Abstract
HIV-1-specific cytotoxic T cell responses are expanded during advanced HIV-1 infection but seem unable to effectively protect the host against disease progression. These cells are able to produce gamma interferon and remain metabolically active but have defective proliferative activities, shortened telomeric DNA, and other signs of accelerated aging. To investigate the molecular mechanisms underlying the premature senescence of HIV-1-specific T cells, we focused here on the expression and function of a group of six nucleoproteins that are responsible for protecting and maintaining the structural integrity of telomeric DNA and are commonly referred to as “shelterin.” We show that in progressive HIV-1 infection, the two major shelterin components TRF2 and TPP1 are selectively reduced in HIV-1-specific CD8 T cells, but not in T cells recognizing alternative viral species. This coincided with increased recruitment of 53BP1, a prominent DNA damage response factor, to telomeric DNA sites and was associated with elevated expression of the tumor suppressor p16INK4a, which causes cellular growth inhibition in response to structural DNA damage. Notably, defective shelterin function and upregulation of p16INK4a remained unaffected by experimental blockade of PD-1, indicating a possibly irreversible structural defect in HIV-1-specific CD8 T cells in progressors that cannot be overcome by manipulation of inhibitory cell-signaling pathways. These data suggest that shelterin dysfunction and ensuing upregulation of the tumor suppressor p16INK4a promote accelerated aging of HIV-1-specific T cells during progressive HIV-1 infection.
INTRODUCTION
In the vast majority of individuals, infection with human immunodeficiency virus type 1 (HIV-1) leads to a chronic progressive infection with ongoing moderate to high-level viremia. This persistent viral replication results in a proinflammatory state that is characterized by high levels of circulating stimulatory cytokines and by massive activation of T cells, B cells, and dendritic cells (2). Moreover, continuous viral replication in lymphoid tissues of the gut leads to destruction of the microanatomical mucosal barrier and facilitates translocation of bacterial antigens, which further augments generalized immune activation (5, 35). In a vicious circle, this immune activation increases the susceptibility of CD4 T cells to HIV-1 infection and stimulates more effective HIV-1 replication. Together, these mechanisms exhaust the regenerative resources of the immune system and cause generalized dysfunction of multiple immune cells. Interestingly, due to alternative mechanisms of immune regulation (15–17), persistent HIV-1 replication and ensuing immune activation seem to affect CD4 and CD8 T cells differently. While CD4 T cells seem to undergo apoptosis and physical elimination, as evidenced by progressively declining cell numbers during chronic infection, CD8 T cells are numerically expanded but acquire a distinct functional and phenotypic profile that closely resembles the aging and senescent cells typically encountered in elderly individuals (6, 13, 38). Functionally, these cells remain metabolically active and are capable of executing specific lymphocellular effector functions, such as gamma interferon secretion, but lose their ability for antigen-specific proliferation and may develop immunosuppressive properties (14). On a molecular level, these changes are combined with a progressive loss of telomere length and a functional decline of telomerase, an enzyme that is selectively expressed in lymphocytes to antagonize telomere erosion and cell aging (42). These specific functional, phenotypic, and molecular properties seem to be particularly pronounced in HIV-1-specific CD8 T cells, which may play an important role in HIV-1 immune control but are particularly vulnerable to HIV-1-associated immune activation due to their direct recognition of HIV-1 antigens (23). Indeed, telomere length in these cells approaches the Hayflick limit of terminal senescence, and the cells are highly dysfunctional during progressive HIV-1 infection (4, 22, 28). The molecular mechanisms that contribute to the specific senescent profile of CD8 T cells in progressive HIV-1 infection are unclear.
In recent years, it has been recognized that telomere stability and integrity are maintained by a group of nucleoproteins that are located at terminal chromosomal DNA segments. This protein complex, termed “shelterin,” consists of six molecules (telomeric-repeat-binding factors 1 and 2 [TRF1 and TRF2], TRF1-interacting nuclear protein 2 [TIN2], repressor/activator protein 1 [RAP1], TPP1, and protection of telomere 1 [POT1]) and is charged with providing a protective cap on telomeric DNA that conserves telomere integrity while simultaneously regulating the enzymatic activity of telomerase (11, 30). In the present study, we analyzed this shelterin complex in antigen-specific CD8 T cells collected from persons with progressive and nonprogressive HIV-1 infection. We show that several of these telomere maintenance factors are selectively reduced in HIV-specific CD8 T cells from progressors, which is associated with signs of a DNA damage response and upregulation of the tumor suppressor p16INK4a, which induces cellular growth arrest and senescence. Thus, the reduced expression of shelterin proteins appears to represent an important structural defect in HIV-1-specific CD8 T cells that may contribute importantly to accelerated aging and immune deficiency associated with progressive HIV-1 infection.
MATERIALS AND METHODS
Patients.
