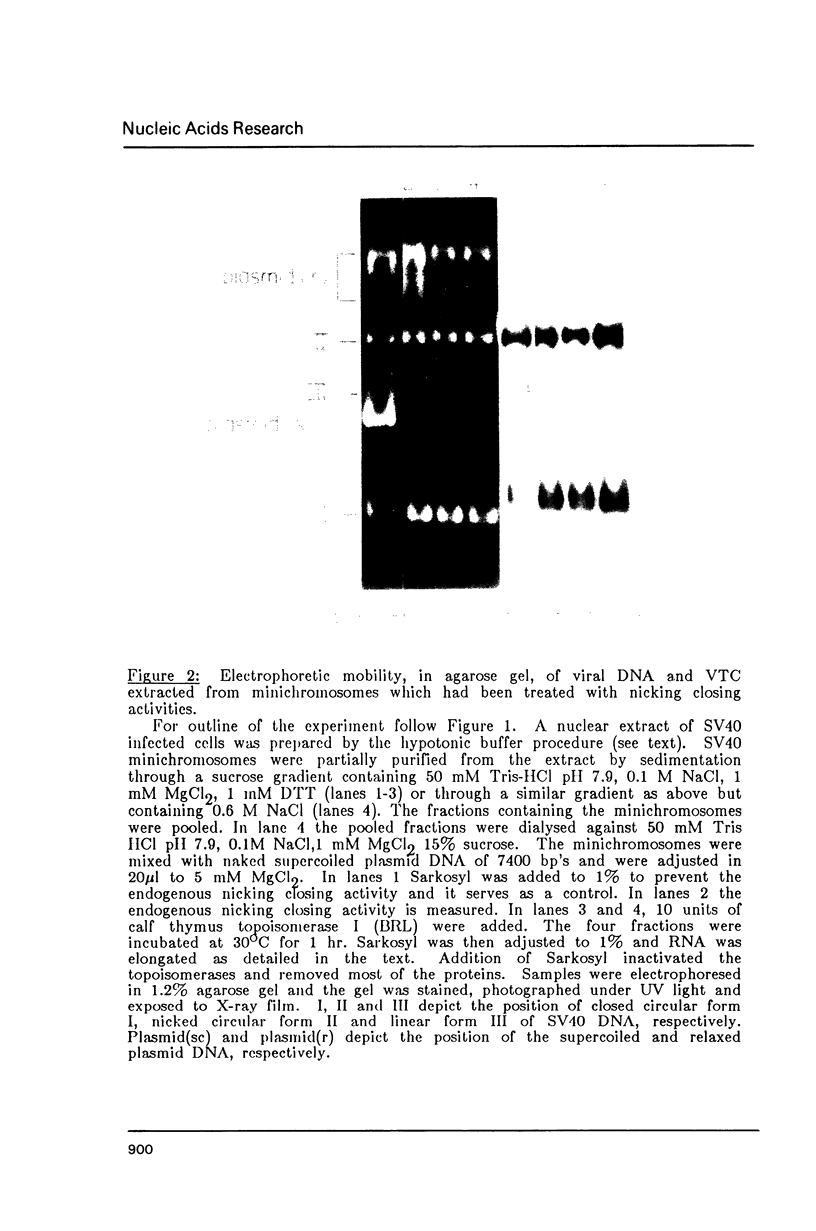




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abulafia R., Ben-Ze'ev A., Hay N., Aloni Y. Control of late simian virus 40 transcription by the attenuation mechanism and transcriptionally active ternary complexes are associated with the nuclear matrix. J Mol Biol. 1984 Feb 5;172(4):467–487. doi: 10.1016/s0022-2836(84)80018-2. [DOI] [PubMed] [Google Scholar]
- Ambrose C., McLaughlin R., Bina M. The flexibility and topology of simian virus 40 DNA in minichromosomes. Nucleic Acids Res. 1987 May 11;15(9):3703–3721. doi: 10.1093/nar/15.9.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choder M., Bratosin S., Aloni Y. A direct analysis of transcribed minichromosomes: all transcribed SV40 minichromosomes have a nuclease-hypersensitive region within a nucleosome-free domain. EMBO J. 1984 Dec 1;3(12):2929–2936. doi: 10.1002/j.1460-2075.1984.tb02234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariglio P., Buss J., Green M. H. Sarkosyl activation of RNA polymerase activity in mitotic mouse cells. FEBS Lett. 1974 Aug 30;44(3):330–333. doi: 10.1016/0014-5793(74)81170-1. [DOI] [PubMed] [Google Scholar]
- Gariglio P., Mousset S. Isolation and partial characterization of a nuclear RNA polymerase - SV40 DNA complex. FEBS Lett. 1975 Aug 1;56(1):149–155. doi: 10.1016/0014-5793(75)80130-x. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits E. B., Bratosin S., Aloni Y. A nucleosome-free region in SV40 minichromosomes. Nature. 1980 May 22;285(5762):263–265. doi: 10.1038/285263a0. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Liu L. F. Association of eukaryotic DNA topoisomerase I with nucleosomes and chromosomal proteins. Nucleic Acids Res. 1983 Jan 25;11(2):461–472. doi: 10.1093/nar/11.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W., Müller U., Eicken I., Wendel I., Zentgraf H. Biochemical and ultrastructural analysis of SV40 chromatin. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):227–244. doi: 10.1101/sqb.1978.042.01.025. [DOI] [PubMed] [Google Scholar]
- Müller U., Zentgraf H., Eicken I., Keller W. Higher order structure of simian virus 40 chromatin. Science. 1978 Aug 4;201(4354):406–415. doi: 10.1126/science.208155. [DOI] [PubMed] [Google Scholar]
- Petryniak B., Lutter L. C. Topological characterization of the simian virus 40 transcription complex. Cell. 1987 Jan 30;48(2):289–295. doi: 10.1016/0092-8674(87)90432-6. [DOI] [PubMed] [Google Scholar]
- Saragosti S., Moyne G., Yaniv M. Absence of nucleosomes in a fraction of SV40 chromatin between the origin of replication and the region coding for the late leader RNA. Cell. 1980 May;20(1):65–73. doi: 10.1016/0092-8674(80)90235-4. [DOI] [PubMed] [Google Scholar]
- Shure M., Pulleyblank D. E., Vinograd J. The problems of eukaryotic and prokaryotic DNA packaging and in vivo conformation posed by superhelix density heterogeneity. Nucleic Acids Res. 1977;4(5):1183–1205. doi: 10.1093/nar/4.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden R. R., Carlson J. O., Pettijohn D. E. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980 Oct;21(3):773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Waldeck W., Theobald M., Zentgraf H. Catenation of DNA by eucaryotic topoisomerase II associated with simian virus 40 minichromosomes. EMBO J. 1983;2(8):1255–1261. doi: 10.1002/j.1460-2075.1983.tb01578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]