HIV-1-infected individuals were recruited at the Massachusetts General Hospital and affiliated hospitals. The clinical and demographical characteristics of the study subjects are summarized in Table 1. These HIV-1 patients were not undergoing antiretroviral therapy at the time of study participation. All subjects gave written informed consent to participate, and the study was approved by the Massachusetts General Hospital Institutional Review Board.
Table 1.
Clinical and demographic characteristics of study subjects
| Subject group | Age (yr) [median(range)] | Sex (male/female) | CD4 T cell count (cells/μl) [median(range)] | Viral load (copies/ml) [median(range)] | Target epitopes of analyzed CTL populations |
|---|---|---|---|---|---|
| Progressors (n = 11) | 51.5 (39–72) | 9/2 | 458 (331–568) | 139,000 (16,400–402,000) | B8-FL8 (FLKEKGGL, HIV-1 nef) |
| B8-EI8 (EIYKRWII, HIV-1 p24) | |||||
| A2-SL9 (SLYNTVATL, HIV-1 p17) | |||||
| B27-KK10 (KRWIILGLNK, HIV-1 p24) | |||||
| A11-QK10 (QVPLRPMTYK,, HIV-1 nef) | |||||
| A11-KK9 (KIRLRPGGK, HIV-1 p17) | |||||
| B7-IL-9 (IPRRIRQGL, HIV-1 gp41) | |||||
| A2-NV9 (NLVPMVATV, CMV pp65) | |||||
| B8-FL9 (FLRGRAYGL, EBV EBNA-3a) | |||||
| Controllers (n = 13) | 45 (34–64) | 12/1 | 843 (328–1,415) | <50 (<50–1,037) | B8-FL8 (FLKEKGGL, HIV-1 nef) |
| B8-EI8 (EIYKRWII, HIV-1 p24) | |||||
| A2-SL9 (SLYNTVATL, HIV-1 p17) | |||||
| B27-KK10 (KRWIILGLNK, HIV-1 p24) | |||||
| B7-IL-9 (IPRRIRQGL, HIV-1 gp41) | |||||
| B57-KF11 (KAFSPEVIPMF, p24) | |||||
| A2-NV9 (NLVPMVATV, CMV pp65) | |||||
| B8-FL9 (FLRGRAYGL, EBV EBNA-3a) |
Cell-sorting and flow cytometry studies.
Peripheral blood mononuclear cells (PBMC) were stained with tetramers/pentamers (refolded with epitopic HIV-1, Epstein-Barr virus [EBV], or cytomegalovirus [CMV] peptides) for 20 min at room temperature, followed by 15 min of surface staining with CD3 and CD8 antibodies, and processed by cell sorting at 70 lb/in2 using a 10-color fluorescence-activated cell sorter (FACS) ARIA instrument. Electronic compensation was performed with antibody-capture beads stained separately with the individual antibodies used in the test samples. Live sorting was performed in an appropriate and specifically designated biosafety hood (Baker hood) according to an approved biosafety sorting protocol.
RT-PCR.
Total RNA from sorted cells was extracted using the RNeasy kit (Qiagen), and cDNA was generated by reverse transcription with random hexameric primers at 50°C for 60 min according to standard procedures. Quantitative SYBR green-based real-time (RT) PCR was then performed using primers described previously (31). The PCR program included 40 cycles, each at 95°C for 30 s, 60°C for 60 s, and 72°C for 30 s. For the semiquantitative assessment of the mRNA expression of Rad17, Ku80, Mre11, Rad50, RPA1, human EST1A (hEST1A), p16INK4a, p21, and p53, real-time quantitative PCR was carried out using commercially available TaqMan Gene Expression Assays. The β-actin gene was used as a housekeeping gene.
Immunofluorescence studies.
HIV-1-specific CD8 T cells were isolated by cell sorting as described above. The cells were then deposited onto positively charged slides, fixed with methanol at −20°C for 20 min, and rehydrated with phosphate-buffered saline (PBS) for 10 min. Following incubation with Target Retrieval Solution (Dako Cytomation) at 95°C for 40 min, the slides were allowed to cool for 20 min at room temperature and incubated with blocking solution for 1 h (1% cold water fish gelatin, 0.5% Triton X, 0.5% donkey serum in PBS) (9). The slides were subsequently stained with primary antibodies (mouse monoclonal anti-TRF1 from Abcam and rabbit polyclonal anti-53BP1 from Novus Biologicals) at 1:300 dilution in blocking solution at 4°C overnight in a moist chamber. Secondary antibodies (Alexa 488-conjugated donkey anti-mouse and Alexa 555-conjugated donkey anti-rabbit polyclonal antibodies from Invitrogen) were applied at 1:500 dilution in blocking solution and incubated at 37°C for 1 h. The cells were washed thoroughly after primary- and secondary-antibody incubations with washing solution (0.1% Triton X, 1% bovine serum albumin [BSA] in PBS). The slides were counterstained with DAPI (4′,6-diamidino-2-phenylindole), mounted with 70% glycerol and 0.5% N-propyl gallate (Sigma-Aldrich) to prevent photobleaching, and visualized using confocal microscopy (LSM510; Zeiss) and Zen Imaging software (Zeiss).
siRNA-mediated gene knockout.
CD4 T cells were nucleofected using the Nucleofector device (Lonza) according to the manufacturer's protocol. Briefly, CD4 T cells were suspended in 100 μl of transfection solution (Lonza), and p16INK4a-specific or control small interfering RNA (siRNA) (Dharmacon) was added at a concentration of 4 nmol/ml. Samples were then transferred into nucleofection cuvettes and transfected using program T-023. Afterward, the cells were resuspended in culture medium supplemented with 20% fetal calf serum. Twenty-four hours after infection, expression of p16 INK4a was assessed using quantitative RT-PCR.
Proliferation assays.
PBMC were first suspended at 106/ml in PBS and incubated at 37°C for 7 min with 0.25 μM carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes). After the addition of serum and washes with PBS, the cells were suspended at 106/ml in medium (RPMI 1640 supplemented with glutamine, 10% human serum, penicillin, and streptomycin). Pools of overlapping HIV-1-specific peptides representing the entire amino acid sequence of HIV-1 Gag were then added at a concentration of 20 ng/ml/peptide. On day 6, cells were harvested, washed with PBS, and stained with tetramers/pentamers and monoclonal antibodies (MAbs) (CD8 allophycocyanin [APC] and CD3 peridinin chlorophyll protein [PerCP]; BD Biosciences). The cells were then washed and fixed in 1% paraformaldehyde and subjected to flow cytometric analysis. In the indicated experiments, PBMC were initially exposed to a PD-L1-blocking antibody for 16 h (0.5 mg/ml; BioLegend Inc.) and washed once before continuing incubation.
Statistics.
Data from different study cohorts were expressed using box-and-whisker plots. Statistical comparisons were made using Student t tests with Bonferroni corrections for multiple comparisons as appropriate.
RESULTS
Shelterin disintegration in HIV-1-specific CD8 T cells.
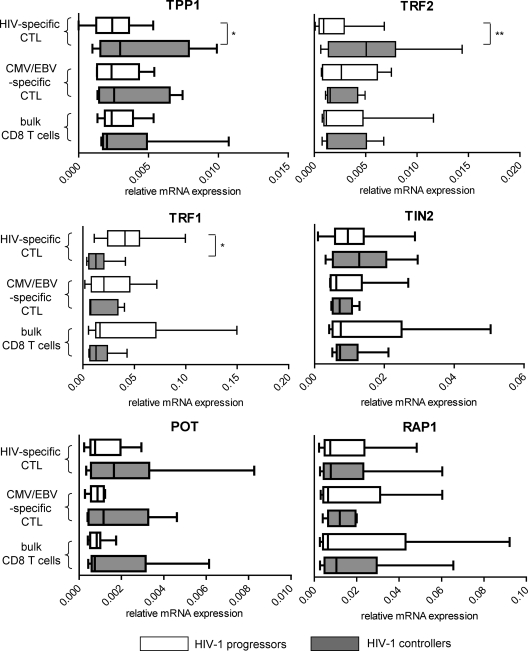
Shelterin proteins represent a group of the six nucleoproteins TRF1, TRF2, TIN2, RAP1, TPP1, and POT1, which are located at telomeric chromosomal DNA ends and have specific functions in protecting telomere integrity and positively or negatively regulating telomerase activity. To investigate shelterin expression in HIV-1-specific CD8 T cells, we initially analyzed the mRNA encoding these proteins in tetramer-positive HIV-1- or CMV/EBV-specific CD8 T cells sorted from untreated HIV-1-infected persons with progressive or spontaneous control of HIV-1 replication. Autologous bulk CD8 T cells were analyzed for reference purposes. As shown in Fig. 1, we observed that the mRNA expression of TRF2 and TPP1, two prominent members of the shelterin complex that are critically involved in telomere homeostasis, was significantly reduced in HIV-1-specific CD8 T cells from progressors compared to those from controllers. There was also a trend toward stronger expression of POT1 mRNA in HIV-1-specific cytotoxic T cells from controllers; however, this did not reach statistical significance (P = 0.09). The mRNA expression pattern of TRF1, a negative regulator of telomerase activity (39), showed the opposite pattern, with significant upregulation in HIV-1-specific CD8 T cells from progressors compared to those from controllers. Notably, the mRNA expression of the analyzed shelterin proteins did not differ between CMV/EBV-specific CD8 T cells or bulk CD8 T cells from the two patient populations. We also analyzed the mRNA expression profile of an array of additional telomere maintenance genes that have adjunct functions in maintaining telomere integrity, including Rad17, Ku80, Mre11, Rad50, RPA1, and hEST1A genes. However, there were no significant differences in the mRNA expression levels for any of these genes between HIV-1 controllers and progressors (data not shown). Overall, these results indicate altered mRNA expression of shelterin components in HIV-1-specific CD8 T cells from progressors and suggest that defective shelterin structure may be involved in the accelerated aging observed previously in HIV-1-specific CD8 T cells.
Fig 1.
Altered expression of shelterin components in HIV-1-specific CD8 T cells from progressors. Box-and-whisker plots show the mRNA expression intensities (the minimum, the maximum, and the 25th, 50th, and 75th percentiles) of the indicated gene products in sorted HIV-1-specific CD8 T cells or autologous CMV/EBV-specific CD8 T cells or bulk CD8 from HIV-1 progressors or controllers. Gene expression was normalized to the housekeeping β-actin gene. Significance was tested using one-way analysis of variance (ANOVA), followed by post hoc analysis using the Tukey test for multiple comparisons (*, P < 0.05; **, P < 0.01).
Shelterin dysfunction leads to a DNA damage response in HIV-1-specific CD8 T cells.
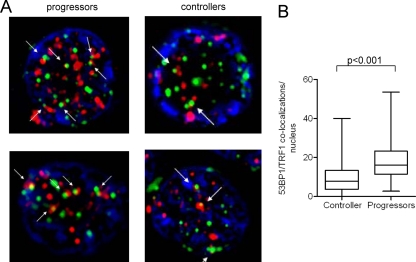
Prior studies have shown that loss of the shelterin proteins can lead to “naked” telomeric DNA that elicits a DNA damage response and leads to the recruitment of DNA repair enzymes to chromosomal DNA ends (7, 19). The telomeric accumulation of such DNA repair factors can be visualized as “telomere dysfunction-induced foci” (TIF) and represents a molecular correlate of shelterin dysfunction (36). To investigate whether the alterations in shelterin composition in HIV-1-specific CD8 T cells from progressors is associated with such a DNA damage response at telomeric DNA sites, we analyzed the subcellular localization of the DNA damage response factor 53BP1. For this purpose, tetramer-positive HIV-1-specific CD8 T cells from controllers and progressors were sorted, and the possible recruitment of 53BP1 to telomeric DNA sites was analyzed by performing coimmunofluorescence staining of these proteins and the telomeric protein TRF1, which exclusively localizes to chromosomal DNA ends. As shown in Fig. 2, we observed that most of the visualized TRF1 molecules at telomeric DNA in HIV-1-specific CD8 T cells from progressors colocalized with 53BP1, indicating that 53BP1 is preferentially recruited to telomeric DNA in these cells. In contrast, molecular sites of TRF1 in HIV-1-specific CD8 T cells from controllers less frequently costained with 53BP1 (Fig. 2). These data are consistent with a cellular DNA damage response that occurs at telomeric DNA sites in HIV-1-specific CD8 T cells from progressors.
Fig 2.
Colocalization of the DNA damage response factor 53BP1 and TRF1 in HIV-1-specific CD8 T cells. Lymphocytes were labeled with anti-53BP1 (red) and anti-TRF1 (green) antibodies; DAPI was used as a nuclear counterstain. (A) Representative examples of 53BP1 and TRF1 staining in sorted tetramer-positive HIV-1-specific CD8 T cells. The arrows indicate costaining of TRF1 and 53BP1 as TIF. The images are shown at a magnification of ×100. (B) Cumulative analysis of 53BP1/TRF1 colocalization in HIV-1-specific CD8 T cells. A total of 120 cell nuclei (a single focal plane for each nucleus) from progressors and controllers (n = 5 each) were analyzed. Significance was tested using a two-sided, unpaired t test.
p16INK4a-mediated growth inhibition in HIV-1-specific CD8 T cells from progressors.
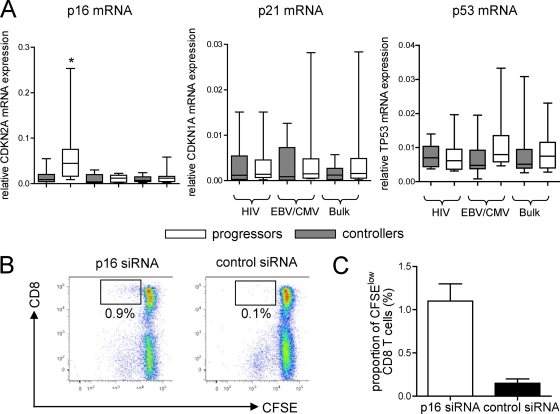
Cellular DNA damage related to shelterin/telomere dysfunction may put cells at risk for malignant transformation and can lead to activation of tumor suppressor mechanisms that can arrest the cell cycle (26). These tumor suppressor mechanisms involve cyclin-dependent kinase inhibitors, such as p16INK4a or p21 and its upstream regulator p53. To analyze whether such tumor suppressor genes are upregulated in HIV-1-specific CD8 T cells from progressors or controllers, we tested their mRNA expression in sorted tetramer-positive HIV-1-specific CD8 T cells from our two study cohorts. Autologous CMV/EBV-specific CD8 T cells and bulk CD8 T cells were analyzed for comparison purposes. While neither p21 nor p53 was differentially expressed between progressors and controllers from any of the analyzed cell compartments (data not shown), we did observe that p16INK4a was significantly upregulated in HIV-1-specific CD8 T cells from progressors (Fig. 3A). This suggests that depletion of shelterin proteins in HIV-1-specific CD8 T cells leads to a DNA damage response that induces upregulation of the tumor suppressor gene p16INK4a. To investigate whether p16INK4a upregulation may be responsible for the defective proliferative activities of HIV-1-specific CD8 T cells described previously, we performed functional T cell proliferation assays after inhibition of p16INK4a using targeted siRNA-mediated gene knockout. As demonstrated in Fig. 3B and C, we found that HIV-1-specific CD8 T cells that had lost their proliferative abilities regained proliferative activity following knockout of p16INK4a, consistent with an inhibitory effect of p16INK4a on cell cycle progression. In conclusion, these experiments suggest that upregulation of p16INK4a can contribute to a functional inhibition of HIV-1-specific CD8 T cell proliferation in progressive infection.
Fig 3.
Upregulation of p16INK4a mRNA in HIV-1-specific CD8 T cells from progressors. (A) Box-and-whisker plots reflect the expression intensities of p16INK4a, p21, and p53 in sorted HIV-1, CMV/EBV, or bulk CD8 T cells from HIV-1 progressors or controllers. Gene expression was normalized to the housekeeping β-actin gene. Significance was tested using one-way ANOVA, followed by post hoc analysis using the Tukey test for multiple comparison (*, P < 0.05). (B and C) p16INK4a inhibits the proliferative activities of HIV-1-specific CD8 T cells. (B) Dot plot of the proliferative activities of CD8 T cells following stimulation with gag peptides in the presence or absence of siRNA-mediated downregulation of p16INK4a. The percentages indicate the proportions of CFSElow CD8 T cells. (C) Proliferative activities of gag-specific CD8 T cells in the presence or absence of p16INK4a downregulation. Cumulative data from 2 independent experiments are shown. The error bars indicate standard deviations.
Shelterin dysfunction and p16INKa upregulation are not affected by PD-L1 blockade.
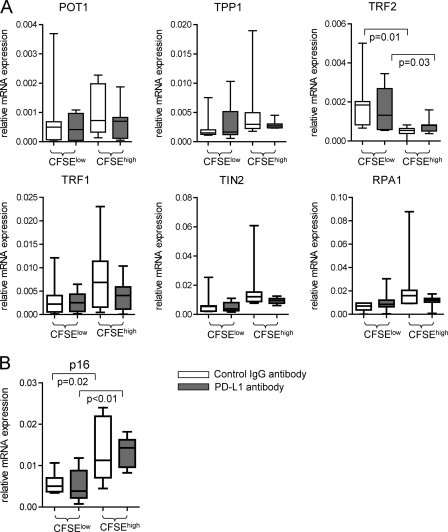
Prior studies have shown that antibody-mediated blockade of PD-1/PD-L1 can lead to increased proliferative activities of HIV-1-specific CD8 T cells from progressors (10, 37) and may improve clinical outcomes of simian immunodeficiency virus (SIV) infection in rhesus macaques (41). These beneficial effects may be related in part to increased telomere length and telomerase activity in HIV-1-specific CD8 T cells following exposure to PD-1/PD-L1-inhibiting antibodies (23). To investigate whether blockade of PD-L1 also affects the expression of shelterin proteins in HIV-1-specific CD8 T cells, we analyzed the mRNA expression of the shelterin components in HIV-1-specific CD8 T cells that proliferated after stimulation with overlapping HIV-1 gag peptides in the presence or absence of PD-L1 antibodies. In agreement with prior work, we observed that the proportion of CD8 T cells proliferating after stimulation with gag was 1.5- to 2.5-fold elevated in the presence of PD-L1 antibodies (data not shown). However, expression of none of the shelterin components was significantly affected by exposure of cells to PD-L1 antibodies (Fig. 4). We also observed no effect of PD-L1 blockade on other telomere maintenance genes, such as Rad17, Ku80, Mre11, Rad50, RPA1, and hEST1A genes (data not shown). As expected, expression of the cell cycle inhibitor p16INK4a was higher in nonproliferating HIV-1-specific CD8 T cells than in proliferating CD8 T cells, but there was no detectable impact of PD-L1 antibodies on p16INK4a expression. Overall, these results suggest that altered expression of shelterin components and upregulation of the cell cycle inhibitor p16INK4a are not related to PD-L1-dependent growth characteristics of HIV-1-specific CD8 T cells and may represent a structural defect in HIV-1-specific CD8 T cells that cannot be overcome by PD-L1 blockade.
Fig 4.
PD-L1 blockade does not correct shelterin dysfunction in HIV-1-specific CD8 T cells. PBMC from HIV-1 progressors were labeled with CFSE and stimulated with overlapping peptides corresponding to HIV-1 gag. On day 6, proliferating CFSElow and nonproliferating CFSEhigh tetramer-positive CD8 T cells were sorted. (A and B) Box-and-whisker plots reflecting shelterin gene expression (A) or p16 expression (B) in proliferating versus nonproliferating HIV-1-specific CD8 T cells in the presence or absence of PD-L1 blocking antibodies. Gene expression data were normalized to the housekeeping β-actin gene. Significance was tested using two-sided unpaired t tests.
DISCUSSION
A growing number of observations indicate a role for accelerated aging in HIV-1 disease pathogenesis (1, 29). Indeed, many of the immunological alterations observed in progressive HIV-1 infection, such as naïve-T-cell losses, elevations in total serum IgG levels, thymic dysfunction, impaired mucosal immunity, and alterations in fat or carbohydrate metabolism, are all reminiscent of typical changes observed in the elderly. However, accelerated aging in HIV-1 infection may be particularly visible in HIV-1-specific cytotoxic T cells, which may be most susceptible to HIV-1-associated immune activation due to direct recognition of HIV-1 antigens. Indeed, previous work indicated a severe shortening of telomeric DNA in HIV-1-specific cytotoxic T cells and associated profound reduction of telomerase activity (23) in comparison to T cells specific for other viral pathogens. The present work extends these findings and demonstrates that telomere shortening in HIV-1-specific CD8 T cells is associated with disintegration of the shelterin complex, which under physiological conditions serves as a protective cap at telomeric DNA ends and regulates the enzymatic activity of telomerase. In the setting of a defective shelterin complex, telomeres are exposed as naked DNA, which is perceived as a DNA double-strand break and triggers the initiation of a DNA damage response to prevent structural DNA damage, such as chromosomal recombination or nonhomologous end-to-end joining of chromosomal DNA ends (12, 40). Such structural chromosomal alternations can result in uncontrolled malignant transformation of cells and represent a serious threat to the host. In this work, we demonstrate that the reduced expression of the shelterin proteins TRF2, TIN2, and, to a lesser extent, POT1 in HIV-1-specific CD8 T cells is indeed associated with signs of a DNA damage response, as evidenced by increased recruitment of the DNA damage response factor 53BP1 to telomeric DNA. Such telomere dysfunction-induced foci can be experimentally induced by ionizing radiation; deletion of protective shelterin proteins, such as TRF2 (32); or high-level cell turnover and associated replicative stress (8). Our work shows that such signs of a DNA damage response at telomeric DNA ends are also detectable in vivo in HIV-1-specific CD8 T cells under conditions of chronic immune activation and high-level viral antigenemia.
An important finding of this study is the selective upregulation of the cyclin-dependent kinase inhibitor p16INK4a in HIV-1-specific CD8 T cells from progressors. p16INK4a is a potent tumor suppressor that can block CDK4- and CDK6-dependent cell cycle progression and is selectively upregulated following cellular DNA damage resulting from various stressors, such as exogenous toxins, ionizing radiation, or activation of oncogenes (33). Although our data do not prove that increased expression of p16INK4a in HIV-1-specific CD8 T cells represents a direct consequence of the observed shelterin defects and the associated exposure of naked telomeric DNA, it is quite likely that elevated p16INK4a expression primarily results from the perceived DNA damage associated with shelterin dysfunction. Indeed, a series of previous studies have indicated that as a cell cycle inhibitor, p16INK4a has a prominent function to protect the host against proliferative expansion of cells with DNA damage and in this way limits the detrimental effects of possible malignant cell transformation. For instance, several studies have demonstrated that reduced p16INK4a expression is detectable in T (21) and B cell lymphoma cells (24) and in a variety of alternative malignant cell entities, which seems to suggest that in the absence of p16INK4a-mediated cellular growth inhibition, expansion of genetically unstable cells and malignant cell growth can indeed occur. The flip side of this protective function of p16INK4a seems to be related to a functional inhibition of the proliferative activities of HIV-1-specific CD8 T cells. Indeed, our work indicates that the proliferative properties of these cells can be expanded upon experimental knockout of p16INK4a expression, and these observations are in line with the increased in vitro proliferation of polyclonal cytotoxic T cells following the silencing of p16INK4a gene expression described previously (27). Moreover, it is important to recognize that the expression of p16INK4a is exponentially elevated in T lymphocytes during the physiological aging process and closely correlated with aging-promoting behavioral characteristics, such as smoking or physical inactivity (25). In addition, increased p16INK4a expression in mice can cause an age-related functional decline of hematopoietic stem cells (18) and pancreatic islet cells (20), which can be attenuated following experimental deletion of the molecule. This indicates that p16INK4a upregulation represents an important component of the physiological aging process that can lead to progressive growth inhibition and functional defects of cells. In essence, this suggests that increased cellular damage response signals in HIV-1-specific CD8 T cells lead to upregulation of p16INK4a and activation of a premature senescence program at the expense of a functional defect in the proliferative activities of these cells. Such a view is consistent with the hypothesized role of tumor suppressor genes in preventing malignant cell transformation by inducing growth inhibition and promoting cellular aging and senescence (3, 26).
The proliferative properties of HIV-1-specific CD8 T cells are impaired in chronic progressive infection, but these functional defects can be corrected in vitro by exogenous interleukin 2 (IL-2) (34), autologous CD4 T helper cell responses (22), or antibody-mediated blockade of the PD-1 signaling pathway (10, 37). In particular, PD-1/PD-L1-blocking antibodies have been shown to increase telomere length and telomerase activity in HIV-1-specific CD8 T cells (23) and led to improved clinical outcomes in SIV-1-infected rhesus macaques (41). This suggests that despite severely shortened telomeres, HIV-1-specific CD8 T cells from persons with progressive infection have not reached an irreversible form of growth arrest and may possibly be susceptible to immunotherapeutic interventions that can improve their functional and antiviral properties. An important finding here is that despite the increased proliferative activities of HIV-1-specific CD8 T cells after exposure to PD-L1 antibodies, there was no detectable change in the expression of shelterin components or p16INK4a. This suggests that shelterin defects and associated p16INK4a increases do not directly influence the PD-1/PD-L1-dependent growth activities of HIV-1-specific CD8 T cells and may represent more definitive and possibly irreversible cellular features that cannot easily be manipulated for immunotherapeutic purposes. In addition, these data may raise safety concerns about the in vivo use of PD-1/PD-L1 antibodies for increasing the proliferative properties of HIV-1-specific CD8 T cells in which shelterin defects and telomeric DNA damage responses may persist.
In conclusion, this report demonstrates a previously unrecognized disintegration of shelterin proteins with associated signs of a DNA damage response and p16INK4a upregulation in HIV-1-specific CD8 T cells from individuals with progressive disease. These findings identify a molecular correlate of the accelerated aging process during HIV-1 infection and may be relevant for the clinical manipulation of HIV-1-specific CD8 T cells for immunotherapeutic purposes.
ACKNOWLEDGMENTS
This study was supported by the U.S. National Institutes of Health (grants AI078799 and AI074415 to X.G.Y. and AI093203 to M.L.). X.G.Y. and M.L. are recipients of the Clinical Scientist Development Award from the Doris Duke Charitable Foundation (grant number 2009034). Recruitment of HIV-1 controllers was supported by the Bill and Melinda Gates Foundation, the Mark and Lisa Schwartz Foundation, and the International HIV Controller Study.
Footnotes
Published ahead of print 7 March 2012
REFERENCES
- 1. Appay V, Almeida JR, Sauce D, Autran B, Papagno L. 2007. Accelerated immune senescence and HIV-1 infection. Exp. Gerontol. 42:432–437 [DOI] [PubMed] [Google Scholar]
- 2. Appay V, Sauce D. 2008. Immune activation and inflammation in HIV-1 infection: causes and consequences. J. Pathol. 214:231–241 [DOI] [PubMed] [Google Scholar]
- 3. Beausejour CM, Campisi J. 2006. Ageing: balancing regeneration and cancer. Nature 443:404–405 [DOI] [PubMed] [Google Scholar]
- 4. Betts MR, et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brenchley JM, et al. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365–1371 [DOI] [PubMed] [Google Scholar]
- 6. Cao W, et al. 2009. Premature aging of T cells is associated with faster HIV-1 disease progression. J. Acquir. Immune Defic. Syndr. 50:137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Celli GB, de Lange T. 2005. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat. Cell Biol. 7:712–718 [DOI] [PubMed] [Google Scholar]
- 8. Cesare AJ, Reddel RR. 2008. Telomere uncapping and alternative lengthening of telomeres. Mech. Ageing Dev. 129:99–108 [DOI] [PubMed] [Google Scholar]
- 9. Chebel A, et al. 2009. Telomere uncapping during in vitro T-lymphocyte senescence. Aging Cell 8:52–64 [DOI] [PubMed] [Google Scholar]
- 10. Day CL, et al. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354 [DOI] [PubMed] [Google Scholar]
- 11. de Lange T. 2005. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19:2100–2110 [DOI] [PubMed] [Google Scholar]
- 12. Dimitrova N, de Lange T. 2006. MDC1 accelerates nonhomologous end-joining of dysfunctional telomeres. Genes Dev. 20:3238–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Effros RB, et al. 1996. Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS 10:F17–F22 [DOI] [PubMed] [Google Scholar]
- 14. Effros RB, Dagarag M, Spaulding C, Man J. 2005. The role of CD8+ T-cell replicative senescence in human aging. Immunol. Rev. 205:147–157 [DOI] [PubMed] [Google Scholar]
- 15. Ferreira C, Barthlott T, Garcia S, Zamoyska R, Stockinger B. 2000. Differential survival of naive CD4 and CD8 T cells. J. Immunol. 165:3689–3694 [DOI] [PubMed] [Google Scholar]
- 16. Foulds KE, et al. 2002. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 168:1528–1532 [DOI] [PubMed] [Google Scholar]
- 17. Homann D, Teyton L, Oldstone MB. 2001. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7:913–919 [DOI] [PubMed] [Google Scholar]
- 18. Janzen V, et al. 2006. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature 443:421–426 [DOI] [PubMed] [Google Scholar]
- 19. Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. 1999. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 283:1321–1325 [DOI] [PubMed] [Google Scholar]
- 20. Krishnamurthy J, et al. 2006. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 443:453–457 [DOI] [PubMed] [Google Scholar]
- 21. Laharanne E, et al. 2010. CDKN2A-CDKN2B deletion defines an aggressive subset of cutaneous T-cell lymphoma. Mod Pathol. 23:547–558 [DOI] [PubMed] [Google Scholar]
- 22. Lichterfeld M, et al. 2004. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J. Exp. Med. 200:701–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lichterfeld M, et al. 2008. Telomerase activity of HIV-1-specific CD8+ T cells: constitutive up-regulation in controllers and selective increase by blockade of PD ligand 1 in progressors. Blood 112:3679–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Y, et al. 2011. Expression of p16(INK4a) prevents cancer and promotes aging in lymphocytes. Blood 117:3257–3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Y, et al. 2009. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell 8:439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Y, Sharpless NE. 2009. Tumor suppressor mechanisms in immune aging. Curr. Opin. Immunol. 21:431–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Migliaccio M, Raj K, Menzel O, Rufer N. 2005. Mechanisms that limit the in vitro proliferative potential of human CD8+ T lymphocytes. J. Immunol. 174:3335–3343 [DOI] [PubMed] [Google Scholar]
- 28. Migueles SA, et al. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061–1068 [DOI] [PubMed] [Google Scholar]
- 29. Onen NF, Overton ET. 2011. A review of premature frailty in HIV-infected persons; another manifestation of HIV-related accelerated aging. Curr. Aging Sci. 4:33–41 [PubMed] [Google Scholar]
- 30. Palm W, de Lange T. 2008. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 42:301–334 [DOI] [PubMed] [Google Scholar]
- 31. Poncet D, et al. 2008. Changes in the expression of telomere maintenance genes suggest global telomere dysfunction in B-chronic lymphocytic leukemia. Blood 111:2388–2391 [DOI] [PubMed] [Google Scholar]
- 32. Rai R, Chang S. 2011. Probing the telomere damage response. Methods Mol. Biol. 735:145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rayess H, Wang MB, Srivatsan ES. 2012. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 130:1715–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shankar P, et al. 2000. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood 96:3094–3101 [PubMed] [Google Scholar]
- 35. Stockmann M, et al. 2000. Mechanisms of epithelial barrier impairment in HIV infection. Ann. N. Y. Acad. Sci. 915:293–303 [DOI] [PubMed] [Google Scholar]
- 36. Takai H, Smogorzewska A, de Lange T. 2003. DNA damage foci at dysfunctional telomeres. Curr. Biol. 13:1549–1556 [DOI] [PubMed] [Google Scholar]
- 37. Trautmann L, et al. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12:1198–1202 [DOI] [PubMed] [Google Scholar]
- 38. van Baarle D, Tsegaye A, Miedema F, Akbar A. 2005. Significance of senescence for virus-specific memory T cell responses: rapid ageing during chronic stimulation of the immune system. Immunol. Lett. 97:19–29 [DOI] [PubMed] [Google Scholar]
- 39. van Steensel B, de Lange T. 1997. Control of telomere length by the human telomeric protein TRF1. Nature 385:740–743 [DOI] [PubMed] [Google Scholar]
- 40. van Steensel B, Smogorzewska A, de Lange T. 1998. TRF2 protects human telomeres from end-to-end fusions. Cell 92:401–413 [DOI] [PubMed] [Google Scholar]
- 41. Velu V, et al. 2009. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458:206–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wolthers KC, et al. 1996. T cell telomere length in HIV-1 infection: no evidence for increased CD4+ T cell turnover. Science 274:1543–1547 [DOI] [PubMed] [Google Scholar]